Attempts of Physical Refining of Sterol-Rich Sunflower Press Oil to Obtain Minimally Processed Edible Oil
Abstract
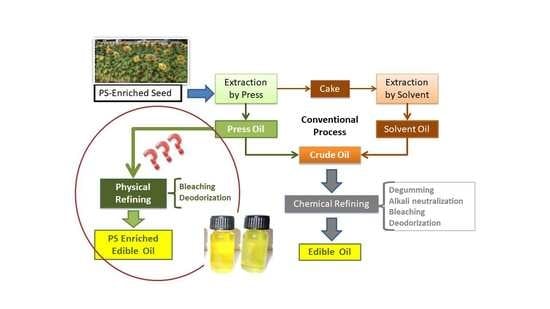
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Oil Samples
2.3. Physical Refining
2.3.1. Bleaching
2.3.2. Neutralization/Deodorization
2.4. Chemical Refining
2.4.1. Phosphoric Degumming
2.4.2. Neutralization
2.4.3. Washes
2.4.4. Bleaching and Deodorization
2.5. Analytical Methods
2.5.1. Elemental Analysis
2.5.2. Analysis of Chlorophylls
2.5.3. Acidity
2.5.4. Fatty Acid Composition and PS Contents
2.5.5. Tocopherols
2.6. Statistical Analyses
3. Results and Discussion
3.1. Bleaching Assays
3.2. Physical Refining (PhR)
3.2.1. Fatty Acid Composition
3.2.2. Phytosterols
3.2.3. Tocopherols
3.3. Chemical Refining (ChR)
3.3.1. Fatty Acid Composition
3.3.2. Phytosterols
3.3.3. Tocopherols
3.4. Pros and Cons of Considering the PO and SO Separately
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Phytosterol | PS |
| Oil obtained by press | PO |
| Solvent extracted oil | SO |
| free fatty acids | FFA |
| Chemical Refining | ChR |
| Physical Refining | PhR |
| phosphorous | P |
| Sunflower oil rich in campesterol | CAM |
| Sunflower oil rich in Δ7-stigmastenol | STIG |
| Conventional sunflower oil | CON |
References
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, K.; Gylling, H.; Kaipiainen, L.; Nissinen, M.J.; Simonen, P. Cholesterol lowering efficacy of plant stanol ester in a new type of product matrix, a chewable dietary supplement. J. Funct. Foods 2017, 30, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.S.; Ferdosh, S.; Haque Akanda, M.J.; Ghafoor, K.; Rukshana, A.H.; Ali, M.E.; Kamaruzzaman, B.Y.; Fauzi, M.B.; Shaarani, S.; Islam Sarker, M.Z. Techniques for the extraction of phytosterols and their benefits in human health: A review. Sep. Sci. Technol. 2018, 53, 2206–2223. [Google Scholar]
- Roman, S.; Sánchez-Siles, L.M.; Siegrist, M. The importance of food naturalness for consumers: Results of a systematic review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]
- Carré, P. Naturalness in the production of vegetable oils and proteins. OCL 2021, 28, 10. [Google Scholar] [CrossRef]
- Harker, M.; Holmberg, N.; Clayton, J.C.; Gibbard, C.L.; Wallace, A.D.; Rawlins, S.; Hellyer, S.A.; Lanot, A.; Safford, R. Enhancement of seed phytosterol levels by expression of an N-terminal truncated Hevea brasiliensis (rubber tree) 3-hydroxy-3-methylglutaryl-CoA reductase. Plant Biotechnol. J. 2003, 1, 113–121. [Google Scholar]
- Yamaya, A.; Endo, Y.; Fujimoto, K.; Kitamura, K. Effects of genetic variability and planting location on the phytosterol content and composition in soybean seeds. Food Chem. 2007, 102, 1071–1075. [Google Scholar] [CrossRef]
- Neelakandan, A.K.; Chamala, S.; Valliyodan, B.; Nes, W.D.; Nguyen, H.T. Metabolic engineering of soybean affords improved phytosterol seed traits. Plant Biotechnol. J. 2012, 10, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Velasco, L.; Fernández-Cuesta, Á.; Fernández-Martínez, J.M. New sunflower seeds with high contents of phytosterols. OCL 2014, 21, D604. [Google Scholar] [CrossRef] [Green Version]
- González Belo, R.; Velasco, L.; Nolasco, S.M.; Izquierdo, N.G. Oil Phytosterol Concentration in Sunflower Presents a Dilution Response with Oil Weight per Grain. J. Am. Oil Chem. Soc. 2019, 96, 1115–1123. [Google Scholar] [CrossRef]
- FAO. Oilseed Production to Rebound in 2020/21. Food Outlook: Biannu. Rep. Glob. Food Markets. 2021, p. 31. Available online: http://www.fao.org/3/cb4479en/cb4479en_oilcrops.pdf (accessed on 13 August 2021).
- Pilorgé, E. Sunflower in the global vegetable oil system: Situation, specificities and perspective. OCL 2020, 27, 34. [Google Scholar] [CrossRef]
- Grompone, M.A. Sunflower and High-oleic Sunflower Oils. In Bailey’s Industrial Oil and Fat Products, 6th ed.; Shahidi, F., Ed.; Wiley-Interscience: Hoboken, NJ, USA, 2020. [Google Scholar]
- Salas, J.J.; Bootello, M.A.; Martínez-Force, E.; Calerón, M.V.; Garcés, R. High stearic sunflower oil: Latest advances and applications. OCL 2021, 28, 35. [Google Scholar] [CrossRef]
- Ayerdi Gotor, A.; Berger, M.; Labalette, F.; Centis, S.; Daydé, J.; Calmon, A. Oleic conversion effect on the tocopherol and phytosterol contents in sunflower oil. Phyton-Int. J. Exp. Bot. 2016, 83, 319–324. [Google Scholar]
- Zhang, H.; Vasanthan, T.; Wettasinghe, M. Enrichment of tocopherols and phytosterols in canola oil during seed germination. J. Agric. Food Chem. 2007, 55, 355–359. [Google Scholar] [CrossRef]
- Fernández-Cuesta, A.; Jan, C.C.; Fernández-Martínez, J.M.; Velasco, L. Variability for seed phytosterols in sunflower germplasm. Crop Sci. 2014, 54, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Konuşkan, D.B. Minor bioactive lipids in cold pressed oils. In Cold Pressed Oils; Academic Press: Cambridge, MA, USA, 2020; pp. 7–14. [Google Scholar]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- Van Hoed, V.; Ali, C.B.; Slah, M.; Verhé, R. Quality differences between pre-pressed and solvent extracted rapeseed oil. Eur. J. Lipid Sci. Technol. 2010, 112, 1241–1247. [Google Scholar] [CrossRef]
- Aguirre, M.R.; Velasco, J.; Ruiz-Méndez, M.V. Characterization of sunflower oils obtained separately by pressing and subsequent solvent extraction from a new line of seeds rich in phytosterols and conventional seeds. OCL 2014, 21, D605. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, M.R.; Ruiz-Méndez, M.V.; Velasco, L.; Dobarganes, M.C. Free sterols and steryl glycosides in sunflower seeds with high phytosterol contents. J. Lipid Sci. Technol. 2012, 114, 1212–1216. [Google Scholar] [CrossRef]
- García-González, A.; Velasco, J.; Velasco, L.; Ruiz-Méndez, M.V. Characterization of press and solvent extraction oils from new sunflower seeds with modified phytosterol compositions. J. Sci. Food Agric. 2020, 101, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius. Standard For Named Vegetable Oils (CODEX STAN 210–1999), 7 July 2018. Available online: http://www.fao.org/input/download/standards/336/CXS_210e_2015.pdf (accessed on 13 August 2021).
- Bai, G.; Ma, C.G.; Chen, X.W. Phytosterols in edible oil: Distribution, analysis and variation during processing. Grain Oil Sci. Technol. 2021, 1, 33–44. [Google Scholar] [CrossRef]
- Verhé, R.; Verleyen, T.; Van Hoed, V.; De Greyt, W. Influence of refining of vegetable oils on minor components. J. Oil Palm. Res. 2006, 4, 168–179. [Google Scholar]
- Azizi, M.; Ghavami, A. The Effects of Refining Operations on Quality and Quantity of Sterols in Canola, Soyabean and Sunflower Seed Oils. J. Food Biosci. Technol. 2020, 10, 11–18. [Google Scholar]
- Gotor, A.A.; Rhazi, L. Effects of refining process on sunflower oil minor components: A review. Oilseeds and fats. Crop. Lipids 2017, 23, D207. [Google Scholar]
- Farr, W.E. Physical refining of vegetable oils. In Green Vegetable Oil Processing; AOCS Press: Urbana, IL, USA, 2014; pp. 159–169. [Google Scholar]
- Kreps, F.; Vrbiková, L.; Schmidt, Š. Influence of industrial physical refining on tocopherol, chlorophyll and beta-carotene content in sunflower and rapeseed oil. Eur. J. Lipid Sci. Technol. 2014, 116, 1572–1582. [Google Scholar] [CrossRef]
- Kovari, K.; Denise, J.; Kemeny, Z.; Recseg, K. Physical refining of sunflower oil. Oléagineux Corps Gras Lipides 2000, 7, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Van Hoed, V.; Depaemelaere, G.; Ayala, J.V.; Santiwattana, P.; Verhé, R.; De Greyt, W. Influence of chemical refining on the major and minor components of rice brain oil. J. Am. Oil Chem. Soc. 2006, 83, 315–321. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, R.; Wang, Z.; Wang, B.; Yang, Y.; Ju, X.; He, R. The effect of refining process on the physicochemical properties and micronutrients of rapeseed oils. PLoS ONE 2019, 14, e0212879. [Google Scholar] [CrossRef] [Green Version]
- Belur, P.D.; Iyyasami, R.; Sampath, C.; Chandrasekhar, V. Refining technologies for edible oils. In Edible Oils; CRC Press: Boca Raton, FL, USA, 2017; pp. 99–128. [Google Scholar]
- Chew, S.C.; Nyam, K.L. Refining of Edible Oils in Lipids and Edible Oils; Academic Press: Cambridge, MA, USA, 2020; pp. 213–241. [Google Scholar]
- Cui, L.; Decker, E.A. Phospholipids in foods: Prooxidants or antioxidants? J. Sci. Food Agric. 2016, 96, 18–31. [Google Scholar] [CrossRef]
- Van Duijn, G. Fate of contaminants during the refining process of vegetable oils and fats: A calculation model. J. Lipid Sci. Technol. 2016, 118, 353–360. [Google Scholar] [CrossRef]
- Zufarov, O.; Schmidt, S.; Sekretár, S. Degumming of rapeseed and sunflower oils. Acta Chim. Slov. 2008, 1, 321–328. [Google Scholar]
- De Greyt, W. Edible oil refining: Current and future technologies. In Edible Oil Process; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 127–151. [Google Scholar]
- Vuorte, M.; Vierros, S.; Kuitunen, S.; Sammalkorpi, M. Adsorption of impurities in vegetable oil: A molecular modelling study. J. Colloid Interface Sci. 2020, 571, 55–65. [Google Scholar] [CrossRef]
- Rossi, M.; Gianazza, M.; Alamprese, C.; Stanga, F. The role of bleaching clays and synthetic silica in palm oil physical refining. Food Chem. 2003, 82, 291–296. [Google Scholar] [CrossRef]
- De Clercq, N.; Moens, K.; Depypere, F.; Ayala, J.V.; Calliauw, G.; De Greyt, W.; Dewettinck, K. Influence of cocoa butter refining on the quality of milk chocolate. J. Food Eng. 2012, 111, 412–419. [Google Scholar] [CrossRef]
- Dimic, E.; Karlovic, D.J.; Turkulov, J. Pretreatment efficiency for physical refining of sunflower seed oil. J. Am. Oil Chem. Soc. 1994, 71, 1357–1361. [Google Scholar] [CrossRef]
- Pokorny, J.; Kalinova, L.; Dysseler, P. Determination of chlorophyll pigments in crude vegetable oils: Results of a collaborative study and the standardized method. Pure Appl. Chem. 1995, 67, 1781–1787. [Google Scholar] [CrossRef]
- ISO 660:2020 Animal and Vegetable Fats and Oils. Determination of Acid Value and Acidity; ISO: Geneva, Switzerland, 2020.
- García-González, A.; Velasco, J.; Velasco, L.; Ruiz-Méndez, M.V. An analytical simplification for faster determination of fatty acid composition and phytosterols in seed oils. Food Anal. Methods 2018, 11, 1234–1242. [Google Scholar] [CrossRef] [Green Version]
- ISO 9936:2016 Animal and Vegetable Fats and Oils. Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography; ISO: Geneva, Switzerland, 2016.
- Łaska-Zieja, B.; Marcinkowski, D.; Golimowski, W.; Niedbała, G.; Wojciechowska, E. Low-Cost Investment with High Quality Performance. Bleaching Earths for Phosphorus Reduction in the Low-Temperature Bleaching Process of Rapeseed Oil. Foods 2020, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, S.R.; Young, E.E.; Were, L.M. Chlorogenic acid oxidation and its reaction with sunflower proteins to form greenenic acid oxidation. Food Sci. Food Saf. 2016, 15, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Carelli, A.A.; Ceci, L.N.; Crapiste, G.H. Phosphorus to phospholipids conversion factors for crude and degummed sunflower oils. J. Am. Oil Chem. Soc. 2002, 79, 1177–1180. [Google Scholar] [CrossRef]
- Lamas, D.L.; Crapiste, G.H.; Constenla, D.T. Changes in quality and composition of sunflower oil during enzymatic degumming process. Food Sci. Technol. 2014, 58, 71–76. [Google Scholar] [CrossRef]
- Verleyen, T.; Sosinska, U.; Ioannidou, S.; Verhé, R.; Dewettinck, K.; Huyghebaert, A.; De Greyt, W. Influence of the vegetable oil refining process on free and esterified sterols. J. Am. Oil Chem. Soc. 2002, 79, 947–953. [Google Scholar] [CrossRef]
| Treatment | Trisyl (%) | Tonsil 114 FF (%) | Tonsil 278 FF (%) | |
|---|---|---|---|---|
| Physical refining | TR1 | 0.1 | - | 1.0 |
| TR2 | 0.1 | 1.0 | - | |
| TR3 | 0.2 | 2.0 | - | |
| Chemical refining | TR4 | 0.2 | 1.0 | - |
| TR5 | 0.3 | 1.0 | - | |
| TR7 | 0.2 | 2.0 | - | |
| TR6 | 0.2 | 1.5 | - |
| Bleaching Treatment | Phosphorous (mg/kg) | ||||
|---|---|---|---|---|---|
| Oil Sample | Trisyl (%) | Tonsil 114 FF (%) | Tonsil 278 FF (%) | Initial | Final |
| 2015 | |||||
| CON | 0.1 | - | 1.0 | 11.7 | 4.2 |
| CAM | 0.1 | - | 1.0 | 9.3 | 3.7 |
| STIG | 0.1 | - | 1.0 | 11.9 | 5.9 |
| 2017 | |||||
| CON | 0.1 | - | 1.0 | 27.3 | 24.2 |
| CAM | 0.1 | - | 1.0 | 15.4 | 10.7 |
| STIG | 0.1 | - | 1.0 | 36.4 | 30.1 |
| STIG | 0.1 | 1.0 | - | 36.4 | 28.9 |
| STIG | 0.2 | 2.0 | - | 36.4 | 24.4 |
| Bleaching Treatment | Phosphorous (mg/kg) | ||||
|---|---|---|---|---|---|
| Oil Sample | Trisyl (%) | Tonsil 114 FF (%) | Tonsil 278 FF (%) | Initial | Final |
| 2015 | |||||
| CON | 0.1 | - | 1.0 | 11.7 | 4.2 |
| CAM | 0.1 | - | 1.0 | 9.3 | 3.7 |
| STIG | 0.1 | - | 1.0 | 11.9 | 5.9 |
| 2017 | |||||
| CON | 0.1 | - | 1.0 | 27.3 | 24.2 |
| CAM | 0.1 | - | 1.0 | 15.4 | 10.7 |
| STIG | 0.1 | - | 1.0 | 36.4 | 30.1 |
| STIG | 0.1 | 1.0 | - | 36.4 | 28.9 |
| STIG | 0.2 | 2.0 | - | 36.4 | 24.4 |
| CAM | STIG | |||
|---|---|---|---|---|
| Bleached | Ph Refined | Bleached | Ph Refined | |
| Acidity (%) | 0.26 ± 0.03a | 0.15 ± 0.05b | 0.18 ± 0.02a | 0.05 ± 0.03b |
| Fatty acid composition (%) | ||||
| C16: 0 | 7.1 ± 0.03a | 7.1 ± 0.05a | 6.1 ± 0.02a | 6.0 ± 0.06a |
| C16: 1 | nd | nd | nd | nd |
| C18: 0 | 2.9 ± 0.03a | 3.0 ± 0.10a | 4.3 ± 0.03a | 4.7 ± 0.11b |
| C18: 1 | 34.7 ± 0.15a | 35.0 ± 0.68a | 39.6 ± 0.15a | 40.0 ± 0.18b |
| C18: 2 | 54.5 ± 0.12a | 54.1 ± 0.68a | 49.0 ± 0.10b | 48.3 ± 0.21a |
| C20: 0 | 0.3 ± 0.02a | 0.3 ± 0.01a | 0.4 ± 0.00b | 0.3 ± 0.03a |
| C22: 0 | 0.4 ± 0.02a | 0.4 ± 0.04a | 0.5 ± 0.00a | 0.5 ± 0.01a |
| C24: 0 | 0.2 ± 0.01a | 0.2 ± 0.01a | 0.2 ± 0.01a | 0.2 ± 0.02a |
| Phytosterols Composition (%) | ||||
| Campesterol | 29.5 ± 0.46a | 29.6 ± 0.43a | 6.4 ± 0.22a | 6.5 ± 0.08a |
| Stigmasterol | 7.5 ± 0.24a | 7.4 ± 0.19a | 8.8 ± 0.27a | 8.4 ± 0.18a |
| β-Sitosterol | 47.9 ± 0.78a | 48.3 ± 0.49a | 49.3 ± 0.29a | 49.6 ± 0.31a |
| ∆5-Avenasterol | 3.3 ± 0.12a | 3.3 ± 0.11a | 1.2 ± 0.09a | 1.2 ± 0.04a |
| ∆7-Stigmastenol | 7.8 ± 0.69a | 7.5 ± 0.48a | 29.7 ± 0.42a | 30.0 ± 0.28a |
| ∆7-Avenasterol | 4.0 ± 0.34a | 3.9 ± 0.35a | 4.6 ± 0.18a | 4.4 ± 0.14a |
| Total PS content (mg kg−1) | 3387 ± 145a | 3285 ± 56a | 4056 ± 53a | 3818 ± 232a |
| Tocopherols (mg·kg−1) | 881 ± 22a | 861 ± 21a | 722 ± 18a | 702 ± 17a |
| CON | CAM | STIG | ||||
|---|---|---|---|---|---|---|
| Crude | Ch Refined | Crude | Ch Refined | Crude | Ch Refined | |
| Press | ||||||
| Acidity (%) | 1.00 ± 0.05a | 0.02 ± 0.01b | 0.99 ± 0.05a | 0.02 ± 0.01b | 1.10 ± 0.05a | 0.02 ± 0.01b |
| Fatty Acids (%) | ||||||
| C16: 0 | 7.0 ± 0.01a | 7.0 ± 0.01a | 6.5 ± 0.01a | 6.6 ± 0.00b | 7.1 ± 0.01a | 7.2 ± 0.01b |
| C16: 1 | 0.2 ± 0.00a | 0.2 ± 0.00a | nd | 0.1 ± 0.00 | 0.1 ± 0.00a | 0.1 ± 0.00a |
| C18: 0 | 3.6 ± 0.01b | 3.5 ± 0.02a | 3.0 ± 0.01b | 2.9 ± 0.01a | 4.1 ± 0.03a | 4.1 ± 0.01a |
| C18: 1 | 30.0 ± 0.02a | 30.3 ± 0.06b | 30.0 ± 0.08a | 30.3 ± 0.03b | 23.8 ± 0.02a | 24.4 ± 0.02b |
| C18: 2 | 57.9 ± 0.05b | 57.7 ± 0.09a | 59.5 ± 0.09b | 59.1 ± 0.05a | 63.6 ± 0.02b | 62.8 ± 0.02a |
| C20: 0 | 0.3 ± 0.00a | 0.3 ± 0.01a | 0.3 ± 0.04b | 0.2 ± 0.00a | 0.3 ± 0.00a | 0.3 ± 0.00a |
| C18: 3 | 0.1 ± 0.00a | 0.1 ± 0.01a | nd | 0.1 ± 0.01 | 0.1 ± 0.00a | 0.1 ± 0.01a |
| C20: 1 | 0.2 ± 0.00a | 0.1 ± 0.01b | 0.1 ± 0.02a | 0.2 ± 0.00a | 0.2 ± 0.00a | 0.2 ± 0.01a |
| C22: 0 | 0.6 ± 0.00a | 0.6 ± 0.01a | 0.4 ± 0.05b | 0.5 ± 0.01a | 0.6 ± 0.01a | 0.6 ± 0.01a |
| C24: 0 | 0.3 ± 0.00a | 0.3 ± 0.01a | 0.1 ± 0.02a | 0.1 ± 0.02a | 0.3 ± 0.00a | 0.3 ± 0.01a |
| Solvent | ||||||
| Acidity (%) | 1.42± 0.05a | 0.08± 0.03b | 1.64 ± 0.10a | 0.10± 0.05b | 1.55 ± 0.05a | 0.09 ± 0.02b |
| Fatty Acids (%) | ||||||
| C16: 0 | 7.3 ± 0.0b | 7.2 ± 0.01a | 6.8 ± 0.03a | 6.7 ± 0.03a | 7.4 ± 0.00a | 7.4 ± 0.04a |
| C16: 1 | 0.2 ± 0.00a | 0.2 ± 0.01a | nd | 0.1 ± 0.00a | 0.1 ± 0.00a | 0.1 ± 0.00a |
| C18: 0 | 3.6 ± 0.01b | 3.5 ± 0.01a | 3.2 ± 0.01a | 3.1 ± 0.04a | 4.1 ± 0.01a | 4.0 ± 0.10a |
| C18: 1 | 29.0 ± 0.03a | 29.3 ± 0.01b | 29.6 ± 0.04b | 27.9 ± 0.07a | 23.6 ± 0.02a | 24.4 ± 0.20b |
| C18: 2 | 58.5 ± 0.06a | 58.5 ± 0.01a | 59.3 ± 0.07a | 59.3 ± 0.09a | 63.3 ± 0.02 | 62.7 ± 0.38a |
| C20: 0 | 0.3 ± 0.00a | 0.3 ± 0.00a | 0.2 ± 0.00a | 0.2 ± 0.00a | 0.3 ± 0.00a | 0.3 ± 0.01a |
| C18: 3 | 0.1 ± 0.00a | 0.1 ± 0.01a | nd | 0.1 ± 0.01a | 0.1 ± 0.00a | 0.1 ± 0.00a |
| C20: 1 | 0.2 ± 0.00a | 0.2 ± 0.00a | 0.2 ± 0.00a | 0.2 ± 0.00a | 0.2 ± 0.00a | 0.2 ± 0.01a |
| C22: 0 | 0.6 ± 0.01b | 0.5 ± 0.01a | 0.5 ± 0.01a | 0.5 ± 0.01a | 0.6 ± 0.00a | 0.6 ± 0.02a |
| C24: 0 | 0.3 ± 0.00a | 0.3 ± 0.00a | 0.2 ± 0.00a | 0.2 ± 0.01a | 0.3 ± 0.00a | 0.3 ± 0.01a |
| CON | CAM | STIG | ||||
|---|---|---|---|---|---|---|
| Crude | Ch Refined | Crude | Ch Refined | Crude | Ch Refined | |
| Press | ||||||
| Phytosterols composition (%) | ||||||
| Campesterol | 6.2 ± 0.06a | 6.1 ± 0.27a | 28.2 ± 0.1b | 26.5 ± 0.39a | 6.2 ± 0.06a | 6.5 ± 0.01b |
| Stigmasterol | 7.8 ± 0.08b | 7.1 ± 0.33a | 8.9 ± 0.29b | 7.7 ± 0.19a | 9.4 ± 0.19a | 9.4 ± 0.17a |
| β-Sitosterol | 61.2 ± 0.44a | 62.6 ± 1.17a | 47.5 ± 0.07b | 46.3 ± 0.74a | 46.6 ± 1.1a | 48.8 ± 0.16b |
| ∆5-Avenasterol | 1.6 ± 0.04 | nd | 3.7 ± 0.07b | 2.6 ± 0.11a | 0.9 ± 0.03 | nd |
| ∆7-Stigmastenol | 18.8 ± 0.51a | 20.3 ± 1.03a | 6.7 ± 0.18a | 12.6 ± 1.24b | 32.5 ± 1.58a | 30.9 ± 0.33a |
| ∆7-Avenasterol | 4.5 ± 0.1b | 3.9 ± 0.19a | 4.9 ± 0.12b | 4.2 ± 0.05a | 4.3 ± 0.28a | 4.5 ± 0.11a |
| Total PS content(mg kg−1) | 4476 ± 136b | 3897 ± 225a | 5225 ± 203b | 3533 ± 63a | 5284 ± 200b | 4547 ± 298a |
| Tocopherols (mg kg−1) | 515 ± 13a | 489 ± 12b | 881 ± 22a | 810 ± 20b | 722 ± 18a | 679 ± 17b |
| Solvent | ||||||
| Phytosterols Composition (%) | ||||||
| Campesterol | 7.3 ± 0.15a | 7.2 ± 0.25a | 23.4 ± 0.19a | 23.2 ± 0.15a | 8.3 ± 0.04a | 8.5 ± 0.19a |
| Stigmasterol | 11.4 ± 0.14b | 10.7 ± 0.15a | 11.0 ± 0.11b | 10.2 ± 0.17a | 12.6 ± 0.25b | 11.2 ± 0.08a |
| β-Sitosterol | 57.9 ± 0.2a | 58.9 ± 0.38b | 50.9 ± 0.35a | 52.7 ± 0.58b | 48.0 ± 0.09a | 48.1 ± 0.26a |
| ∆5-Avenasterol | 1.7 ± 0.04 | nd | 3.3 ± 0.03b | 2.5 ± 0.13a | 1.5 ± 0.05b | 1.2 ± 0.03a |
| ∆7-Stigmastenol | 17.3 ± 0.46a | 18.8 ± 0.22b | 7.7 ± 0.64a | 7.6 ± 0.48a | 24.8 ± 0.48a | 26.4 ± 0.09b |
| ∆7-Avenasterol | 4.4 ± 0.19a | 4.3 ± 0.27a | 3.6 ± 0.05a | 3.9 ± 0.18b | 4.6 ± 0.22a | 4.7 ± 0.14a |
| Total PS content(mg kg−1) | 7272 ± 343b | 5858 ± 310a | 6678 ± 387b | 4602 ± 238a | 9249 ± 669b | 6610 ± 458a |
| Tocopherols (mg kg−1) | 517 ± 12a | 480 ± 12b | 912 ± 23a | 871 ± 21b | 784 ± 20a | 713 ± 17b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-González, A.; Velasco, J.; Velasco, L.; Ruiz-Méndez, M.V. Attempts of Physical Refining of Sterol-Rich Sunflower Press Oil to Obtain Minimally Processed Edible Oil. Foods 2021, 10, 1901. https://doi.org/10.3390/foods10081901
García-González A, Velasco J, Velasco L, Ruiz-Méndez MV. Attempts of Physical Refining of Sterol-Rich Sunflower Press Oil to Obtain Minimally Processed Edible Oil. Foods. 2021; 10(8):1901. https://doi.org/10.3390/foods10081901
Chicago/Turabian StyleGarcía-González, Aída, Joaquín Velasco, Leonardo Velasco, and M. Victoria Ruiz-Méndez. 2021. "Attempts of Physical Refining of Sterol-Rich Sunflower Press Oil to Obtain Minimally Processed Edible Oil" Foods 10, no. 8: 1901. https://doi.org/10.3390/foods10081901
APA StyleGarcía-González, A., Velasco, J., Velasco, L., & Ruiz-Méndez, M. V. (2021). Attempts of Physical Refining of Sterol-Rich Sunflower Press Oil to Obtain Minimally Processed Edible Oil. Foods, 10(8), 1901. https://doi.org/10.3390/foods10081901