Effect of Filter Types on Physicochemical Properties, Volatile Compounds, and Sensory Evaluations of Purified Water by Point-of-Use Water Treatment
Abstract
1. Introduction
2. Materials and Methods
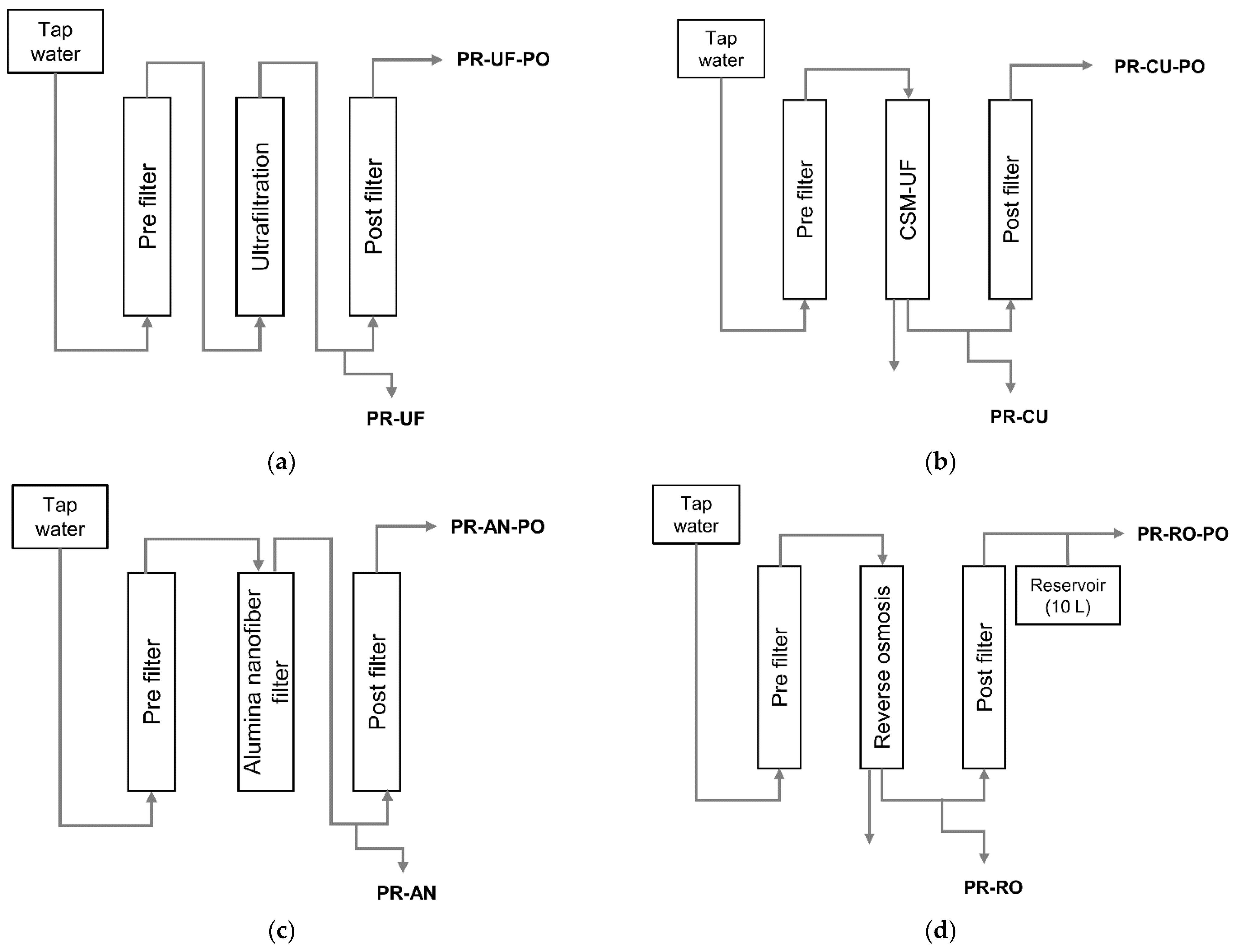
2.1. Water Filtration System
2.2. Mineral and Anion Analyses of Purified Water
2.3. Identification of Volatile Compounds of Purified Water by GC-MS
2.4. Polarized Sensory Positioning Test of Purified Tap Water
2.5. Consumer Acceptance Test
2.6. Statistical Analysis
3. Results and Discussion
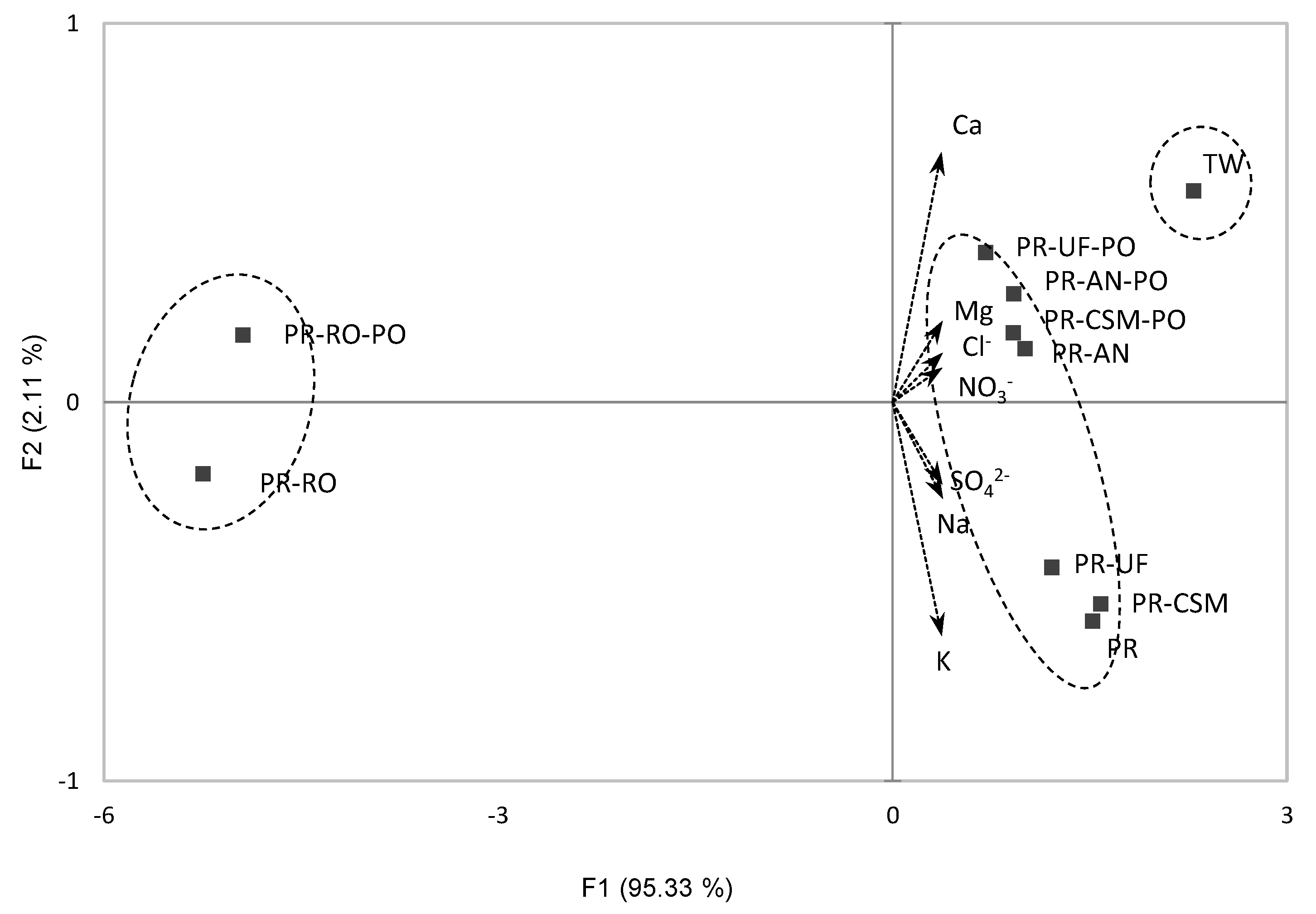
3.1. Chemical Analysis of Filtered Water
3.2. Volatile Organic Compounds in Filtered Tap Water
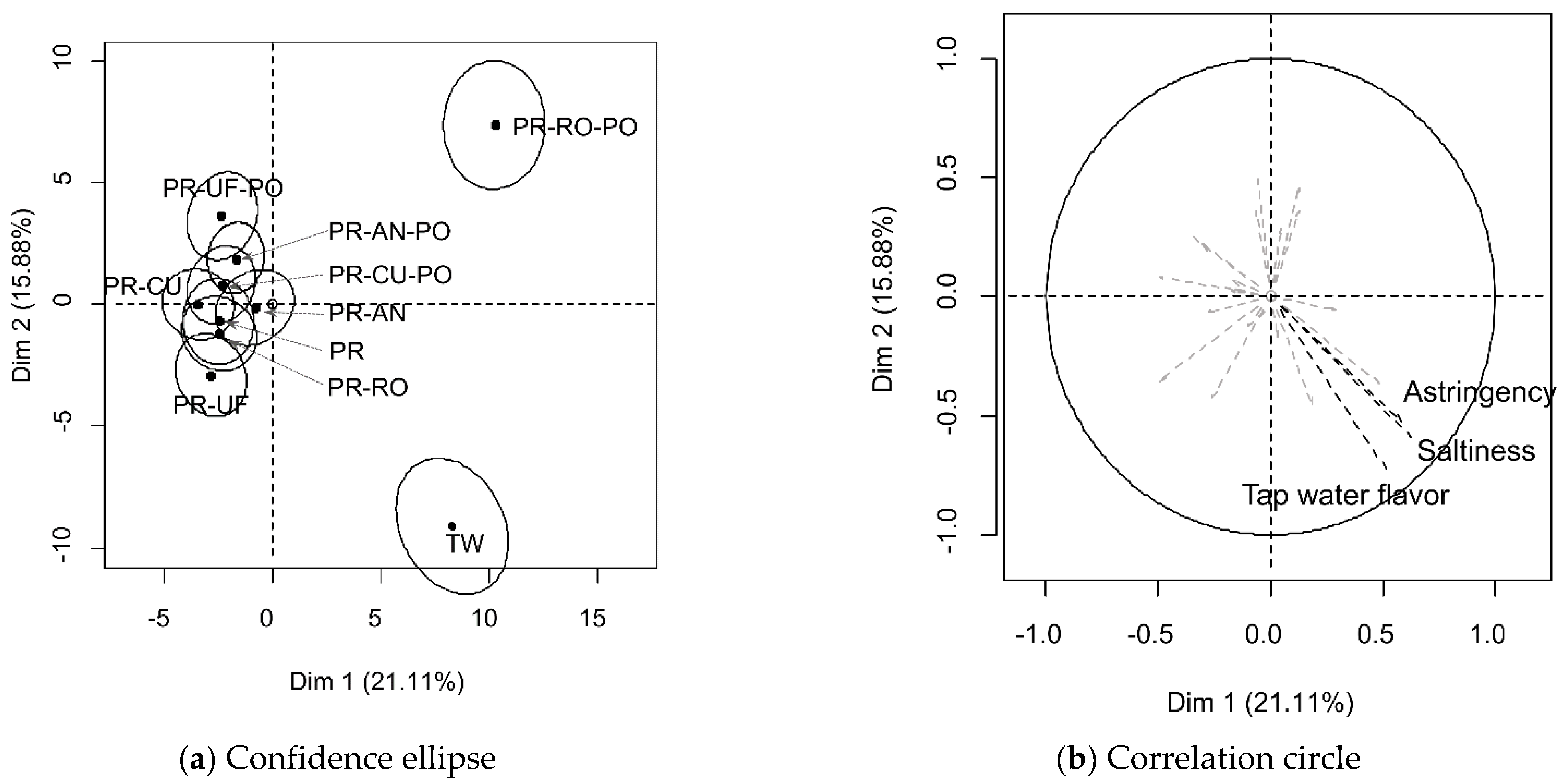
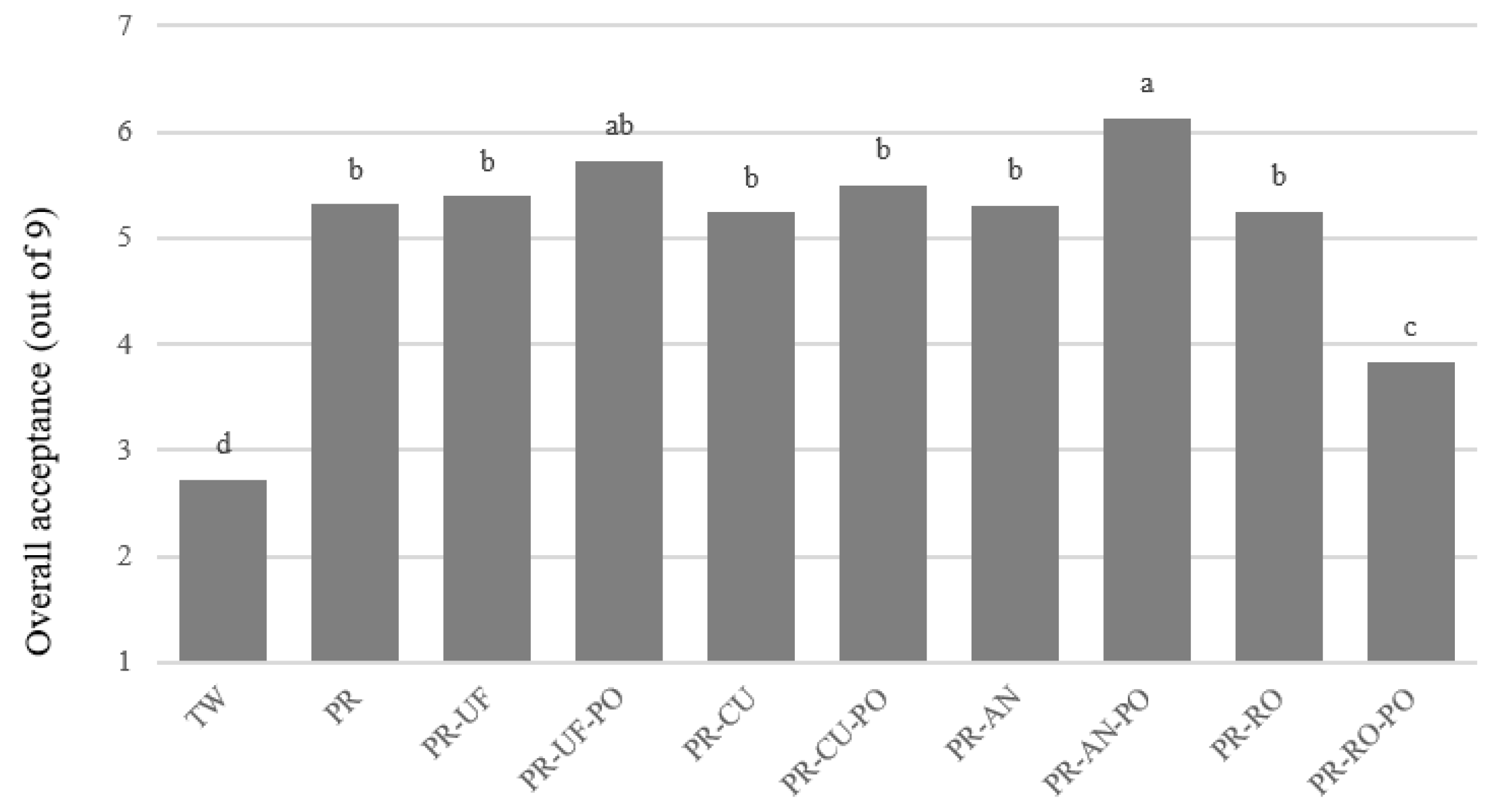
3.3. Discrimination and Consumer Acceptability of Purified Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doria, M.F. Bottled water versus tap water: Understanding consumers’ preferences. J. Water Health 2006, 4, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Doria, M.F. Factors influencing public perception of drinking water quality. Water Policy 2010, 12, 1–19. [Google Scholar] [CrossRef]
- Hu, Z.; Morton, L.W.; Mahler, R.L. Bottled Water: United States Consumers and Their Perceptions of Water Quality. Int. J. Environ. Res. Public Health 2011, 8, 565–578. [Google Scholar] [CrossRef]
- Johnstone, N.; Serret, Y. Determinants of bottled and purified water consumption: Results based on an OECD survey. Water Policy 2012, 14, 668–679. [Google Scholar] [CrossRef]
- Brown, K.W.; Gessesse, B.; Butler, L.J.; MacIntosh, D.L. Potential effectiveness of point-of-use filtration to address risks to drinking water in the United States. Environ. Health Insights 2017, 11, 1178630217746997. [Google Scholar] [CrossRef]
- Luo, Q.; Chen, X.; Wei, Z.; Xu, X.; Wang, D.; Wang, Z. Simultaneous and high-throughput analysis of iodo-trihalomethanes, haloacetonitriles, and halonitromethanes in drinking water using solid-phase microextraction/gas chromatography-mass spectrometry: An optimization of sample preparation. J. Chromatogr. A 2014, 1365, 45–53. [Google Scholar] [CrossRef]
- March, H.; Garcia, X.; Domene, E.; Sauri, D. Tap Water, Bottled Water or In-Home Water Treatment Systems: Insights on Household Perceptions and Choices. Water 2020, 12, 1310. [Google Scholar] [CrossRef]
- Min, S.; Kim, S.-I. Study on Improvement of tap water drinking rate of Seoul city Tap water’Arisu’through usage and recognition analysis. J. Digit. Converg. 2018, 16, 399–404. [Google Scholar]
- Ikem, A. Measurement of volatile organic compounds in bottled and tap waters by purge and trap GC–MS: Are drinking water types different? J. Food Compos. Anal. 2010, 23, 70–77. [Google Scholar] [CrossRef]
- Jones, A.Q.; Majowicz, S.E.; Edge, V.L.; Thomas, M.K.; MacDougall, L.; Fyfe, M.; Atashband, S.; Kovacs, S.J. Drinking water consumption patterns in British Columbia: An investigation of associations with demographic factors and acute gastrointestinal illness. Sci. Total Environ. 2007, 388, 54–65. [Google Scholar] [CrossRef]
- Marketsandmarkets. Point-of-Use Water Treatment Systems Market by Device (Tabletop, Faucet-Mounted, Countertop, Under-the-Sink) Technology (RO, Ultrafiltration, Distillation, Disinfection, Filtration), Application (Residential, Non-Residential), and Region—Forecast to 2023; MarketsandMarkets Research Private Ltd.: Hadapsar, India, 2020. [Google Scholar]
- Stalter, D.; O’Malley, E.; Von Gunten, U.; Escher, B.I. Point-of-use water filters can effectively remove disinfection by-products and toxicity from chlorinated and chloraminated tap water. Environ. Sci. Water Res. Technol. 2016, 2, 875–883. [Google Scholar] [CrossRef]
- Serpieri, N.; Moneti, G.; Pieraccini, G.; Donati, R.; Mariottini, E.; Dolara, P. Chemical and microbiological characterization of drinking water after filtration with a domestic-size charcoal column and ultraviolet sterilization. Urban Water 2000, 2, 13–20. [Google Scholar] [CrossRef]
- Nriagu, J.; Xi, C.; Siddique, A.; Vincent, A.; Shomar, B. Influence of Household Water Filters on Bacteria Growth and Trace Metals in Tap Water of Doha, Qatar. Sci. Rep. 2018, 8, 8268. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yu, R.; Jiang, J.; Ding, Z.; Ma, J.; Liang, R. Robust immobilization of anionic silver nanoparticles on cellulose filter paper toward a low-cost point-of-use water disinfection system with improved anti-biofouling properties. RSC Adv. 2021, 11, 4873–4882. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Tang, C.Y.; Ma, J.; Liu, M.; Ping, M.; Chen, M.; Wu, Z. Modification of microfiltration membranes by alkoxysilane polycondensation induced quaternary ammonium compounds grafting for biofouling mitigation. J. Membr. Sci. 2018, 549, 165–172. [Google Scholar] [CrossRef]
- Van Geluwe, S.; Braeken, L.; Van der Bruggen, B. Ozone oxidation for the alleviation of membrane fouling by natural organic matter: A review. Water Res. 2011, 45, 3551–3570. [Google Scholar] [CrossRef]
- USAPHC. Alumina Nanofilter Filters in Drinking Water Treatment; US Army Public Health Center: Aberdeen Proving Ground, MD, UDA, 2011. [Google Scholar]
- MFDS. Korean Food Standard Codex Method 2.2.1. Analysis of Micronutrients; Ministry of Food and Drug Safety: Cheongju-is, Korea, 2019.
- Protocol 300.1, U.S. EPA. Determination of Inorganic Anions in Drinking Water by Ion Chromatography; U.S. Environmental Protection Agency: Washington, DC, USA, 1997.
- Zhang, C.; Qi, M.; Shao, Q.; Zhou, S.; Fu, R. Analysis of the volatile compounds in Ligusticum chuanxiong Hort. using HS-SPME–GC-MS. J. Pharm. Biomed. Anal. 2007, 44, 464–470. [Google Scholar] [CrossRef]
- McLafferty, F.W.; Turecek, F. Interpretation of Mass Spectra; University Science Books: Sausalito, CA, USA, 1993. [Google Scholar]
- Teillet, E.; Schlich, P.; Urbano, C.; Cordelle, S.; Guichard, E. Sensory methodologies and the taste of water. Food Qual. Prefer. 2010, 21, 967–976. [Google Scholar] [CrossRef]
- Teillet, E. 10—Polarized sensory positioning (PSP) as a sensory profiling technique. In Rapid Sensory Profiling Techniques; Delarue, J., Lawlor, J.B., Rogeaux, M., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 215–225. [Google Scholar]
- Williams, E.J. Experimental Designs Balanced for the Estimation of Residual Effects of Treatments. Aust. J. Chem. 1949, 2, 149–168. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 38254. [Google Scholar] [CrossRef]
- Cadoret, M.; Husson, F. Construction and evaluation of confidence ellipses applied at sensory data. Food Qual. Prefer. 2013, 28, 106–115. [Google Scholar] [CrossRef]
- Kai, Z.; Yuesi, W.; Tianxue, W.; Yousef, M.; Frank, M. Properties of nitrate, sulfate and ammonium in typical polluted atmospheric aerosols (PM10) in Beijing. Atmos. Res. 2007, 84, 67–77. [Google Scholar] [CrossRef]
- Lee, I.; Lee, K.-L.; Lim, T.-H.; Park, J.-J.; Cheon, S. Determination of geosmin and 2-MIB in Nakdong River using headspace solid phase microextraction and GC-MS. Anal. Sci. Technol. 2013, 26, 326–332. [Google Scholar] [CrossRef][Green Version]
- Korean Ministry of Environment. Drinking Water Management Act; Ministry of Environment: Sejong-si, Korea, 2019.
- Chowdhury, S.; Rodriguez, M.J.; Serodes, J. Model development for predicting changes in DBP exposure concentrations during indoor handling of tap water. Sci. Total Environ. 2010, 408, 4733–4743. [Google Scholar] [CrossRef]
- Padmaja, K.; Cherukuri, J.; Reddy, M.A. Conventional to cutting edge technologies in Drinking Water Purification—A REVIEW. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 9375–9385. [Google Scholar]
- Kwak, H.S.; Kim, M.; Lee, Y.; Jeong, Y. Identification of key sensory attributes for consumer acceptance and instrumental quality of aseptic-packaged cooked rice. Int. J. Food Sci. Technol. 2015, 50, 691–699. [Google Scholar] [CrossRef]
| Sample | pH | Conductivity (µS/cm) | Mineral (mg/L) | Anion (mg/L) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ca | Na | K | Mg | Cl− | NO3− | SO42− | |||
| TW | 7.13 ± 0.05 bc | 67.97 ± 0.17 c | 6.52 ± 0.08 a1) | 3.53 ± 0.17 abc | 1.05 ± 0.01 b | 0.91 ± 0.00 a | 7.85 ± 0.00 a | 3.64 ± 0.00 a | 4.58 ± 0.02 a |
| PR | 7.17 ± 0.01 b | 67.10 ± 0.16 c | 4.22 ± 0.04 cd | 3.75 ± 0.15 ab | 1.15 ± 0.02 ab | 0.85 ± 0.00 a | 7.67 ± 0.02 c | 2.79 ± 0.02 b | 4.24 ± 0.00 b |
| PR-UF | 7.21 ± 0.01 abA | 67.93 ± 0.12 cB | 3.94 ± 0.15 dB2) | 3.42 ± 0.23 abcNS | 1.07 ± 0.08 abA | 0.83 ± 0.08 aNS | 7.68 ± 0.01 cB | 2.88 ± 0.00 bA | 3.98 ± 0.00 dA |
| PR-UF-PO | 7.06 ± 0.00 cB | 70.17 ± 0.00 bA | 4.50 ± 0.00 bcA | 3.25 ± 0.00 c | 0.86 ± 0.00 cB | 0.89 ± 0.00 a | 7.85 ± 0.00 aA | 2.52 ± 0.00 cdB | 2.76 ± 0.00 hB |
| PR-CSM | 7.27 ± 0.04 aNS | 68.77 ± 0.41 bcA | 4.40 ± 0.17 bcNS3) | 3.86 ± 0.25 aNS | 1.17 ± 0.05 aA | 0.87 ± 0.05 aNS | 7.60 ± 0.01 dB | 2.81 ± 0.00 bA | 3.94 ± 0.00 eA |
| PR-CSM-PO | 7.21 ± 0.01 ab | 67.33 ± 0.26 cB | 4.54 ± 0.10 b | 3.50 ± 0.14 abc | 0.91 ± 0.00 cB | 0.84 ± 0.00 a | 7.81 ± 0.00 bA | 2.63 ± 0.06 cB | 3.14 ± 0.00 fgB |
| PR-AN | 7.16 ± 0.01 bcNS | 68.37 ± 0.45 bcNS | 4.37 ± 0.09 bcB | 3.32 ± 0.01 bcB | 0.85 ± 0.02 cNS | 0.82 ± 0.01 aB | 7.66 ± 0.00 cB | 2.83 ± 0.00 bA | 4.11 ± 0.01 cA |
| PR-AN-PO | 7.20 ± 0.02 ab | 68.83 ± 0.12 bc | 4.59 ± 0.04 bA | 3.35 ± 0.00 bcA | 0.87 ± 0.02 c | 0.89 ± 0.01 aA | 7.80 ± 0.00 bA | 2.52 ± 0.08 dB | 3.43 ± 0.01 fB |
| PR-RO | 6.31 ± 0.04 eB | 56.93 ± 0.25 dB | 0.00 ± 0.00 fB | 0.77 ± 0.10 dNS | 0.21 ± 0.04 dNS | 0.00 ± 0.00 cB | 0.32 ± 0.00 fB | 0.61 ± 0.00 eA | 0.00 ± 0.00 iNS |
| PR-RO-PO | 6.55 ± 0.04 dA | 129.63 ± 1.76 aA | 0.73 ± 0.00 eA | 0.76 ± 0.02 d | 0.23 ± 0.00 d | 0.19 ± 0.00 bA | 1.29 ± 0.00 eA | 0.00 ± 0.00 fB | 0.00 ± 0.00 i |
| Compounds | RT (min) (1) | RI (1) | Content (µg/Kg of Water) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TW | PR | PR-UF | PR-CU | PR-AN | PR-RO | PR-UF-PO | PR-CU-PO | PR-AN-PO | PR-RO-PO | I.D. (1) | |||
| Acids (3) | |||||||||||||
| Ethyl caprylate | 20.137 | 1188 | 2.40 | ND | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| Ethyl 6-bromohexanoate | 20.235 | 1191 | ND | 1.00 | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| Phenylacrylic acid | 24.005 | 1329 | 0.30 | ND | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| Aldehyde (1) | |||||||||||||
| 2-Hexenal | 5.489 | <800 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | MS/RI |
| Hydrocarbons (19) | |||||||||||||
| Hexane | 3.349 | <800 | 0.10 | ND | 0.10 | ND | 0.10 | 0.10 | 0.20 | 0.20 | 0.20 | 0.10 | MS/RI |
| Chloroform | 3.561 | <800 | 0.07 | 0.30 | 0.60 | 0.30 | 0.10 | 0.10 | ND | ND | ND | ND | MS |
| Methylcyclopentane | 3.658 | <800 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.10 | MS |
| 4-Methyl-1-hexene | 3.675 | <800 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| 3-Methyl-pentane | 3.693 | <800 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| 4-Methyl-1-pentene | 4.059 | <800 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.10 | MS |
| Cyclohexane | 4.156 | <800 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.10 | MS |
| 2-Ethyl-1-butene | 4.259 | <800 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.10 | MS |
| Bromodichloromethane | 5.014 | <800 | 3.00 | ND | ND | ND | 3.30 | ND | ND | ND | ND | ND | MS |
| Toluene | 6.617 | <800 | 0.20 | ND | ND | 0.20 | 0.10 | ND | ND | ND | ND | ND | MS/RI |
| Propenyl vinyl acetylene | 6.702 | <800 | ND | ND | ND | ND | 0.10 | ND | ND | ND | ND | ND | MS |
| Cycloheptatriene | 6.851 | <800 | ND | ND | ND | 0.10 | ND | ND | ND | ND | ND | ND | MS |
| Dibromochloromethane | 7.469 | <800 | 2.50 | ND | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| Chlorobenzene | 9.077 | 845 | ND | ND | ND | 0.30 | ND | ND | ND | ND | ND | ND | MS |
| 1,2-Dimethyl-benzene | 9.901 | 869 | 0.10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| 1,4-Dimethyl-benzene | 9.958 | 870 | 0.20 | ND | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| 1,3-Dimethyl-benzene | 10.593 | 887 | 0.10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| Anethole | 21.762 | 1247 | 3.20 | 2.40 | 1.30 | 0.50 | ND | 0.30 | ND | ND | ND | ND | MS |
| Estragole | 22.964 | 1289 | 0.50 | 0.80 | ND | 0.30 | ND | ND | ND | ND | ND | ND | MS/RI |
| Heterocyclics (8) | |||||||||||||
| 3-Methyltetrahydrofuran | 3.372 | <800 | ND | ND | ND | ND | ND | 0.10 | ND | ND | ND | ND | MS |
| Benzoimide | 4.516 | <800 | ND | ND | ND | ND | ND | 0.30 | ND | ND | ND | ND | MS |
| 6-Aminotetralin | 4.602 | <800 | ND | ND | ND | ND | ND | 0.30 | ND | ND | ND | ND | MS |
| 1-Methyl-2-aminobenzimidazole | 4.642 | <800 | 0.30 | ND | ND | ND | ND | 0.30 | ND | ND | ND | ND | MS |
| Permetrinic acid methylaminde | 5.272 | <800 | 2.00 | ND | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| Benzenehexanamine | 6.542 | <800 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| 7-Morpholinoindolizidine | 7.99 | 810 | ND | ND | ND | ND | ND | 0.90 | ND | ND | ND | ND | MS |
| Auramine | 17.705 | 1105 | 1.60 | ND | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| Total content | 14.97 | 4.50 | 2.00 | 1.70 | 3.70 | 2.40 | 0.20 | 0.20 | 0.20 | 0.50 | |||
| Sample | C1 (n = 27) | C2 (n = 47) | C3 (n = 16) |
|---|---|---|---|
| TW | 3.52 b | 1.91 b | 3.75 b |
| PR | 5.07 a | 5.26 a | 5.94 a |
| PR-UF | 4.74 ab | 5.28 a | 6.56 a |
| PR-CU | 4.30 ab | 5.28 a | 6.75 a |
| PR-AN | 4.59 ab | 5.38 a | 6.31 a |
| PR-RO | 4.87 a | 5.11 a | 6.31 a |
| PR-UF-PO | 5.37 a | 5.70 a | 6.44 a |
| PR-CU-PO | 5.48 a | 5.28 a | 6.19 a |
| PR-AN-PO | 5.48 a | 6.13 a | 7.25 a |
| PR-RO-PO | 5.41 a | 2.09 b | 6.31 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-R.; Heo, J.; Kim, S.S.; Shin, E.-C.; Boo, C.G.; Kwak, H.S. Effect of Filter Types on Physicochemical Properties, Volatile Compounds, and Sensory Evaluations of Purified Water by Point-of-Use Water Treatment. Foods 2021, 10, 1958. https://doi.org/10.3390/foods10081958
Kim M-R, Heo J, Kim SS, Shin E-C, Boo CG, Kwak HS. Effect of Filter Types on Physicochemical Properties, Volatile Compounds, and Sensory Evaluations of Purified Water by Point-of-Use Water Treatment. Foods. 2021; 10(8):1958. https://doi.org/10.3390/foods10081958
Chicago/Turabian StyleKim, Mi-Ran, JeongAe Heo, Sang Sook Kim, Eui-Cheol Shin, Chang Guk Boo, and Han Sub Kwak. 2021. "Effect of Filter Types on Physicochemical Properties, Volatile Compounds, and Sensory Evaluations of Purified Water by Point-of-Use Water Treatment" Foods 10, no. 8: 1958. https://doi.org/10.3390/foods10081958
APA StyleKim, M.-R., Heo, J., Kim, S. S., Shin, E.-C., Boo, C. G., & Kwak, H. S. (2021). Effect of Filter Types on Physicochemical Properties, Volatile Compounds, and Sensory Evaluations of Purified Water by Point-of-Use Water Treatment. Foods, 10(8), 1958. https://doi.org/10.3390/foods10081958






