Effects of Herring Milt Hydrolysates and Fractions in a Diet-Induced Obesity Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Supplementation
2.2. Animals and Dietary Treatment
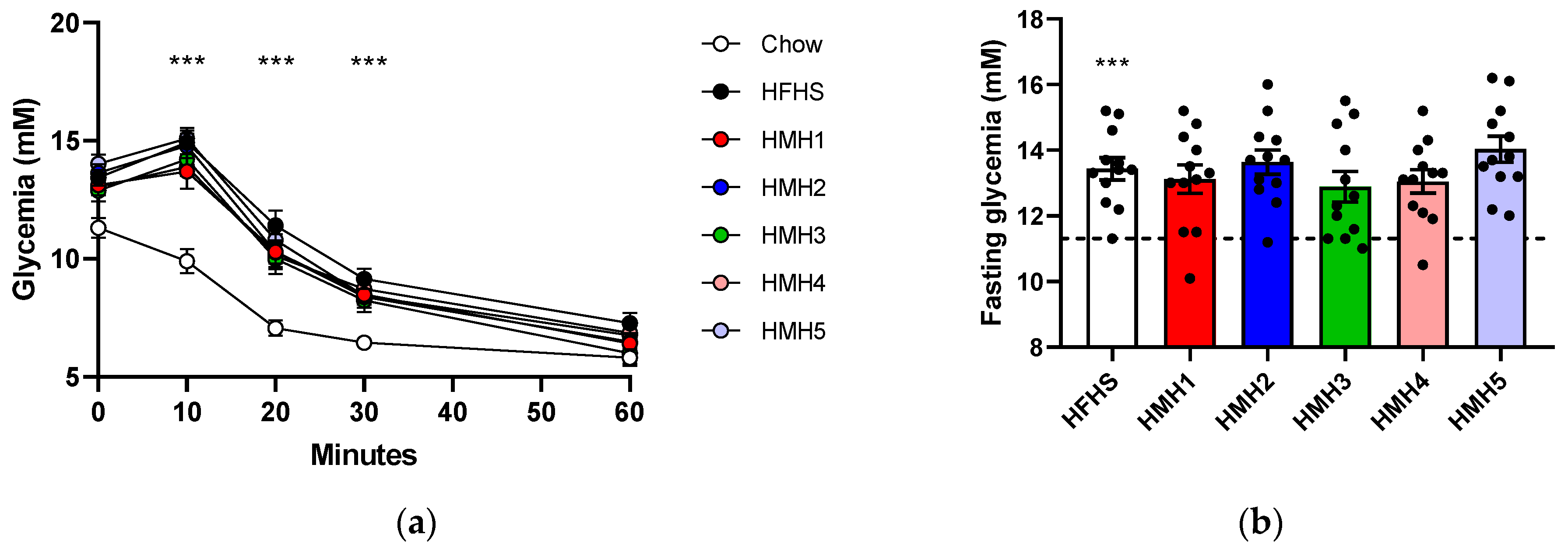
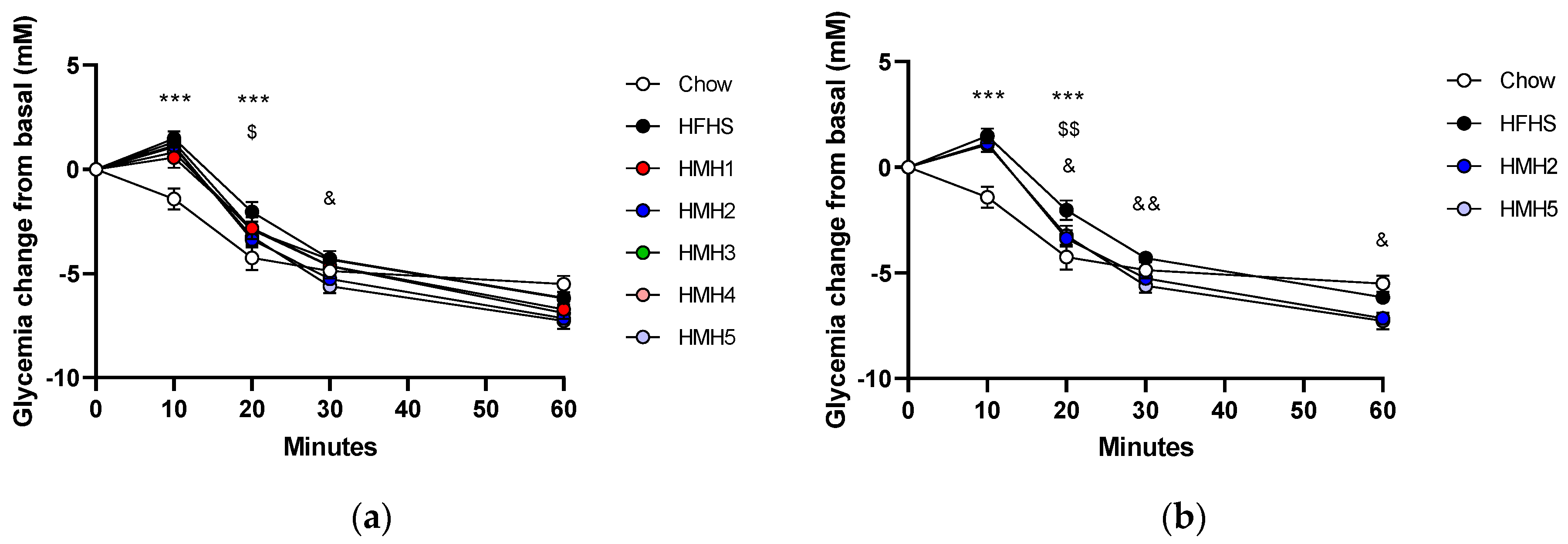
2.3. Insulin Tolerance Test
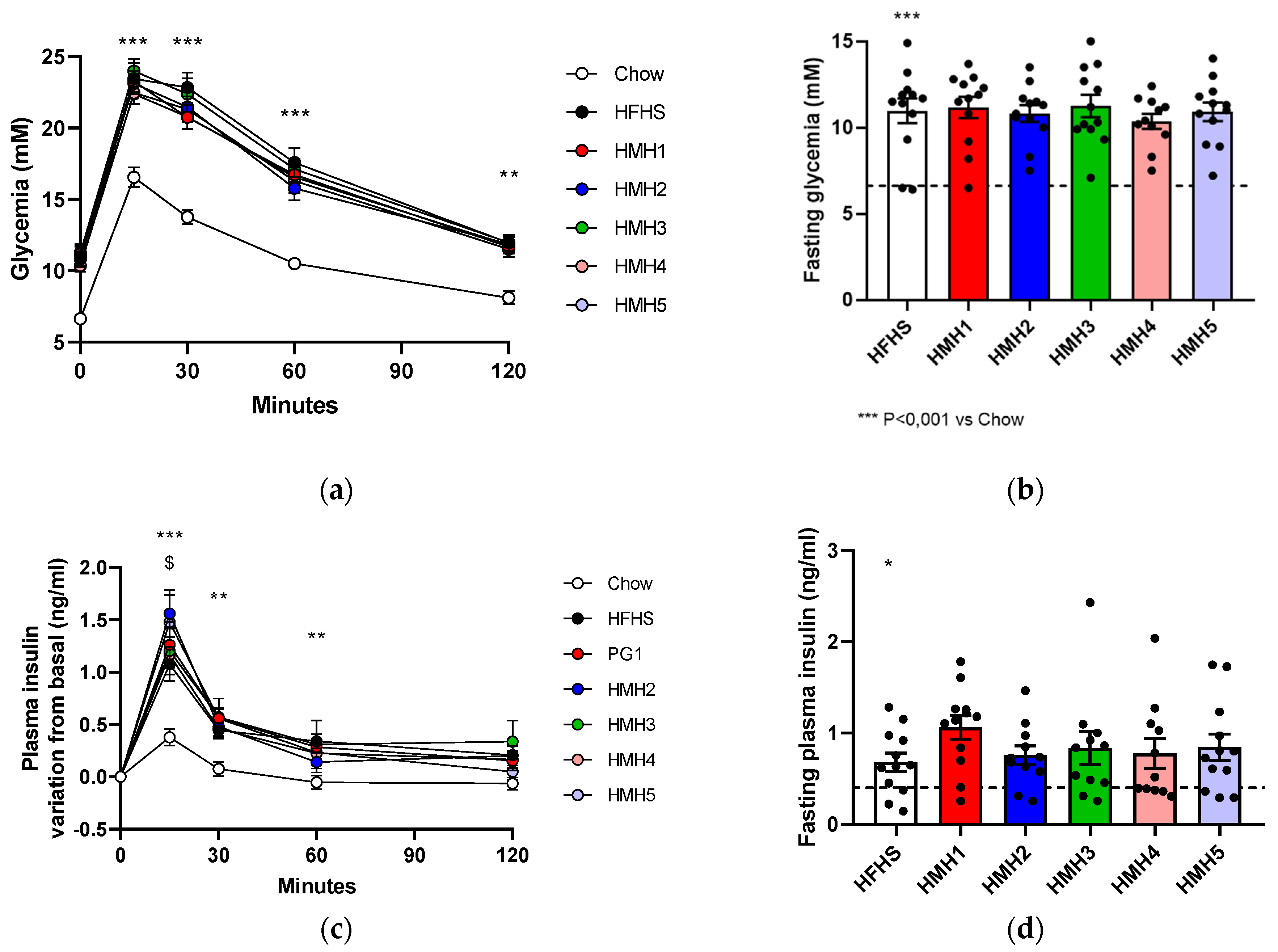
2.4. Glucose Tolerance Test
2.5. Insulin Measurement
2.6. Gut Microbiota Analysis
2.7. Statistical Analyses
3. Results and Discussion
3.1. Physiological Effects
3.2. Insulin Tolerance
3.3. Glucose Tolerance
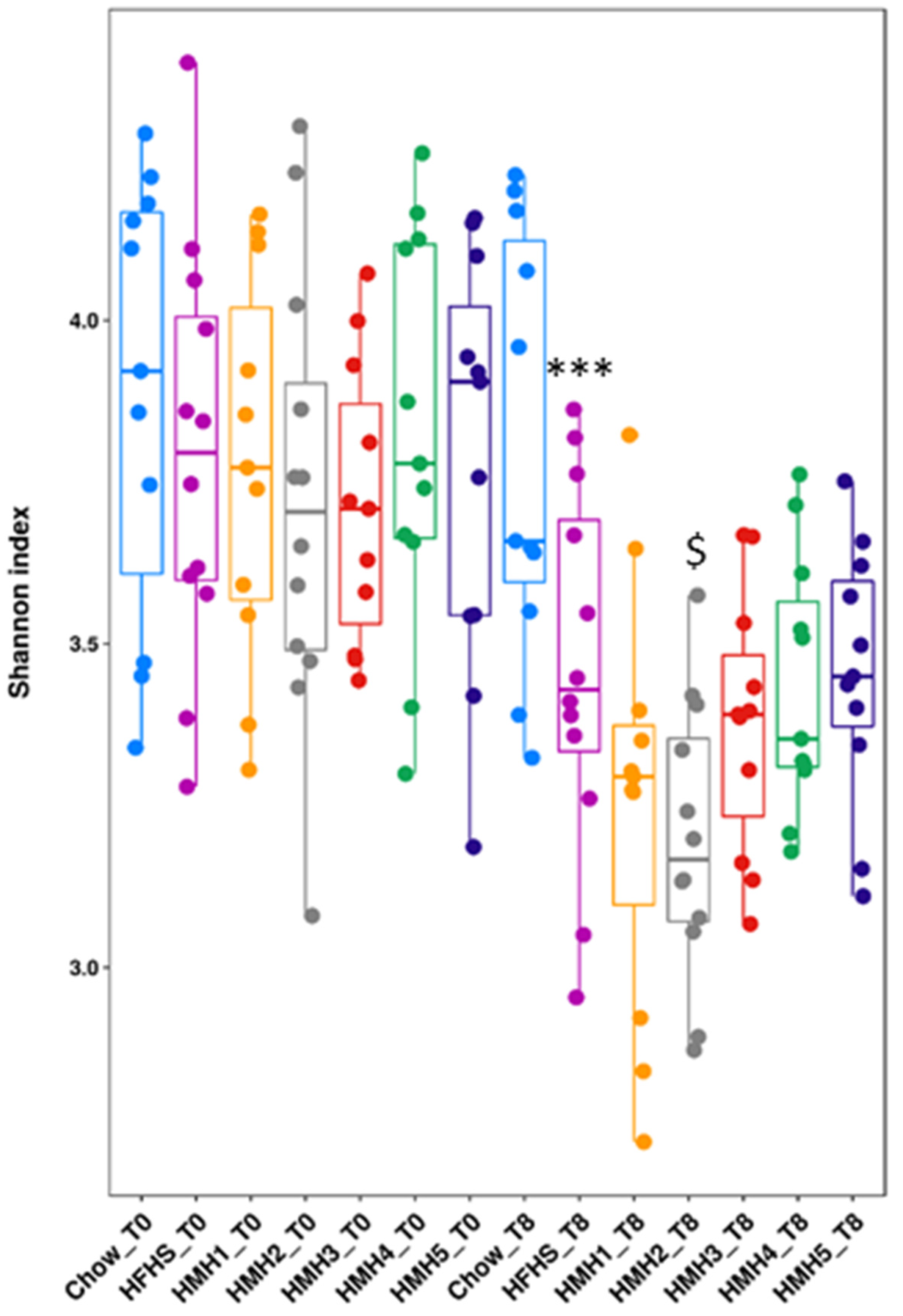
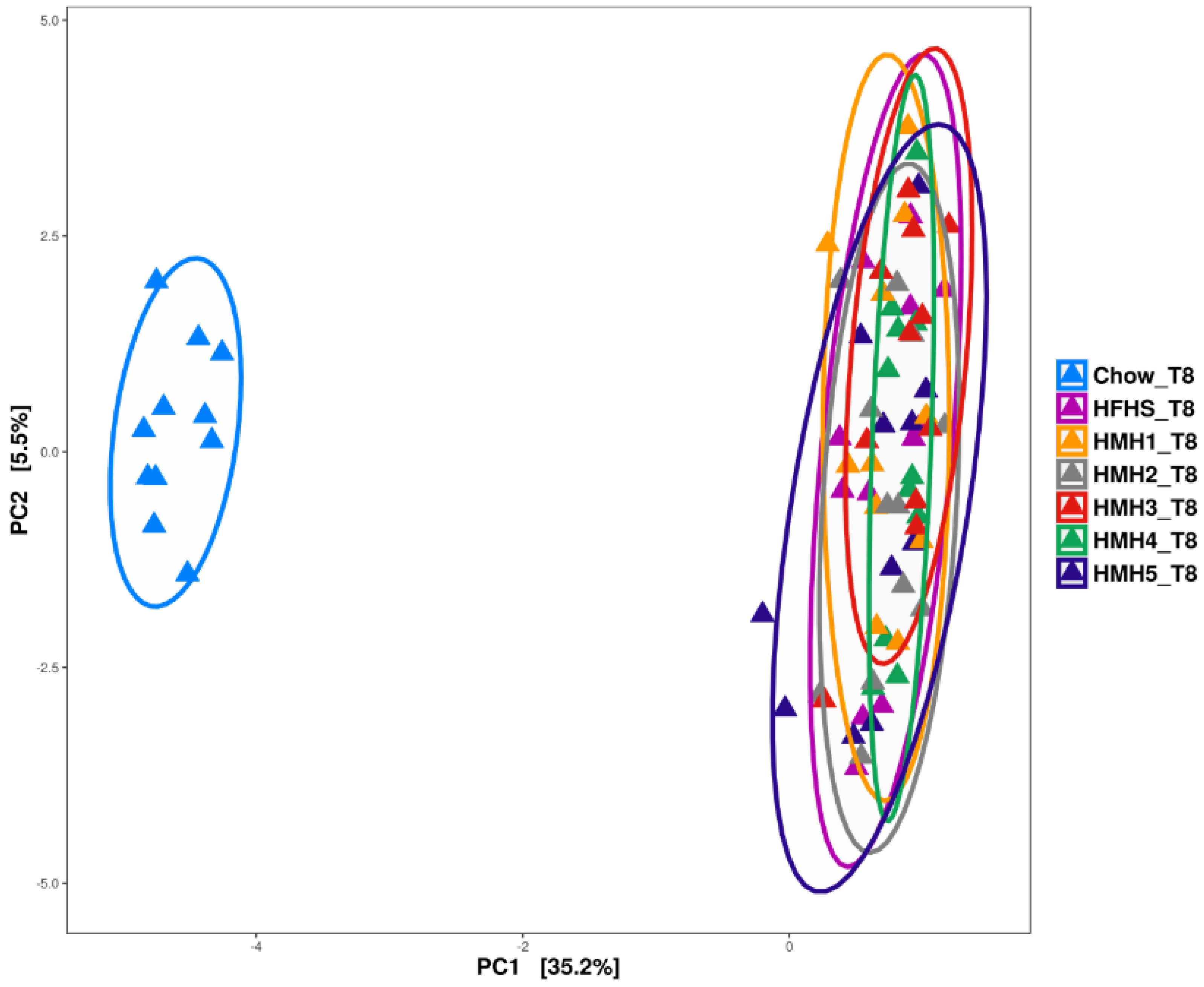
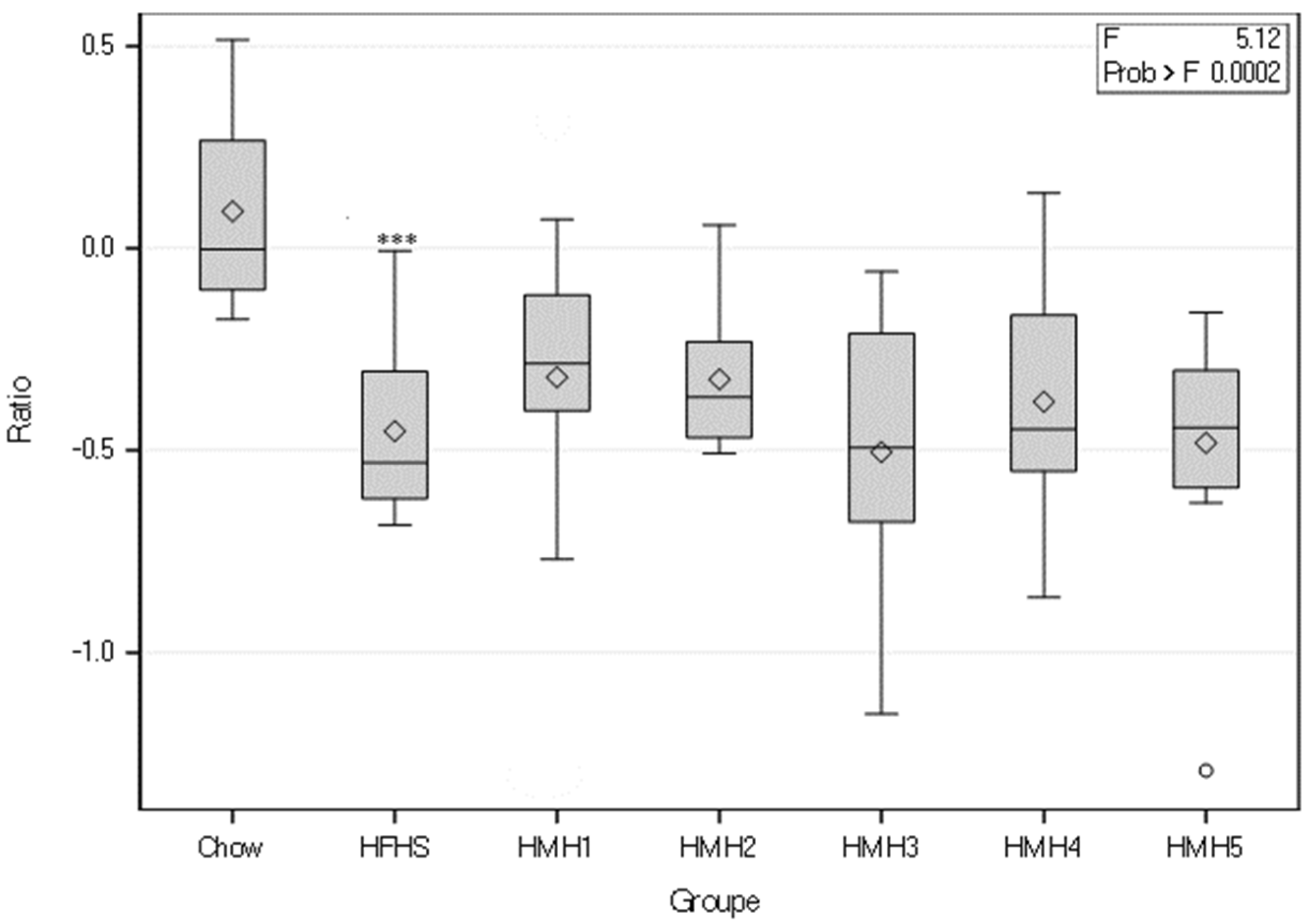
3.4. Gut Microbiota Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistique Canada. Syndrome Métabolique Chez les Adultes, 2012 à. 2015. Available online: https://www150.statcan.gc.ca/n1/pub/82-625-x/2014001/article/14123-fra.htm (accessed on 11 January 2021).
- NCEP. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- CCDI Steering Committee; Branchard, B.; Deb-Rinker, P.; Dubois, A.; Lapointe, P.; O’Donnell, S.; Pelletier, L.; Williams, G. At-A-Glance—How Healthy are Canadians? A brief update. Health Promot. Chronic Dis. Prev. Can. 2018, 38, 385–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Suivi des Progrès dans la lutte Contre les Maladies non Transmissibles. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/259805/9789242513028-fre.pdf?sequence=1 (accessed on 13 January 2021).
- Morgan, M.; Zamora, N.; Hindmarsh, M. An Inconvenient Truth: A Sustainable Healthcare System Requires Chronic Disease Prevention and Management Transformation. Heal. 2007, 7, 6–23. [Google Scholar] [CrossRef] [PubMed]
- WHO. 2021. Integrated Chronic Disease Prevention and Control. 2021. Available online: https://www.who.int/chp/about/integrated_cd/en/ (accessed on 1 July 2021).
- Karimi, G.; Heidari, Z.; Firouzi, S.; Haghighatdoost, F. A Systematic Review and Meta-Analysis of the Association Be-tween Fish Consumption and Risk of Metabolic Syndrome. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 717–729. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, F.B.; Manson, J.E. Marine Omega-3 Supplementation and Cardiovascular Disease: An Updated Meta-Analysis of 13 Randomized Controlled Trials Involving 127 477 Participants. J. Am. Hear. Assoc. 2019, 8, e013543. [Google Scholar] [CrossRef]
- Yang, B.; Shi, M.-Q.; Li, Z.-H.; Yang, J.-J.; Li, D. Fish, Long-Chain n-3 PUFA and Incidence of Elevated Blood Pressure: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2016, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Chevrier, G.; Mitchell, P.L.; Rioux, L.-E.; Hasan, F.; Jin, T.; Roblet, C.R.; Doyen, A.; Pilon, G.; St-Pierre, P.; Lavigne, C.; et al. Low-Molecular-Weight Peptides from Salmon Protein Prevent Obesity-Linked Glucose Intolerance, Inflammation, and Dyslipidemia in LDLR−/−/ApoB100/100 Mice. J. Nutr. 2015, 145, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, C.H.; Wang, T.Y.; Hung, C.C.; Chen, M.C.; Hsu, K.C. Improvement of Glycemic Control in Streptozoto-cin-Induced Diabetic Rats by Atlantic Salmon Skin Gelatin Hydrolysate as the Dipeptidyl-Peptidase IV Inhibitor. Food Funct. 2015, 6, 1887–1892. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Hsieh, C.-H.; Hung, C.-C.; Jao, C.-L.; Chen, M.-C.; Hsu, K.-C. Fish Skin Gelatin Hydrolysates as Dipeptidyl Peptidase IV Inhibitors and Glucagon-Like Peptide-1 Stimulators Improve Glycaemic Control in Diabetic Rats: A Comparison Between Warm- and Cold-Water Fish. J. Funct. Foods 2015, 14, 330–340. [Google Scholar] [CrossRef]
- Jun, S.-Y.; Park, P.-J.; Jung, W.-K.; Kim, S.-K. Purification and Characterization of an Antioxidative Peptide from Enzy-matic Hydrolysate of Yellowfin Sole (Limanda Aspera) Frame Protein. Eur. Food Res. Technol. 2004, 219, 20–26. [Google Scholar]
- Je, J.-Y.; Park, P.-J.; Kim, S.-K. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res. Int. 2005, 38, 45–50. [Google Scholar] [CrossRef]
- Doyen, A.; Beaulieu, L.; Saucier, L.; Pouliot, Y.; Bazinet, L. Demonstration of in vitro anticancer properties of peptide fractions from a snow crab by-products hydrolysate after separation by electrodialysis with ultrafiltration membranes. Sep. Purif. Technol. 2011, 78, 321–329. [Google Scholar] [CrossRef]
- Durand, R.; Fraboulet, E.; Marette, A.; Bazinet, L. Simultaneous double cationic and anionic molecule separation from herring milt hydrolysate and impact on resulting fraction bioactivities. Sep. Purif. Technol. 2019, 210, 431–441. [Google Scholar] [CrossRef]
- Durand, R.; Ouellette, A.; Houde, V.P.; Guénard, F.; Varin, T.V.; Marcotte, B.; Pilon, G.; Fraboulet, E.; Vohl, M.C.; Marette, A.; et al. Animal and Cellular Studies Demonstrate Some of the Beneficial Impacts of Herring Milt Hydrolysates on Obesity-Induced Glucose Intolerance and Inflammation. Nutrients 2020, 12, 3235. [Google Scholar] [CrossRef]
- Durand, R.; Pellerin, G.; Thibodeau, J.; Frabouletc, E.; Marette, A.; Bazinet, L. Screening for Metabolic Syndrome Applica-tion of a Herring By-Product Hydrolysate after its Separation by Electrodialysis with Ultrafiltration Membrane and Identification of Novel Anti-Inflammatory Peptides. Sep. Purif. Technol. 2020, 235, 116205. [Google Scholar] [CrossRef]
- Thibodeau, J.; Benoit, N.; Perreault, V.; Bazinet, L. Scale-Up and Long-Term Study of Electrodialysis with Ultrafiltration Membrane (EDUF) for the Separation of a Herring Milt Hydrolysate. Membranes 2021, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, J.; Mohamed, M.; Bakar, A. A systematic review on different models of inducing obesity in animals: Advantages and limitations. J. Adv. Veter. Anim. Res. 2020, 7, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Hart, D.A.; Reimer, R.A.; Seerattan, R.A.; Waters-Banker, C.; Sibole, S.C.; Herzog, W. High-Fat high-sucrose diet leads to dynamic structural and inflammatory alterations in the rat vastus lateralis muscle. J. Orthop. Res. 2016, 34, 2069–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, K.H.; Hart, D.A.; Seerattan, R.A.; Reimer, R.A.; Herzog, W. High-Fat/High-Sucrose diet-induced obesity results in joint-specific development of osteoarthritis-like degeneration in a rat model. Bone Jt. Res. 2018, 7, 274–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierard, M.M.; Conotte, S.; Tassin, A.; Boutry, S.; Uzureau, P.; Boudjeltia, K.Z.; Legrand, A. Interactions of exercise training and high-fat diet on adiponectin forms and muscle receptors in mice. Nutr. Metab. 2016, 13, 75. [Google Scholar] [CrossRef]
- Choi, S.-C.; Brown, J.; Gong, M.; Ge, Y.; Zadeh, M.; Li, W.; Croker, B.P.; Michailidis, G.; Garrett, T.J.; Mohamadzadeh, M.; et al. Gut microbiota dysbiosis and altered tryptophan catabolism contribute to autoimmunity in lupus-susceptible mice. Sci. Transl. Med. 2020, 12, eaax2220. [Google Scholar] [CrossRef]
- Peet, R. The Measurement of Species Diversity. Annu. Rev. Ecol. Syst. 1974, 5, 285–307. [Google Scholar] [CrossRef]
- Storlien, L.; Kraegen, E.; Chisholm, D.; Ford, G.; Bruce, D.; Pascoe, W. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 1987, 237, 885–888. [Google Scholar] [CrossRef]
- Taouis, M.; Dagou, C.; Ster, C.; Durand, G.; Pinault, M.; Delarue, J. n-3 Polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am. J. Physiol. Metab. 2002, 282, E664–E671. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Nair, S.; Gagnon, J. Herring Milt and Herring Milt Protein Hydrolysate Are Equally Effective in Improving Insulin Sensitivity and Pancreatic Beta-Cell Function in Diet-Induced Obese- and Insulin-Resistant Mice. Mar. Drugs 2020, 18, 635. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.J.; Haynes, T.E.; Kohli, R.; Hu, J.; Shi, W.; Spencer, T.; Carroll, R.J.; Meininger, C.J.; Wu, G. Dietary L-Arginine Supplementation Reduces Fat Mass in Zucker Diabetic Fatty Rats. J. Nutr. 2005, 135, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial Ecology: Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [Green Version]
- Bervoets, L.; Van Hoorenbeeck, K.; Kortleven, I.; Van Noten, C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Di Iorio, B.R.; Rocchetti, M.T.; De Angelis, M.; Cosola, C.; Marzocco, S.; Di Micco, L.; di Bari, I.; Accetturo, M.; Vacca, M.; Gobbetti, M.; et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Se-rum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J. Clin. Med. 2019, 8, 1424. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Ishaq, S.; Zhao, F.-Q.; Wright, A.-D.G. Colonic inflammation accompanies an increase of β-catenin signaling and Lachnospiraceae/Streptococcaceae bacteria in the hind gut of high-fat diet-fed mice. J. Nutr. Biochem. 2016, 35, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef]
- Kameyama, K.; Itoh, K. Intestinal Colonization by a Lachnospiraceae Bacterium Contributes to the Development of Diabetes in Obese Mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Dig. Liver Dis. 2016, 48, e268. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitert, M.D.; Mousa, A.; Barrett, H.L.; Naderpoor, N.; De Courten, B. Altered Gut Microbiota Composition Is Associated With Back Pain in Overweight and Obese Individuals. Front. Endocrinol. 2020, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xu, Z.; Deng, Q.; Huang, Q.; Wang, X.; Huang, F. Beneficial effects of flaxseed polysaccharides on metabolic syndrome via gut microbiota in high-fat diet fed mice. Food Res. Int. 2020, 131, 108994. [Google Scholar] [CrossRef]
- Haasbroek, K.; Takabe, W.; Yagi, M.; Yonei, Y. High-Fat Diet Induced Dysbiosis & Amelioration by Astaxanthin. Med. Sci. 2019, 48–49, 58–66. [Google Scholar] [CrossRef]
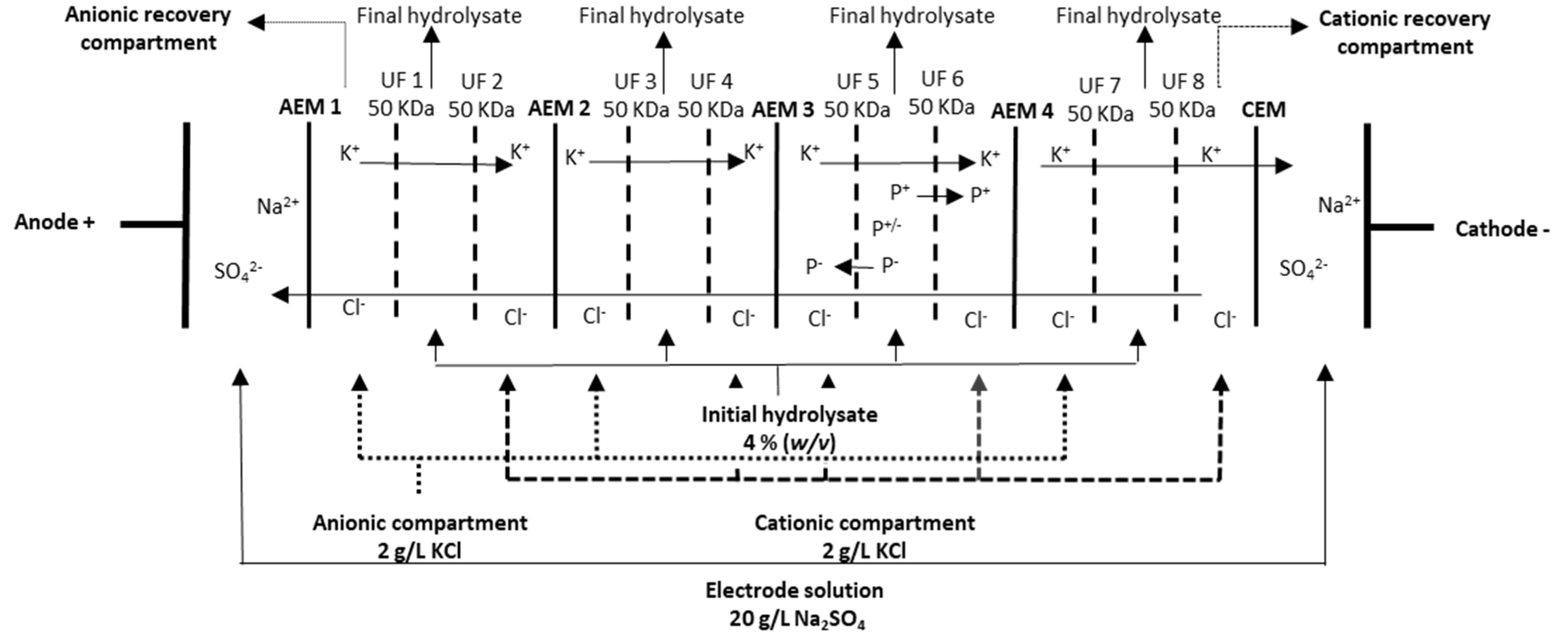

| HMH1 | HMH2 | HMH3 | HMH4 | HMH5 | |
|---|---|---|---|---|---|
| Protein/peptide (%) | 93.8 * | 48.3 * | 47.0 * | 93.1 | 74.4 |
| Lipids (%) | - | 18.5 * | 26.0 * | - | - |
| Nucleic acid (%) | 7.3 * | 27.3 * | 7.0 * | - | - |
| Astaxanthin (ppm) | - | - | 500 * | - | - |
| Chow | HFHS | HMH1 | HMH2 | HMH3 | HMH4 | HMH5 | |
|---|---|---|---|---|---|---|---|
| Total weight gain (g) | 3.38 ± 0.64 *** | 8.47 ± 2.29 | 9.79 ± 2.47 | 9.42 ± 2.14 | 9.65 ± 3.48 | 9.72 ± 2.28 | 9.98 ± 2.91 |
| Total energy intake (kcal) | 518.16 ± 4.76 *** | 626.87 ± 14.14 | 621.97 ± 14.62 | 629.13 ± 8.32 | 667.97 ± 16.66 | 660.80 ± 20.06 | 639.22 ± 14.03 |
| Visceral fat pad (g) | 0.88 ± 0.18 *** | 2.58 ± 0.85 | 2.92 ± 1.14 | 2.85 ± 0.65 | 2.81 ± 1.17 | 2.85 ± 0.89 | 2.90 ± 0.99 |
| Subcutaneous fat pad (g) | 0.27 ± 0.04 *** | 0.61 ± 0.21 | 0.69 ± 0.24 | 0.67 ± 0.14 | 0.70 ± 0.31 | 0.68 ± 0.20 | 0.74 ± 0.26 |
| Brown adipose tissue (g) | 0.07 ± 0.01 ** | 0.10 ± 0.03 | 0.10 ± 0.03 | 0.10 ± 0.02 | 0.11 ± 0.03 | 0.10 ± 0.02 | 0.10 ± 0.02 |
| Total lean mass (g) | 18.9 ± 1.0 | 19.6 ± 1.3 | 20.0 ± 1.6 | 19.9 ± 1.2 | 20.2 ± 2.1 | 20.9 ± 1.3 | 19.9 ± 1.1 |
| Total fat mass (g) | 2.3 ± 0.9 *** | 6.2 ± 2.1 | 6.6 ± 2.5 | 7.1 ± 2.0 | 6.4 ± 3.1 | 6.9 ± 2.2 | 6.4 ± 2.3 |
| Gastroc (2) (g) | 0.25 ± 0.02 | 0.26 ± 0.02 | 0.26 ± 0.02 | 0.26 ± 0.01 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.26 ± 0.02 |
| Soleus (2) (g) | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Brain (g) | 0.44 ± 0.01 | 0.42 ± 0.02 | 0.44 ± 0.01 @ | 0.44 ± 0.01 | 0.44 ± 0.02 | 0.44 ± 0.01 | 0.42 ± 0.02 |
| Heart (g) | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.02 | 0.13 ± 0.01 | 0.13 ± 0.01 |
| Kidneys (2) (g) | 0.32 ± 0.03 | 0.33 ± 0.03 | 0.36 ± 0.06 | 0.35 ± 0.03 | 0.35 ± 0.05 | 0.35 ± 0.04 | 0.33 ± 0.03 |
| Liver (g) | 1.05 ± 0.07 | 1.08 ± 0.13 | 1.01 ± 0.20 | 1.05 ± 0.13 | 1.03 ± 0.16 | 1.06 ± 0.14 | 1.07 ± 0.14 |
| Pancreas (g) | 0.26 ± 0.01 | 0.28 ± 0.02 | 0.31 ± 0.07 | 0.29 ± 0.04 | 0.31 ± 0.07 | 0.29 ± 0.05 | 0.27 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benoit, N.; Dubois, M.-J.; Pilon, G.; Varin, T.V.; Marette, A.; Bazinet, L. Effects of Herring Milt Hydrolysates and Fractions in a Diet-Induced Obesity Model. Foods 2021, 10, 2046. https://doi.org/10.3390/foods10092046
Benoit N, Dubois M-J, Pilon G, Varin TV, Marette A, Bazinet L. Effects of Herring Milt Hydrolysates and Fractions in a Diet-Induced Obesity Model. Foods. 2021; 10(9):2046. https://doi.org/10.3390/foods10092046
Chicago/Turabian StyleBenoit, Noémie, Marie-Julie Dubois, Geneviève Pilon, Thibault V. Varin, André Marette, and Laurent Bazinet. 2021. "Effects of Herring Milt Hydrolysates and Fractions in a Diet-Induced Obesity Model" Foods 10, no. 9: 2046. https://doi.org/10.3390/foods10092046
APA StyleBenoit, N., Dubois, M.-J., Pilon, G., Varin, T. V., Marette, A., & Bazinet, L. (2021). Effects of Herring Milt Hydrolysates and Fractions in a Diet-Induced Obesity Model. Foods, 10(9), 2046. https://doi.org/10.3390/foods10092046







