A Sensitive SERS Method for Determination of Pymetrozine in Apple and Cabbage Based on an Easily Prepared Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Fabrication of AgNPs and Functionalization with Different Chemicals
2.3. Characterization of Non-Modified and Modified AgNPs
2.4. Preparation of Standard Solutions
2.5. Sample Preparation
2.6. SERS Measurement and Data Analysis
2.7. The Correlation of SERS with LC-MS/MS
3. Results and Discussion
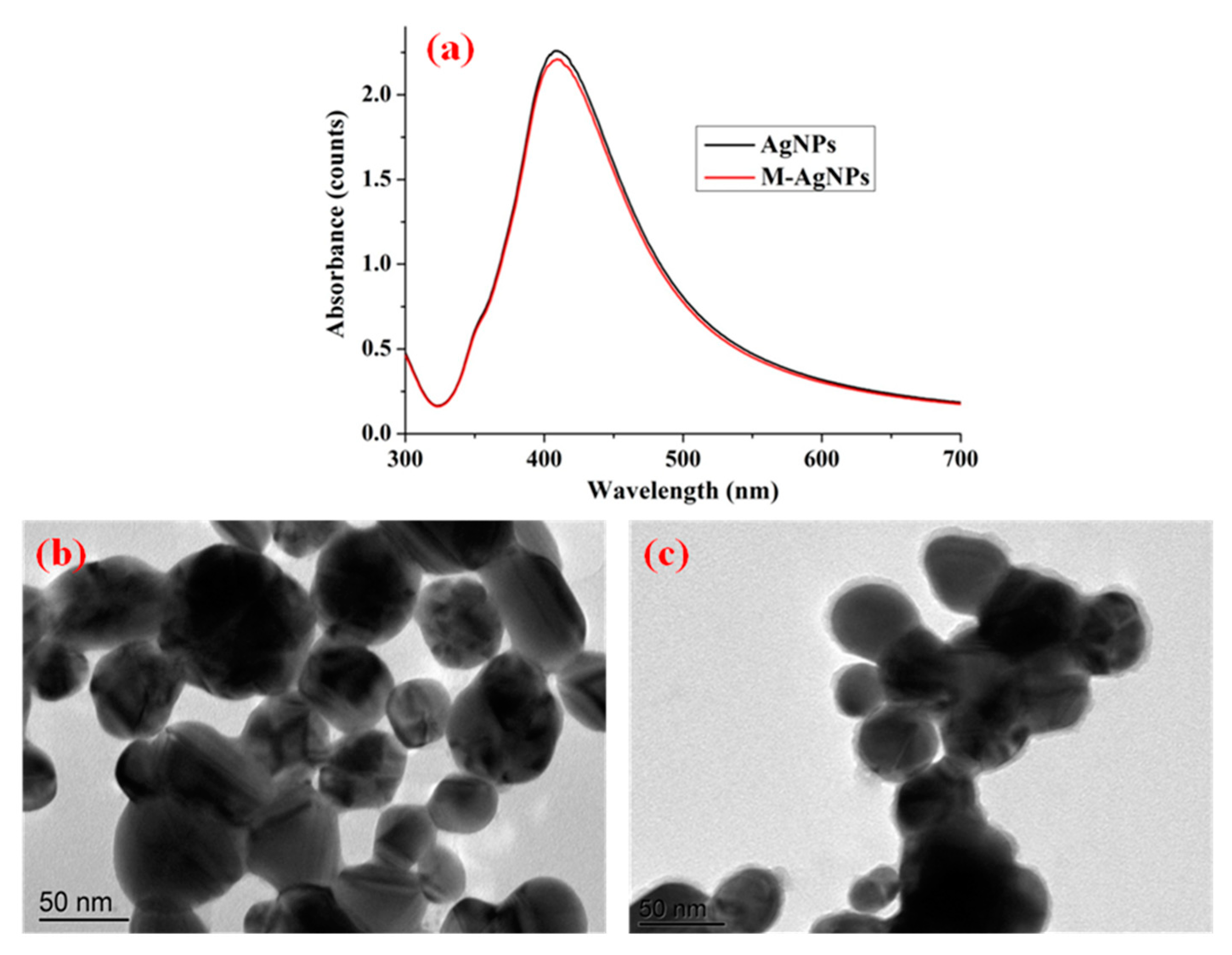
3.1. Characterization of AgNPs and M-AgNPs
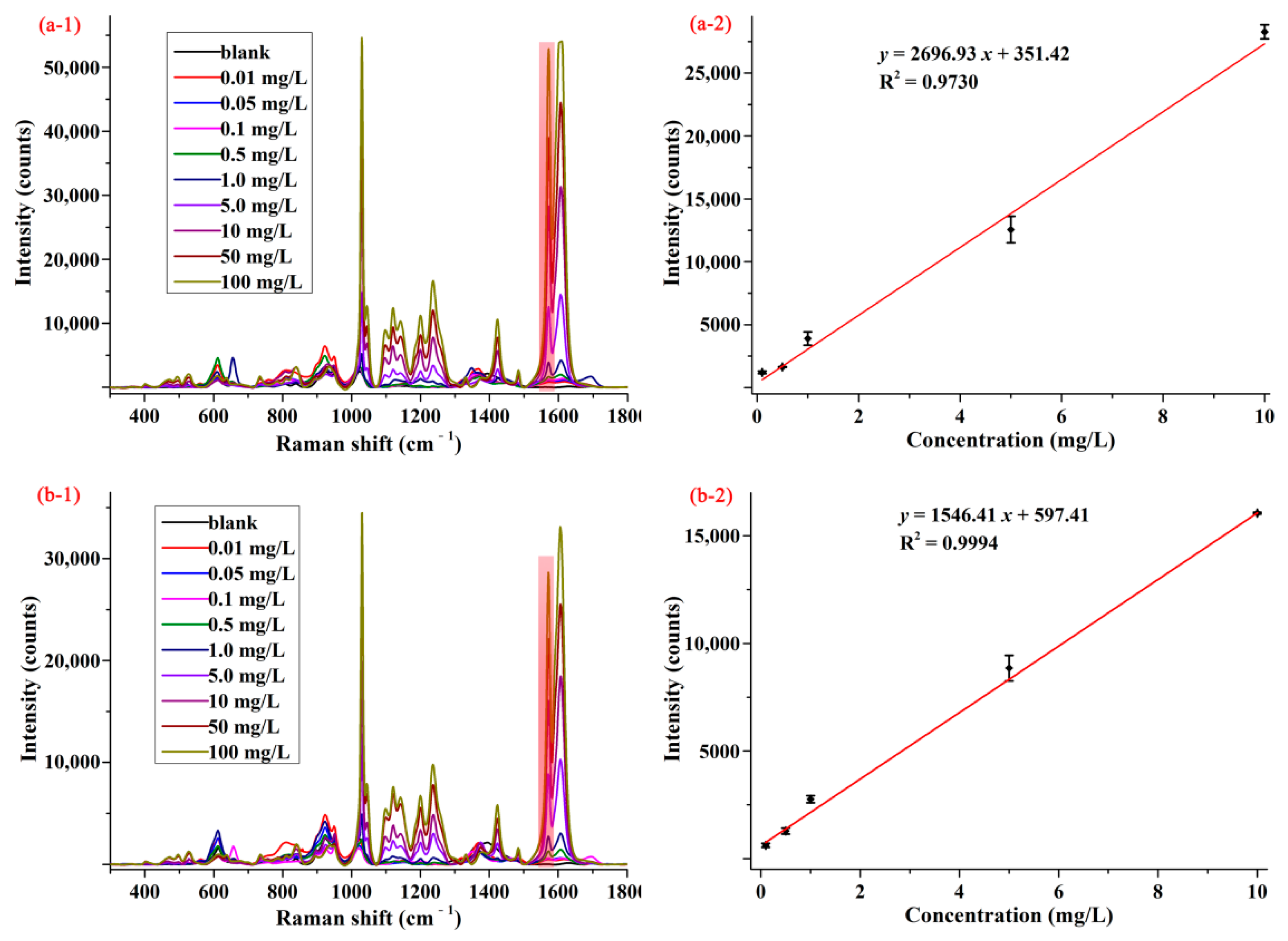
3.2. SERS Activity of Pymetrozine on AgNPs and the M-AgNPs
3.3. Detection of Pymetrozine in Matrix Standard Solution and the Spiked Samples
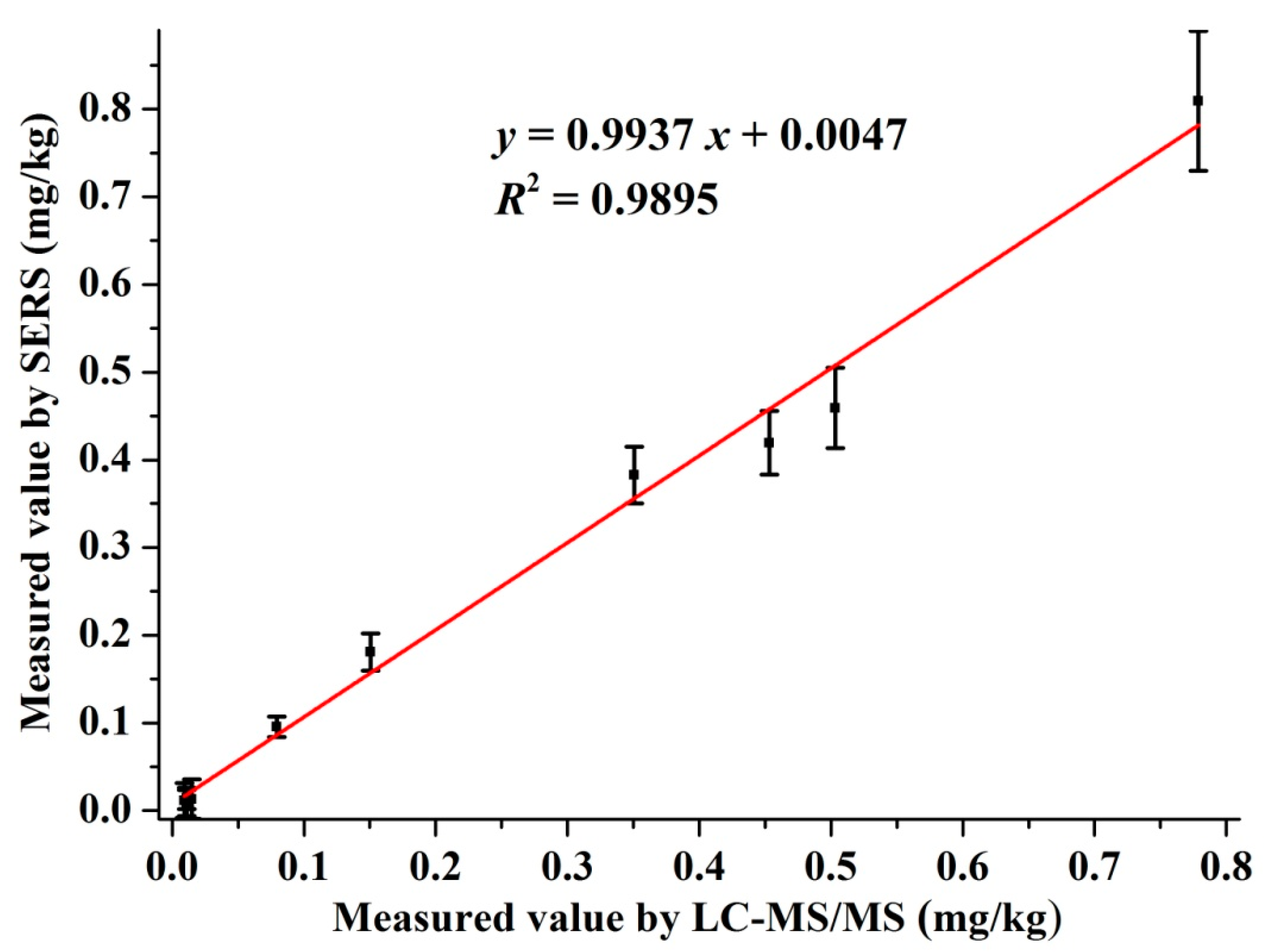
3.4. The Validation of SERS with LC-MS/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, S.; Hu, J.; Guo, P.; Liu, M.; Wu, R. Rapid detection of chlorpyriphos residue in rice by surface-enhanced Raman scattering. Anal. Methods 2015, 7, 4334–4339. [Google Scholar] [CrossRef]
- Chai, L.-K.; Elie, F.; Jinang, C. Determination of 24 pesticides residues in mineral and peat soils by modified QuEChERS method and gas chromatography. Int. J. Environ. Anal. Chem. 2013, 94, 519–530. [Google Scholar] [CrossRef]
- Huang, S.; Yan, W.; Liu, M.; Hu, J. Detection of difenoconazole pesticides in pak choi by surface-enhanced Raman scattering spectroscopy coupled with gold nanoparticles. Anal. Methods 2016, 8, 4755–4761. [Google Scholar] [CrossRef]
- Jang, J.; Rahman, M.; Ko, A.-Y.; El-Aty, A.M.A.; Park, J.-H.; Cho, S.-K.; Shim, J.-H. A matrix sensitive gas chromatography method for the analysis of pymetrozine in red pepper: Application to dissipation pattern and PHRL. Food Chem. 2013, 146, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Jam, N.A.; Saber, M. Sublethal effects of imidacloprid and pymetrozine on the functional response of the aphid parasitoid, Lysiphlebus fabarum. Èntomol. Gen. 2018, 38, 173–190. [Google Scholar] [CrossRef]
- Wang, L.-X.; Niu, C.-D.; Salgado, V.L.; Lelito, K.; Stam, L.; Jia, Y.-L.; Zhang, Y.; Gao, C.-F.; Wu, S.-F. Pymetrozine activates TRPV channels of brown planthopper Nilaparvata lugens. Pestic. Biochem. Physiol. 2018, 153, 77–86. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) 2015/401. Off. J. Eur. Union. 2015. L 71/114. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32015R0401&qid=1610337784804 (accessed on 10 January 2021).
- CFDA; MOA; NHFPC. National Food Safety Standard-Maximum Residue Limits for Pesticides in Food. Chinese National Standard GB 2763-2016. 18 December 2016. Available online: http://bz.cfsa.net.cn/staticPages/0D64FDAF-B210-43F4-B01D-8A97B276BCAF.html (accessed on 10 January 2021). (In Chinese).
- Sheng, E.Z.; Tan, Y.T.; Lu, Y.X.; Xiao, Y.; Li, Z.X. Sensitive time-resolved fluorescence immunoassay for quantitative determination of Oxyfluorfen in food and environmental samples. Front. Chem. 2021, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Xu, P.; Li, J.; Wang, H. Dissipation and residue of pymetrozine in rice field ecosystem. Environ. Monit. Assess. 2015, 187, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Jia, G.; Zeng, L.; Zhao, S.; Ge, S.; Long, X.; Zhang, Y.; Hu, D. Monitoring residue levels and dietary risk assessment of pymetrozine for Chinese consumption of cauliflower. Biomed. Chromatogr. 2019, 338, e4455. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Zhang, Y.-J.; Li, X.; Dong, C.; Liu, H.-Y.; Miao, L.-J.; Low, P.J.; Gao, Z.-X.; Hosmane, N.S.; Wu, A.-G. Rapid and sensitive colorimetric sensing of the insecticide pymetrozine using melamine-modified gold nanoparticles. Anal. Methods 2018, 10, 417–421. [Google Scholar] [CrossRef]
- Jia, D.; Gao, J.; Wang, L.; Gao, Y.; Ye, B. Electrochemical behavior of the insecticide pymetrozine at an electrochemically pretreated glassy carbon electrode and its analytical application. Anal. Methods 2015, 7, 9100–9107. [Google Scholar] [CrossRef]
- Mercan, H.; Yilmaz, E.; Inam, R. Determination of insecticide pymetrozine by differential pulse polarography/application to lake water and orange juice. J. Hazard. Mater. 2007, 141, 700–706. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, C.; Pu, S.; Wang, C.; Cheng, F.; Wang, Y.; Fan, M. Rapid and direct detection of illicit dyes on tainted fruit peel using a PVA hydrogel surface enhanced Raman scattering substrate. Anal. Methods 2016, 8, 4816–4820. [Google Scholar] [CrossRef]
- Yaseen, T.; Sun, D.-W.; Pu, H.; Pan, T.-T. Detection of Omethoate Residues in Peach with Surface-Enhanced Raman Spectroscopy. Food Anal. Methods 2018, 11, 2518–2527. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.-W.; Pu, H.; Wei, Q. Surface-enhanced Raman scattering of core-shell Au@Ag nanoparticles aggregates for rapid detection of difenoconazole in grapes. Talanta 2019, 191, 449–456. [Google Scholar] [CrossRef]
- Pan, T.; Guo, W.; Lu, P.; Hu, D. In situ and rapid determination of acetamiprid residue on cabbage leaf using surface-enhanced Raman scattering. J. Sci. Food Agric. 2020, 101. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.W.; Pu, H.; Wei, Q. Two-dimensional Au@Ag nanodot array for sensing dual-fungicides in fruit juices with surface-enhanced Raman spectroscopy technique. Food Chem. 2019, 310, 125923–125932. [Google Scholar] [CrossRef]
- Wu, L.; Pu, H.; Huang, L.; Sun, D.-W. Plasmonic nanoparticles on metal-organic framework: A versatile SERS platform for adsorptive detection of new coccine and orange II dyes in food. Food Chem. 2020, 328, 127105–127113. [Google Scholar] [CrossRef]
- Pan, T.-T.; Sun, D.-W.; Pu, H.; Wei, Q.-Y. Simple Approach for the Rapid Detection of Alternariol in Pear Fruit by Surface-Enhanced Raman Scattering with Pyridine-Modified Silver Nanoparticles. J. Agric. Food Chem. 2018, 66, 2180–2187. [Google Scholar] [CrossRef]
- Pan, T.-T.; Sun, D.-W.; Pu, H.; Wei, Q.; Xiao, W.; Wang, Q.-J. Detection of A. alternata from pear juice using surface-enhanced Raman spectroscopy based silver nanodots array. J. Food Eng. 2017, 215, 147–155. [Google Scholar] [CrossRef]
- Jiao, T.; Hassan, M.; Zhu, J.; Ali, S.; Ahmad, W.; Wang, J.; Lv, C.; Chen, Q.; Li, H. Quantification of deltamethrin residues in wheat by Ag@ZnO NFs-based surface-enhanced Raman spectroscopy coupling chemometric models. Food Chem. 2021, 337, 127652–127659. [Google Scholar] [CrossRef]
- Hassan, M.; Zareef, M.; Jiao, T.; Liu, S.; Xu, Y.; Viswadevarayalu, A.; Li, H.; Chen, Q. Signal optimized rough silver nanoparticle for rapid SERS sensing of pesticide residues in tea. Food Chem. 2021, 338, 127796–127802. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhao, Q.; Meng, G.; Wang, X.; Hu, X.; Han, F.; Lei, Y. Silver nanoparticle-assembled micro-bowl arrays for sensitive SERS detection of pesticide residue. Nanotechnology 2020, 31, 205303. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Li, J.; Xu, T.; Lai, K.; Ding, Q.; Lin, H.; Sun, L.; Lin, M. A spectroscopic approach to detect and quantify phosmet residues in Oolong tea by surface-enhanced Raman scattering and silver nanoparticle substrate. Food Chem. 2020, 312, 126016–126022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Bai, Y.; Yao, S.; Wei, Z.; Zhang, M.; Wang, L.; Wang, L. Superhydrophobic SERS substrates based on silver dendrite-decorated filter paper for trace detection of nitenpyram. Anal. Chim. Acta 2019, 1049, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kutsanedzie, F.Y.; Hassan, M.; Zhu, J.; Ahmad, W.; Li, H.; Chen, Q. Mesoporous silica supported orderly-spaced gold nanoparticles SERS-based sensor for pesticides detection in food. Food Chem. 2020, 315, 126300–126306. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the Synthesis and Assembly of Silver Nanostructures for Plasmonic Applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Pu, H.; Sun, D.-W. Cysteamine modified core-shell nanoparticles for rapid assessment of oxamyl and thiacloprid pesticides in milk using SERS. J. Food Meas. Charact. 2020, 14, 2021–2029. [Google Scholar] [CrossRef]
- Hussainabc, A.; Puabc, H.; Sunabcd, D.W. SERS detection of sodium thiocyanate and benzoic acid preservatives in liquid milk using cysteamine functionalized core-shelled nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117994. [Google Scholar] [CrossRef]
- Hussain, N.; Puabc, H.; Hussain, A.; Sunabcd, D.W. Rapid detection of ziram residues in apple and pear fruits by SERS based on octanethiol functionalized bimetallic core-shell nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 236, 118357. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, T.; Ruan, W.; Jung, Y.M.; Cong, Q.; Zhao, B. A simple strategy to improve the sensitivity of probe molecules on SERS substrates. Talanta 2019, 195, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, T.; Ruan, W.; Zhao, B.; Cong, Q. Controlling the orientation of probe molecules on surface-enhanced Raman scattering substrates: A novel strategy to improve sensitivity. Anal. Chim. Acta 2017, 994, 65–72. [Google Scholar] [CrossRef]
- Shen, G.; Hu, X.; Hu, Y. Kinetic study of the degradation of the insecticide pymetrozine in a vegetable-field ecosystem. J. Hazard. Mater. 2009, 164, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, J.; Tao, Z.; Eremin, S.A.; Hua, X.; Wang, M. Development of fluorescence polarization immunoassay for Imidacloprid in environmental and agricultural samples. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]


| Samples | Spiked Level (mg/kg) | Recovery (%) | RSD (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Intra-Day | Inter-Day | |||||
| Range | AVG | Range | AVG | Day 1 | Day 2 | D1 + D2 | ||
| Apple | 0.2 | 74.50–95.50 | 83.50 | 70.50–93.00 | 83.00 | 8.86 | 8.43 | 8.16 |
| 2.0 | 72.45–100.35 | 87.15 | 70.40–96.90 | 83.61 | 10.90 | 10.07 | 10.07 | |
| 10 | 78.95–98.53 | 87.70 | 71.59–98.12 | 82.42 | 7.17 | 12.18 | 9.83 | |
| Cabbage | 0.2 | 70.50–99.50 | 86.40 | 75.50–104.00 | 84.30 | 10.35 | 11.42 | 10.33 |
| 2.0 | 73.40–99.25 | 83.34 | 75.20–98.50 | 86.84 | 9.66 | 9.55 | 9.24 | |
| 10 | 70.92–98.30 | 83.90 | 71.15–97.79 | 82.24 | 11.63 | 11.01 | 10.24 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, T.-T.; Guo, M.-T.; Guo, W.; Lu, P.; Hu, D.-Y. A Sensitive SERS Method for Determination of Pymetrozine in Apple and Cabbage Based on an Easily Prepared Substrate. Foods 2021, 10, 1874. https://doi.org/10.3390/foods10081874
Pan T-T, Guo M-T, Guo W, Lu P, Hu D-Y. A Sensitive SERS Method for Determination of Pymetrozine in Apple and Cabbage Based on an Easily Prepared Substrate. Foods. 2021; 10(8):1874. https://doi.org/10.3390/foods10081874
Chicago/Turabian StylePan, Ting-Tiao, Mei-Ting Guo, Wang Guo, Ping Lu, and De-Yu Hu. 2021. "A Sensitive SERS Method for Determination of Pymetrozine in Apple and Cabbage Based on an Easily Prepared Substrate" Foods 10, no. 8: 1874. https://doi.org/10.3390/foods10081874
APA StylePan, T.-T., Guo, M.-T., Guo, W., Lu, P., & Hu, D.-Y. (2021). A Sensitive SERS Method for Determination of Pymetrozine in Apple and Cabbage Based on an Easily Prepared Substrate. Foods, 10(8), 1874. https://doi.org/10.3390/foods10081874





