Benefits of Polyphenols and Methylxanthines from Cocoa Beans on Dietary Metabolic Disorders
Abstract
1. Introduction
2. Taxonomy
3. Bean Morphology
4. Bean Composition
4.1. Polyphenols
- ο
- Total polyphenol content by the Folin–Ciocalteu method
- ο
- Total anthocyanidins content by the analysis of pH variation
- ο
- Total flavonoids content by AlCl3 method
- ο
- Total proanthocyanidins by Vanillin, DMACA, butanol-HCl or bovine serum albumin (BSA) methods
- ο
- Thin layer chromatography
- ο
- High performance liquid chromatography (Identification with or without mass spectrometry detector/Determination of the polymerization degree of tannin by thiolysis such as phloroglucinolysis)
4.2. Methylxanthines
- Spectrophotometric methods such as AOAC Micro Bailey–Andrew method and Morton–Stubb method
- Thin-layer chromatography
- Gas Chromatography
- LC (Liquid chromatography) or HPLC (High performance liquid chromatography) with or without MS (mass spectrometry detector) for identifications
- Capillary electrophoresis
5. Health Benefits of Polyphenols and Methylxanthines from Cocoa
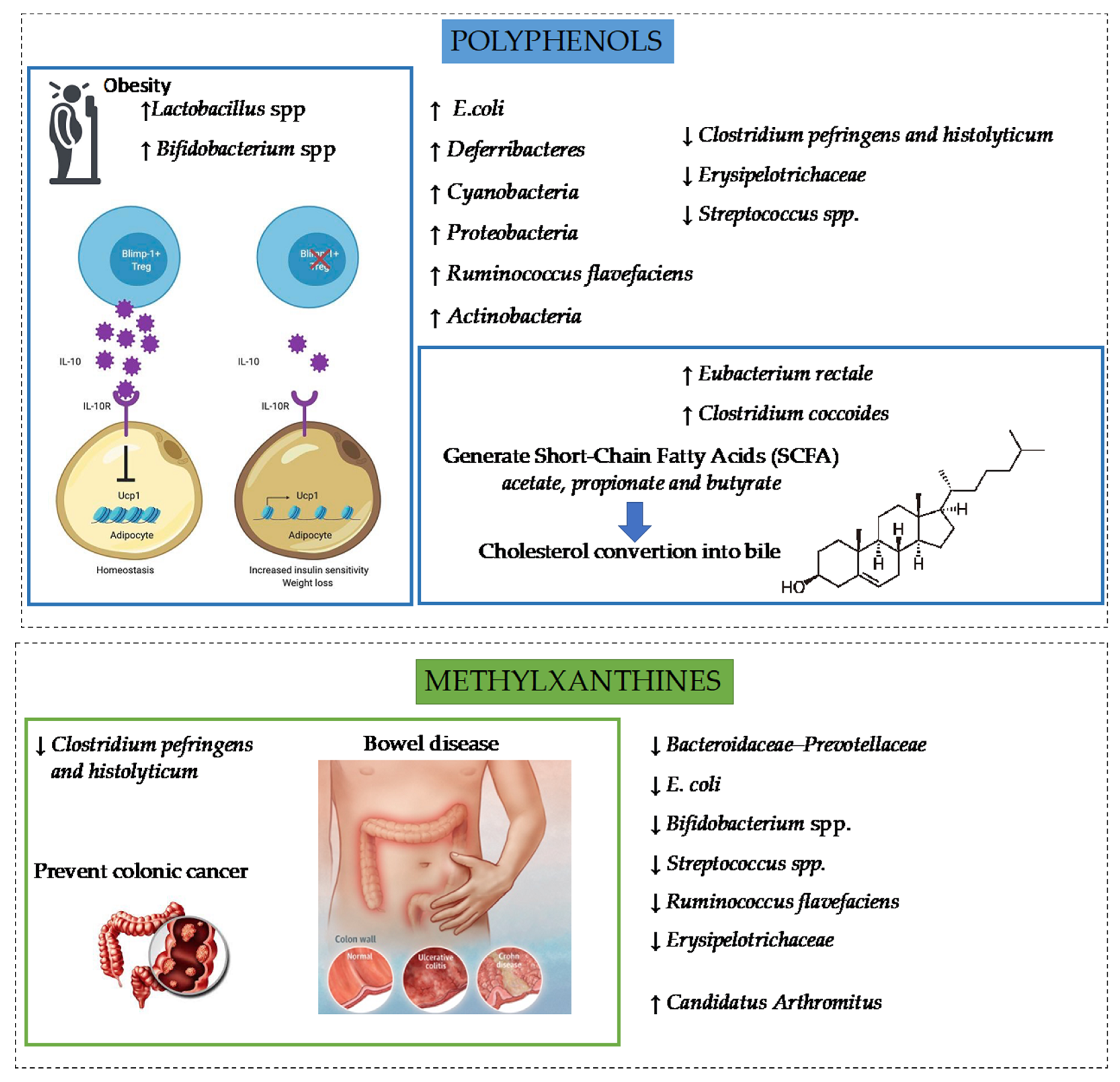
5.1. Cocoa and Intestinal Inflammation
5.2. Cocoa and Obesity
5.3. Cocoa and Diabetes
5.4. Cocoa and Gut Microbiota
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lachenaud, P.; Sounigo, O.; Sallée, B. Les Cacaoyers Spontanés de Guyane Française: État Des Recherches. Acta Bot. Gall. 2005, 152, 325–346. [Google Scholar] [CrossRef]
- Beg, M.S.; Ahmad, S.; Jan, K.; Bashir, K. Status, Supply Chain and Processing of Cocoa—A Review. Trends Food Sci. Technol. 2017, 66, 108–116. [Google Scholar] [CrossRef]
- Andújar, I.; Recio, M.C.; Giner, R.M.; Ríos, J.L. Cocoa Polyphenols and Their Potential Benefits for Human Health. Oxid. Med. Cell Longev. 2012, 2012, 906252. [Google Scholar] [CrossRef]
- Jean-Marie, E.; Bereau, D.; Poucheret, P.; Guzman, C.; Boudard, F.; Robinson, J.-C. Antioxidative and Immunomodulatory Potential of the Endemic French Guiana Wild Cocoa “Guiana”. Foods 2021, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Wollgast, J.; Anklam, E. Review on Polyphenols in Theobroma cacao: Changes in Composition during the Manufacture of Chocolate and Methodology for Identification and Quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Latif, R. Chocolate/Cocoa and Human Health: A Review. Neth. J. Med. 2013, 71, 63–68. [Google Scholar]
- Rabadan-Chávez, G.; Quevedo-Corona, L.; Garcia, A.M.; Reyes-Maldonado, E.; Jaramillo-Flores, M.E. Cocoa Powder, Cocoa Extract and Epicatechin Attenuate Hypercaloric Diet-Induced Obesity through Enhanced β-Oxidation and Energy Expenditure in White Adipose Tissue. J. Funct. Foods 2016, 20, 54–67. [Google Scholar] [CrossRef]
- Crichton, G.E.; Elias, M.F.; Dearborn, P.; Robbins, M. Habitual Chocolate Intake and Type 2 Diabetes Mellitus in the Maine-Syracuse Longitudinal Study: (1975–2010): Prospective Observations. Appetite 2017, 108, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Żyżelewicz, D.; Zakłos-Szyda, M.; Juśkiewicz, J.; Bojczuk, M.; Oracz, J.; Budryn, G.; Miśkiewicz, K.; Krysiak, W.; Zduńczyk, Z.; Jurgoński, A. Cocoa Bean (Theobroma cacao L.) Phenolic Extracts as PTP1B Inhibitors, Hepatic HepG2 and Pancreatic β-TC3 Cell Cytoprotective Agents and Their Influence on Oxidative Stress in Rats. Food Res. Int. 2016, 89 Pt 2, 946–957. [Google Scholar] [CrossRef]
- Mirpuri, J.; Raetz, M.; Sturge, C.R.; Wilhelm, C.L.; Benson, A.; Savani, R.C.; Hooper, L.V.; Yarovinsky, F. Proteobacteria-Specific IgA Regulates Maturation of the Intestinal Microbiota. Gut Microbes 2014, 5, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Verna, R. The History and Science of Chocolate. Malays. J. Pathol. 2013, 35, 111–121. [Google Scholar] [PubMed]
- Cuatrecasas, J. Cacao and Its Allies: A Taxonomic Revision of the Genus Theobroma; Contributions from the United States National Herbarium; Smithsonian Institution Press: Washington, DC, USA, 1964; Volume 35. [Google Scholar]
- Silva, C.R.S.; Venturieri, G.A.; Figueira, A. Description of Amazonian Theobroma L. Collections, Species Identification, and Characterization of Interspecific Hybrids. Acta Bot. Bras. 2004, 18, 333–341. [Google Scholar] [CrossRef][Green Version]
- Sounigo, O.; Lachenaud, P.; Bastide, P.; Cilas, C.; N’Goran, J.; Lanaud, C. Assessment of the Value of Doubled Haploids as Progenitors in Cocoa (Theobroma cacao L.) Breeding. J. Appl. Genet. 2003, 44, 339–353. [Google Scholar]
- Motamayor, J.C.; Risterucci, A.M.; Heath, M.; Lanaud, C. Cacao Domestication II: Progenitor Germplasm of the Trinitario Cacao Cultivar. Heredity 2003, 91, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R. Taxonomy/Classification of Cocoa. 2018, pp. 1–16. Available online: https://biblio1.iita.org/handle/20.500.12478/4366 (accessed on 22 June 2021).
- Laurent, V.; Risterucci, A.M.; Lanaud, C. Genetic Diversity in Cocoa Revealed by CDNA Probes. Theor. Appl. Genet. 1994, 88, 193–198. [Google Scholar] [CrossRef]
- N’Goran, J.A.K.; Laurent, V.; Risterucci, A.M.; Lanaud, C. Comparative Genetic Diversity Studies of Theobroma cacao L. Using RFLP and RAPD Markers. Heredity 1994, 73, 589–597. [Google Scholar] [CrossRef]
- Ronning, C.M.; Schnell, R.J. Allozyme Diversity in a Germplasm Collection of Theobroma cacao L. J. Hered. 1994, 85, 291–295. [Google Scholar] [CrossRef]
- Motamayor, J.C.; Lachenaud, P.; Da Silva e Mota, J.W.; Loor, R.; Kuhn, D.N.; Brown, J.S.; Schnell, R.J. Geographic and Genetic Population Differentiation of the Amazonian Chocolate Tree (Theobroma cacao L). PLoS ONE 2008, 3, e3311. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.; Bekele, F.; Schnell, R.J. Field Guide to the ICS Clones of Trinidad; Tropical Agricultural Research and Higher Education Center: Turrialba, Costa Rica, 2004. [Google Scholar]
- Toxopeus, H. Botany, Types and Populations. In Cocoa, 4th ed.; Wiley Online Library: Hoboken, NJ, USA, 2001; pp. 11–37. [Google Scholar]
- Fowler, M.S.; Coutel, F. Cocoa beans: From tree to factory. In Beckett’s Industrial Chocolate Manufacture and Use; Wiley-Blackwell: Hoboken, NJ, USA, 2008; pp. 9–49. ISBN 978-1-118-92359-7. [Google Scholar]
- Kadow, D. The Biochemistry of Cocoa Flavor—A Holistic Analysis of Its Development along the Processing Chain. J. Appl. Bot. Food Qual. 2020, 93, 300–312. [Google Scholar] [CrossRef]
- Badrie, N.; Bekele, F.; Sikora, E.; Sikora, M. Cocoa Agronomy, Quality, Nutritional, and Health Aspects. Crit. Rev. Food Sci. Nutr. 2015, 55, 620–659. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, H.; Kato, M.; Crozier, A. Distribution, Biosynthesis and Catabolism of Methylxanthines in Plants. In Methylxanthines, Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 200, pp. 11–31. ISBN 978-3-642-13442-5. [Google Scholar]
- Agus, B.A.P.; Mohamad, N.; Hussain, N. Composition of Unfermented, Unroasted, Roasted Cocoa Beans and Cocoa Shells from Peninsular Malaysia. J. Food Meas. Charact. 2018, 12, 2581–2589. [Google Scholar] [CrossRef]
- Oracz, J.; Zyzelewicz, D.; Nebesny, E. The Content of Polyphenolic Compounds in Cocoa Beans (Theobroma cacao L.), Depending on Variety, Growing Region, and Processing Operations: A Review. Crit. Rev. Food Sci. Nutr. 2013, 55, 1176–1192. [Google Scholar] [CrossRef]
- Rusconi, M.; Conti, A. Theobroma cacao L., the Food of the Gods: A Scientific Approach beyond Myths and Claims. Pharmacol. Res. 2010, 61, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Andres-Lacueva, C.; Monagas, M.; Khan, N.; Izquierdo-Pulido, M.; Urpi-Sarda, M.; Permanyer, J.; Lamuela-Raventós, R.M. Flavanol and Flavonol Contents of Cocoa Powder Products: Influence of the Manufacturing Process. J. Agric. Food Chem. 2008, 56, 3111–3117. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid Chromatographic/Electrospray Ionization Tandem Mass Spectrometric Study of the Phenolic Composition of Cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.L. Cocoa and Chocolate in Human Health and Disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef] [PubMed]
- Hurst, W.J.; Glinski, J.A.; Miller, K.B.; Apgar, J.; Davey, M.H.; Stuart, D.A. Survey of the Trans-Resveratrol and Trans-Piceid Content of Cocoa-Containing and Chocolate Products. J. Agric. Food Chem. 2008, 56, 8374–8378. [Google Scholar] [CrossRef]
- Elwers, S.; Zambrano, A.; Rohsius, C.; Lieberei, R. Differences between the Content of Phenolic Compounds in Criollo, Forastero and Trinitario Cocoa Seed (Theobroma cacao L.). Eur. Food Res. Technol. 2009, 229, 937–948. [Google Scholar] [CrossRef]
- D’Souza, R.N.; Grimbs, S.; Behrends, B.; Bernaert, H.; Ullrich, M.S.; Kuhnert, N. Origin-Based Polyphenolic Fingerprinting of Theobroma cacao in Unfermented and Fermented Beans. Food Res. Int. 2017, 99, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, L.C.; Londoño-Londoño, J.; Gil, A. Comparison of Polyphenol, Methylxanthines and Antioxidant Activity in Theobroma cacao Beans from Different Cocoa-Growing Areas in Colombia. Food Res. Int. 2014, 60, 273–280. [Google Scholar] [CrossRef]
- Camu, N.; Winter, T.D.; Addo, S.K.; Takrama, J.S.; Bernaert, H.; Vuyst, L.D. Fermentation of Cocoa Beans: Influence of Microbial Activities and Polyphenol Concentrations on the Flavour of Chocolate. J. Sci. Food Agric. 2008, 88, 2288–2297. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Borges, G.; Nagai, C.; Jackson, M.C.; Yokota, T.; Crozier, A.; Ashihara, H. Profiles of Phenolic Compounds and Purine Alkaloids during the Development of Seeds of Theobroma cacao cv. Trinitario. J. Agric. Food Chem. 2013, 61, 427–434. [Google Scholar] [CrossRef]
- Yen, D.T.K.; Ha, N.V.H. Effects of Maturity Stages and Fermentation of Cocoa Beans on Total Phenolic Contents and Antioxidant Capacities in Raw Cocoa Powder. Vietnam. J. Biotechnol. 2016, 14, 743–752. [Google Scholar] [CrossRef]
- do Carmo Brito, B.d.N.; Chisté, R.C.; da Silva Pena, R.; Gloria, M.B.A.; Lopes, A.S. Bioactive Amines and Phenolic Compounds in Cocoa Beans Are Affected by Fermentation. Food Chem. 2017, 228, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Efraim, P.; Barreto Alves, A.; Jardim, D. Review: Polyphenols in Cocoa and Derivatives: Factors of Variation and Health Effects. Braz. J. Food Technol. 2011, 14, 181–201. [Google Scholar] [CrossRef]
- Santander Muñoz, M.; Rodríguez Cortina, J.; Vaillant, F.E.; Escobar Parra, S. An Overview of the Physical and Biochemical Transformation of Cocoa Seeds to Beans and to Chocolate: Flavor Formation. Crit. Rev. Food Sci. Nutr. 2020, 60, 1593–1613. [Google Scholar] [CrossRef]
- De Taeye, C.; Eyamo Evina, V.J.; Caullet, G.; Niemenak, N.; Collin, S. Fate of Anthocyanins through Cocoa Fermentation. Emergence of New Polyphenolic Dimers. J. Agric. Food Chem. 2016, 64, 8876–8885. [Google Scholar] [CrossRef]
- Le, X.T.; Huynh, M.T.; Pham, T.N.; Than, V.T.; Toan, T.Q.; Bach, L.G.; Trung, N.Q. Optimization of Total Anthocyanin Content, Stability and Antioxidant Evaluation of the Anthocyanin Extract from Vietnamese Carissa carandas L. Fruits. Processes 2019, 7, 468. [Google Scholar] [CrossRef]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of Pigment and Flavanol Content with Antioxidant Properties in Selected Aged Regional Wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of Antioxidant Activity of Vanillin by Using Multiple Antioxidant Assays. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2011, 1810, 170–177. [Google Scholar] [CrossRef]
- Da Silva, L.A.L.; Pezzini, B.R.; Soares, L. Spectrophotometric Determination of the Total Flavonoid Content in Ocimum basilicum L. (Lamiaceae) Leaves. Pharm. Mag. 2015, 11, 96–101. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. Protein Precipitation Method for the Quantitative Determination of Tannins. J. Agric. Food Chem. 1978, 26, 809–812. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The Conversion of Procyanidins and Prodelphinidins to Cyanidin and Delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Analytical Dataset of Ecuadorian Cocoa Shells and Beans. Data Brief 2018, 22, 56–64. [Google Scholar] [CrossRef]
- Kennedy, J.; Jones, G. Analysis of Proanthocyanidin Cleavage Products Following Acid-Catalysis in the Presence of Excess Phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Bartella, L.; Di Donna, L.; Napoli, A.; Siciliano, C.; Sindona, G.; Mazzotti, F. A Rapid Method for the Assay of Methylxanthines Alkaloids: Theobromine, Theophylline and Caffeine, in Cocoa Products and Drugs by Paper Spray Tandem Mass Spectrometry. Food Chem. 2019, 278, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Kongor, J.E.; Hinneh, M.; de Walle, D.V.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors Influencing Quality Variation in Cocoa (Theobroma cacao) Bean Flavour Profile—A Review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Davrieux, F.; Assemat, S.; Sukha, D.; Bastianelli, D.; Renaud, B.; Cros, E. Genotype Characterization of Cocoa into Genetic Groups through Caffeine and Theobromine Content Predicted by NIRS. In Proceedings of the 12th International Conference, Auckland, New Zealand, 9–15 April 2005; pp. 14–19. [Google Scholar]
- Cruz, J.; Leite, P.; Soares, S.; Bispo, E. Bioactive Compounds in Different Cocoa (Theobroma cacao L.) Cultivars during Fermentation. Food Sci. Technol. 2015, 35, 279–284. [Google Scholar] [CrossRef]
- Dang, Y.K.T.; Nguyen, H.V.H. Effects of Maturity at Harvest and Fermentation Conditions on Bioactive Compounds of Cocoa Beans. Plant Foods Hum. Nutr. 2019, 74, 54–60. [Google Scholar] [CrossRef]
- Hurst, W.; Martin, R.; Tarka, S. Analytical Methods for Quantitation of Methylxanthines. Prog. Clin. Biol. Res. 1984, 158, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, U.M.; Wijesekera, R.O. A Rapid Micro-Method for the Separation, Identification and Estimation of the Purine Bases: Caffeine, Theobromine and Theophylline. J. Chromatogr. 1968, 32, 75–86. [Google Scholar] [CrossRef]
- Soares, J.-B.; Pimentel-Nunes, P.; Roncon-Albuquerque, R.; Leite-Moreira, A. The Role of Lipopolysaccharide/Toll-like Receptor 4 Signaling in Chronic Liver Diseases. Hepatol. Int. 2010, 4, 659–672. [Google Scholar] [CrossRef]
- Majer, O.; Liu, B.; Barton, G.M. Nucleic Acid-Sensing TLRs: Trafficking and Regulation. Curr. Opin. Immunol. 2017, 44, 26–33. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H. Basic Immunology: Glossary; Elsevier Masson: Paris, France, 2008; ISBN 978-2-8101-0023-1. [Google Scholar]
- Honda, K.; Takaoka, A.; Taniguchi, T. Type I Inteferon Gene Induction by the Interferon Regulatory Factor Family of Transcription Factors. Immunity 2006, 25, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, C. Stress oxydatif et inflammation. Rev. Fr. Lab. 1995, 1995, 87–92. [Google Scholar] [CrossRef]
- Bowie, A.; O’Neill, L.A. Oxidative Stress and Nuclear Factor-KappaB Activation: A Reassessment of the Evidence in the Light of Recent Discoveries. Biochem. Pharmacol. 2000, 59, 13–23. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ro, J.Y. Signal Pathway of Cytokines Produced by Reactive Oxygen Species Generated from Phorbol Myristate Acetate-Stimulated HMC-1 Cells. Scand. J. Immunol. 2005, 62, 25–35. [Google Scholar] [CrossRef]
- Pan, M.-H.; Lai, C.-S.; Wu, J.-C.; Ho, C.-T. Molecular Mechanisms for Chemoprevention of Colorectal Cancer by Natural Dietary Compounds. Mol. Nutr. Food Res. 2011, 55, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.-L.; Olivier, Y.; Trouillas, P. Free Radical Scavenging by Natural Polyphenols: Atom versus Electron Transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Buckley, A.; Turner, J.R. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef] [PubMed]
- Rossin, D.; Barbosa-Pereira, L.; Iaia, N.; Sottero, B.; Danzero, A.C.; Poli, G.; Zeppa, G.; Biasi, F. Protective Effect of Cocoa Bean Shell against Intestinal Damage: An Example of Byproduct Valorization. Antioxidants 2021, 10, 280. [Google Scholar] [CrossRef]
- Xu, M.; Li, X.-X.; Wang, L.; Wang, M.; Zhang, Y.; Li, P.-L. Contribution of Nrf2 to Atherogenic Phenotype Switching of Coronary Arterial Smooth Muscle Cells Lacking CD38 Gene. Cell. Physiol. Biochem. 2015, 37, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Iaia, N.; Rossin, D.; Sottero, B.; Venezia, I.; Poli, G.; Biasi, F. Efficacy of Theobromine in Preventing Intestinal CaCo-2 Cell Damage Induced by Oxysterols. Arch. Biochem. Biophys. 2020, 694, 108591. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Cilleros, D.; Ramos, S.; López-Oliva, M.E.; Escrivá, F.; Álvarez, C.; Fernández-Millán, E.; Martín, M.Á. Cocoa Diet Modulates Gut Microbiota Composition and Improves Intestinal Health in Zucker Diabetic Rats. Food Res. Int. 2020, 132, 109058. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Shi, Y.; Huang, W.; Lao, C.; Zou, Z.; Pan, S.; Huang, Z. Theobromine Mitigates IL-1β-Induced Oxidative Stress, Inflammatory Response, and Degradation of Type II Collagen in Human Chondrocytes. Int. Immunopharmacol. 2020, 82, 106226. [Google Scholar] [CrossRef]
- Midttun, H.L.E.; Ramsay, A.; Mueller-Harvey, I.; Williams, A.R. Cocoa Procyanidins Modulate Transcriptional Pathways Linked to Inflammation and Metabolism in Human Dendritic Cells. Food Funct. 2018, 9, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Stepniak, D.; Koning, F. Celiac Disease—Sandwiched between Innate and Adaptive Immunity. Hum. Immunol. 2006, 67, 460–468. [Google Scholar] [CrossRef]
- Bayardo, M.; Punzi, F.; Bondar, C.; Chopita, N.; Chirdo, F. Transglutaminase 2 Expression Is Enhanced Synergistically by Interferon-γ and Tumour Necrosis Factor-α in Human Small Intestine. Clin. Exp. Immunol. 2012, 168, 95–104. [Google Scholar] [CrossRef]
- Kramer, K.; Yeboah-Awudzi, M.; Magazine, N.; King, J.M.; Xu, Z.; Losso, J.N. Procyanidin B2 Rich Cocoa Extracts Inhibit Inflammation in Caco-2 Cell Model of in Vitro Celiac Disease by down-Regulating Interferon-Gamma- or Gliadin Peptide 31-43-Induced Transglutaminase-2 and Interleukin-15. J. Funct. Foods 2019, 57, 112–120. [Google Scholar] [CrossRef]
- Larché, M.; Akdis, C.A.; Valenta, R. Immunological Mechanisms of Allergen-Specific Immunotherapy. Nat. Rev. Immunol. 2006, 6, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Abril-Gil, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M. Effect of a Cocoa-Enriched Diet on Immune Response and Anaphylaxis in a Food Allergy Model in Brown Norway Rats. J. Nutr. Biochem. 2016, 27, 317–326. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Stern, J.H.; Scherer, P.E. The Cell Biology of Fat Expansion. J. Cell Biol. 2015, 208, 501–512. [Google Scholar] [CrossRef]
- Chew, S.C.; Nyam, K.L. Chapter 6—Refining of edible oils. In Lipids and Edible Oils; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 213–241. ISBN 978-0-12-817105-9. [Google Scholar]
- Boden, G. Obesity and Free Fatty Acids (FFA). Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef]
- Zhou, H.; Urso, C.; Jadeja, V. Saturated Fatty Acids in Obesity-Associated Inflammation. J. Inflamm Res. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Leyva-Soto, A.; Chavez-Santoscoy, R.A.; Lara-Jacobo, L.R.; Chavez-Santoscoy, A.V.; Gonzalez-Cobian, L.N. Daily Consumption of Chocolate Rich in Flavonoids Decreases Cellular Genotoxicity and Improves Biochemical Parameters of Lipid and Glucose Metabolism. Molecules 2018, 23, 2220. [Google Scholar] [CrossRef]
- Yoon, M. The Role of PPARα in Lipid Metabolism and Obesity: Focusing on the Effects of Estrogen on PPARα Actions. Pharmacol. Res. 2009, 60, 151–159. [Google Scholar] [CrossRef]
- Coronado-Cáceres, L.J.; Rabadán-Chávez, G.; Quevedo-Corona, L.; Hernández-Ledesma, B.; Garcia, A.M.; Mojica, L.; Lugo-Cervantes, E. Anti-Obesity Effect of Cocoa Proteins (Theobroma cacao L.) Variety “Criollo” and the Expression of Genes Related to the Dysfunction of White Adipose Tissue in High-Fat Diet-Induced Obese Rats. J. Funct. Foods 2019, 62, 103519. [Google Scholar] [CrossRef]
- Cheng, C.-F.; Ku, H.-C.; Lin, H. PGC-1α as a Pivotal Factor in Lipid and Metabolic Regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef]
- Jang, Y.J.; Koo, H.J.; Sohn, E.-H.; Kang, S.C.; Rhee, D.-K.; Pyo, S. Theobromine Inhibits Differentiation of 3T3-L1 Cells during the Early Stage of Adipogenesis via AMPK and MAPK Signaling Pathways. Food Funct. 2015, 6, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Mitani, T.; Watanabe, S.; Yoshioka, Y.; Katayama, S.; Nakamura, S.; Ashida, H. Theobromine Suppresses Adipogenesis through Enhancement of CCAAT-Enhancer-Binding Protein β Degradation by Adenosine Receptor A1. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2017, 1864, 2438–2448. [Google Scholar] [CrossRef]
- Ali, F.; Ismail, A.; Esa, N.M.; Pei, C. Cocoa Polyphenols Treatment Ameliorates Visceral Obesity by Reduction Lipogenesis and Promoting Fatty Acid Oxidation Genes in Obese Rats through Interfering with AMPK Pathway. Eur. J. Lipid Sci. Technol. 2016, 118, 564–575. [Google Scholar] [CrossRef]
- Dobrzyn, P.; Dobrzyn, A.; Miyazaki, M.; Cohen, P.; Asilmaz, E.; Hardie, D.G.; Friedman, J.M.; Ntambi, J.M. Stearoyl-CoA Desaturase 1 Deficiency Increases Fatty Acid Oxidation by Activating AMP-Activated Protein Kinase in Liver. Proc. Natl. Acad. Sci. USA 2004, 101, 6409–6414. [Google Scholar] [CrossRef]
- Acheson, K.J.; Gremaud, G.; Meirim, I.; Montigon, F.; Krebs, Y.; Fay, L.B.; Gay, L.-J.; Schneiter, P.; Schindler, C.; Tappy, L. Metabolic Effects of Caffeine in Humans: Lipid Oxidation or Futile Cycling? Am. J. Clin. Nutr. 2004, 79, 40–46. [Google Scholar] [CrossRef]
- García-Merino, J.Á.; Moreno-Pérez, D.; de Lucas, B.; Montalvo-Lominchar, M.G.; Muñoz, E.; Sánchez, L.; Naclerio, F.; Herrera-Rocha, K.M.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E.; et al. Chronic Flavanol-Rich Cocoa Powder Supplementation Reduces Body Fat Mass in Endurance Athletes by Modifying the Follistatin/Myostatin Ratio and Leptin Levels. Food Funct. 2020, 11, 3441–3450. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Avila, J.A.; Rodrigo García, J.; González Aguilar, G.A.; De la Rosa, L.A. The Antidiabetic Mechanisms of Polyphenols Related to Increased Glucagon-Like Peptide-1 (GLP1) and Insulin Signaling. Molecules 2017, 22, 903. [Google Scholar] [CrossRef]
- Papadimitriou, A.; Silva, K.C.; Peixoto, E.B.M.I.; Borges, C.M.; Lopes de Faria, J.M.; Lopes de Faria, J.B. Theobromine Increases NAD+/Sirt-1 Activity and Protects the Kidney under Diabetic Conditions. Am. J. Physiol. Ren. Physiol. 2015, 308, F209–F225. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Millán, E.; Cordero-Herrera, I.; Ramos, S.; Escrivá, F.; Alvarez, C.; Goya, L.; Martín, M.A. Cocoa-Rich Diet Attenuates Beta Cell Mass Loss and Function in Young Zucker Diabetic Fatty Rats by Preventing Oxidative Stress and Beta Cell Apoptosis. Mol. Nutr. Food Res. 2015, 59, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.C.; Villagarcía, H.; Nazar, A.; Arbeláez, L.G.; Massa, M.L.; Del Zotto, H.; Ríos, J.L.; Schinella, G.R.; Francini, F. Cacao Extract Enriched in Polyphenols Prevents Endocrine-Metabolic Disturbances in a Rat Model of Prediabetes Triggered by a Sucrose Rich Diet. J. Ethnopharmacol. 2020, 247, 112263. [Google Scholar] [CrossRef]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular Actions of Insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef]
- Klover, P.J.; Mooney, R.A. Hepatocytes: Critical for Glucose Homeostasis. Int. J. Biochem. Cell Biol. 2004, 36, 753–758. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Cocoa Flavonoids Improve Insulin Signalling and Modulate Glucose Production via AKT and AMPK in HepG2 Cells. Mol. Nutr. Food Res. 2013, 57, 974–985. [Google Scholar] [CrossRef]
- Bowser, S.M.; Moore, W.T.; McMillan, R.P.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Hulver, M.W.; Neilson, A.P. High-Molecular-Weight Cocoa Procyanidins Possess Enhanced Insulin-Enhancing and Insulin Mimetic Activities in Human Primary Skeletal Muscle Cells Compared to Smaller Procyanidins. J. Nutr. Biochem. 2017, 39, 48–58. [Google Scholar] [CrossRef]
- Kawakami, Y.; Watanabe, Y.; Mazuka, M.; Yagi, N.; Sawazaki, A.; Koganei, M.; Natsume, M.; Kuriki, K.; Morimoto, T.; Asai, T.; et al. Effect of Cacao Polyphenol-Rich Chocolate on Postprandial Glycemia, Insulin, and Incretin Secretion in Healthy Participants. Nutrition 2021, 85, 111128. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martín, M.Á.; Escrivá, F.; Álvarez, C.; Goya, L.; Ramos, S. Cocoa-Rich Diet Ameliorates Hepatic Insulin Resistance by Modulating Insulin Signaling and Glucose Homeostasis in Zucker Diabetic Fatty Rats. J. Nutr. Biochem. 2015, 26, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, X.; Li, L. Human Gut Microbiome: The Second Genome of Human Body. Protein Cell 2010, 1, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Guarner, F.; Malagelada, J.-R. Gut Flora in Health and Disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Camps-Bossacoma, M.; Massot-Cladera, M.; Rigo-Adrover, M.; Franch, À.; Pérez-Cano, F.J.; Castell, M. Effect of Cocoa’s Theobromine on Intestinal Microbiota of Rats. Mol. Nutr. Food Res. 2017, 61, 1700238. [Google Scholar] [CrossRef] [PubMed]
- Klinder, A.; Shen, Q.; Heppel, S.; Lovegrove, J.A.; Rowland, I.; Tuohy, K.M. Impact of Increasing Fruit and Vegetables and Flavonoid Intake on the Human Gut Microbiota. Food Funct. 2016, 7, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping Faecal Gut Microbiota Composition by the Intake of Trans-Resveratrol and Quercetin in High-Fat Sucrose Diet-Fed Rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.C.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Flavanol Monomer-Induced Changes to the Human Faecal Microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef]
- Beppu, L.Y.; Mooli, R.G.R.; Qu, X.; Marrero, G.J.; Finley, C.A.; Fooks, A.N.; Mullen, Z.P.; Frias, A.B., Jr.; Sipula, I.; Xie, B.; et al. Tregs Facilitate Obesity and Insulin Resistance via a Blimp-1/IL-10 Axis. Available online: https://insight.jci.org/articles/view/140644/ga (accessed on 22 June 2021).
- Wiese, M.; Bashmakov, Y.; Chalyk, N.; Nielsen, D.S.; Krych, Ł.; Kot, W.; Klochkov, V.; Pristensky, D.; Bandaletova, T.; Chernyshova, M.; et al. Prebiotic Effect of Lycopene and Dark Chocolate on Gut Microbiome with Systemic Changes in Liver Metabolism, Skeletal Muscles and Skin in Moderately Obese Persons. BioMed Res. Int. 2019, 2019, 4625279. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Bobryshev, Y.V.; Kozarov, E.; Sobenin, I.A.; Orekhov, A.N. Intestinal Mucosal Tolerance and Impact of Gut Microbiota to Mucosal Tolerance. Front. Microbiol. 2015, 5, 781. [Google Scholar] [CrossRef]
- Camps-Bossacoma, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M. Gut Microbiota in a Rat Oral Sensitization Model: Effect of a Cocoa-Enriched Diet. Oxid. Med. Cell. Longev. 2017, 2017, 7417505. [Google Scholar] [CrossRef]
- de Oliveira Araújo, F.; de Castro Moreira, M.E.; Lima, C.F.; Toledo, R.C.; de Sousa, A.R.; Veloso, M.P.; de Freitas, P.G.; Dos Santos, M.H.; de Souza, E.C.; Mantovani, H.C.; et al. Bacupari (Garcinia brasiliensis) Extract Modulates Intestinal Microbiota and Reduces Oxidative Stress and Inflammation in Obese Rats. Food Res. Int. 2019, 122, 199–208. [Google Scholar] [CrossRef]
- Hossain, M.N.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Impact of Encapsulating Probiotics with Cocoa Powder on the Viability of Probiotics during Chocolate Processing, Storage, and in Vitro Gastrointestinal Digestion. J. Food Sci. 2021, 86, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Homayouni Rad, A.; Rasouli Pirouzian, H. Optimization of Prebiotic Sucrose-Free Milk Chocolate Formulation by Mixture Design. J. Food Sci. Technol. 2021, 58, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic Evaluation of Cocoa-Derived Flavanols in Healthy Humans by Using a Randomized, Controlled, Double-Blind, Crossover Intervention Study. Am. J. Clin. Nutr. 2010, 93, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Percival, R.S.; Devine, D.A.; Duggal, M.S.; Chartron, S.; Marsh, P.D. The Effect of Cocoa Polyphenols on the Growth, Metabolism, and Biofilm Formation by Streptococcus Mutans and Streptococcus Sanguinis. Eur. J. Oral Sci. 2006, 114, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Inflammatory Bowel Disease. Available online: https://ultra-dd.org/tissue-platform/diseases/inflammatory-bowel-disease (accessed on 5 August 2021).
- Dépistage du Cancer du Côlon: Qui Devrait être Dépisté et Comment. Available online: https://www.a7la-home.com/fr/colon-cancer-screening/ (accessed on 5 August 2021).
- Dabke, K.; Hendrick, G.; Devkota, S. The Gut Microbiome and Metabolic Syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Rachmawaty; Mu’nisa, A.; Hasri; Pagarra, H.; Hartati; Maulana, Z. Active Compounds Extraction of Cocoa Pod Husk (Thebroma Cacaol.) and Potential as Fungicides. J. Phys. Conf. Ser. 2018, 1028, 012013. [Google Scholar] [CrossRef]
- Sotelo, C.L.; Alvis, B.A.; Arrázola, P.G. Evaluation of Epicatechin, Theobromine and Caffeine in Cacao Husks (Theobroma cacao L.), Determination of the Antioxidant Capacity. Rev. Colomb. Cienc. Hortíc. 2015, 9, 124–134. [Google Scholar] [CrossRef]
- Shashikiran, N.; Subba Reddy, V.; Srikanth, R. Chocolate Mouth Rinse: Effect on Plaque Accumulation and Mutans Streptococci Counts When Used by Children. J. Indian Soc. Pedod. Prev. Dent. 2008, 26, 67. [Google Scholar] [CrossRef]
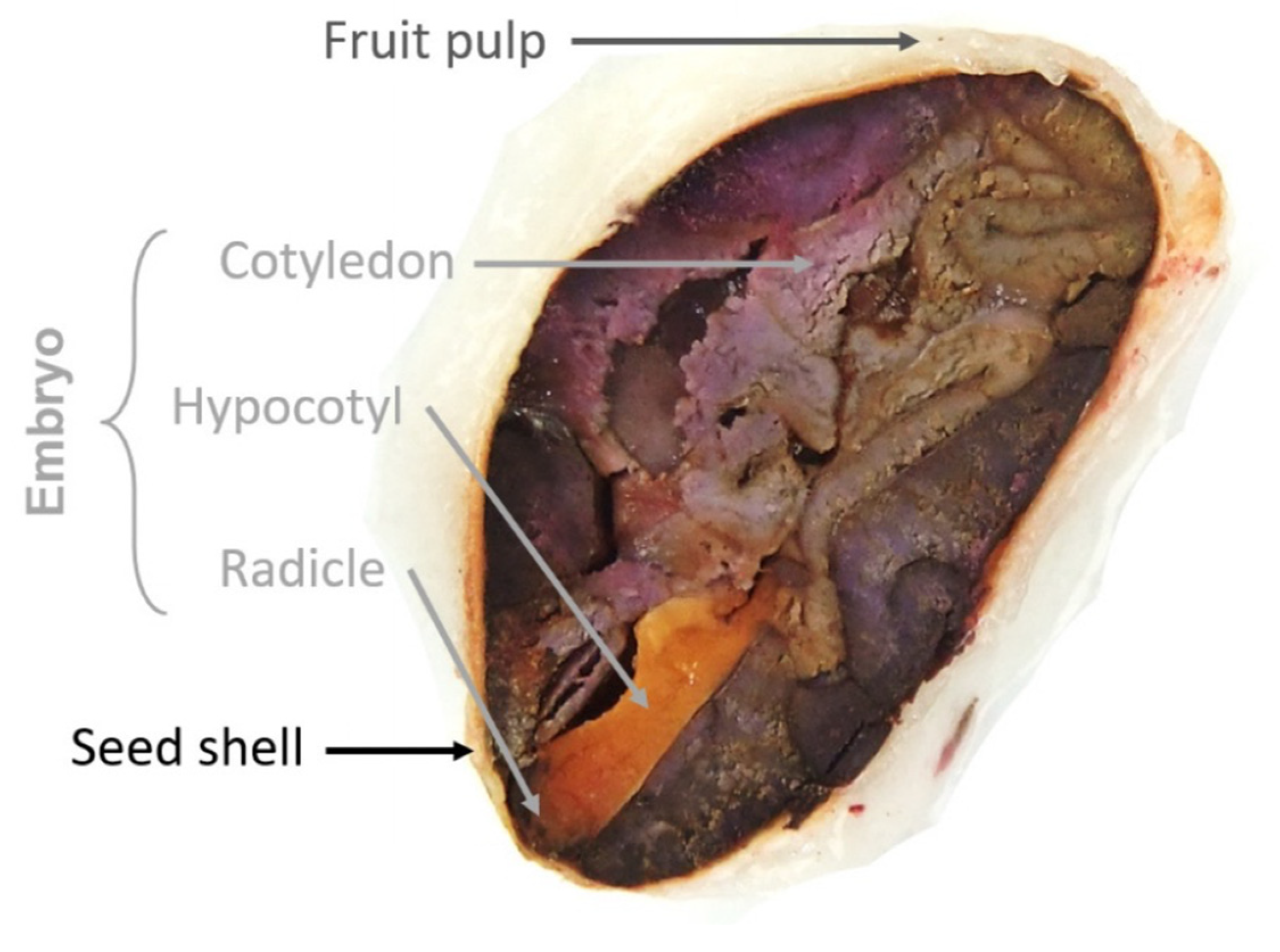
| Almond (%) | Shell (%) | |
|---|---|---|
| Moisture | 2–50 | 4–11 |
| Fat | 48–57 | 2–6 |
| Protein | 10–22 | 13–20 |
| Starch | 6–9 | 6.5–9 |
| Fiber | 2–3.2 | 13–19 |
| Polyphenol | 14–20 | - |
| Theobromine | 0.8–2.7 | 0.2–1.3 |
| Caffeine | 0.1–0.8 | 0.04–0.6 |
| Ash | 2.6–4.2 | 6.5–20.7 |
| Impact of Polyphenols or/and Methylxanthines from Cocoa | Metabolic Pathway Involved | Sources | |
|---|---|---|---|
| Intestinal inflammation | Protection against redox stressors, Prevention of intestinal tigh junction, Reduction of inflammation caused by oxysterols, | Upregulation of Nrf2 expression, Decrease of IL-8 and MCP-1 levels, Restoring Bax/Bcl XL proteins, Avoid decrease of Claudin 1, occludin, and JAM-A levels | [70,72] |
| Reduction of inflammation in colon | Decrease of TNF-α, IL-6 and MCP-1 levels | [73] | |
| Reduction of inflammation | Induce cellular NF-κB activation by inhibiting the IκBα activation, nuclear p65 accumulation and promoter activation | [74] | |
| Reduction of inflammatory markers such as IL-12, IL-23, IL-6, IL-28, IL-29, JAK2 and STAT4 | Modulation of 150 gene expressions, downregulation of gene KEGG JAK-STAT pathway | [75] | |
| Reduction of celiac disease markers | Decrease of the levels of TG2, IL-15, IL-1β, IL-6, IL-8 induced by IFN-γ or pep 31–43 | [78] | |
| Reduction of prevention of food allergy IgE | Upregulation of the gene expression of IgE receptor FcεRI, decrease of rat mast cell protease II (RMCP-II) levels, prevention of the synthesis and decrease of anti-ovalbumin IgE, | [80] | |
| Obesity | Reduction of adiposity, dyslipidemia, plasma triglyceride levels and increase HDL-cholesterol | Upregulation of PPARγ, PGC1α and SIRT1 | [86,87,88] |
| Reduction in lipid accumulation and inhibition of the differentiation of preadipocytes | Decrease the expression of PPARγ and C/EBPα and C/EBPβ, activation of AMPK and inhibition of the JNK pathway | [89,90] | |
| Promotion of β-oxidation, lipogenesis of FFA and activation of lipogenic and fatty oxidation enzymes | Activation of AMPK pathway, Down-regulation of the expression of Acaca Mcat, Fasn and Scd1 genes | [87,91] | |
| Improve lipolysis pathway and release of FFA and glycerol | Antagonism potential of adenosine receptors, Enhance the effects of catecholamines, Modulation of the release of noradrenalin, Activation of β-adrenergic receptors | [93] | |
| Influence satiety and energy expenditure, reduce hypercaloric diet induced-leptin resistance, activation of fat body loss | Down regulation of leptin gene expression | [94] | |
| Diabetes | Prevention against ECM accumulation of kidney | Increase of SIRT-1 level by reducing activation of NOX-4 which have blocked the PARP-1 activation and restoration of NAD+ levels | [97] |
| Protection against death-inducing factors on β-cells, Reduction of apoptosis of β-cell mass and delay on the progression of T2D | Antioxidative actions (mainly glutathione peroxidase) | [98] | |
| Increase of HOMA-IR index | Decrease of P-Akt/Akt and P-eNOS/eNOS ratios, decrease of insulin resistance receptor phosphorylation (IRS-1, Ser 307, and Ser 636/639) and activation of the GSK3/GS pathway | [99,101] | |
| Modulation of glucose metabolism with or without insulin | Activation of GLUT-4 activities, Increase of GLP-1 activity, Increase of PEPCK and GK levels, | [103,104,105] | |
| Reduction in postprandial plasma glucose elevation. | Increase insulin secretion and activation of GLP-1 activity | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jean-Marie, E.; Bereau, D.; Robinson, J.-C. Benefits of Polyphenols and Methylxanthines from Cocoa Beans on Dietary Metabolic Disorders. Foods 2021, 10, 2049. https://doi.org/10.3390/foods10092049
Jean-Marie E, Bereau D, Robinson J-C. Benefits of Polyphenols and Methylxanthines from Cocoa Beans on Dietary Metabolic Disorders. Foods. 2021; 10(9):2049. https://doi.org/10.3390/foods10092049
Chicago/Turabian StyleJean-Marie, Elodie, Didier Bereau, and Jean-Charles Robinson. 2021. "Benefits of Polyphenols and Methylxanthines from Cocoa Beans on Dietary Metabolic Disorders" Foods 10, no. 9: 2049. https://doi.org/10.3390/foods10092049
APA StyleJean-Marie, E., Bereau, D., & Robinson, J.-C. (2021). Benefits of Polyphenols and Methylxanthines from Cocoa Beans on Dietary Metabolic Disorders. Foods, 10(9), 2049. https://doi.org/10.3390/foods10092049








