Non-Specific Binding and Cross-Reaction of ELISA: A Case Study of Porcine Hemoglobin Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Indirect sELISA
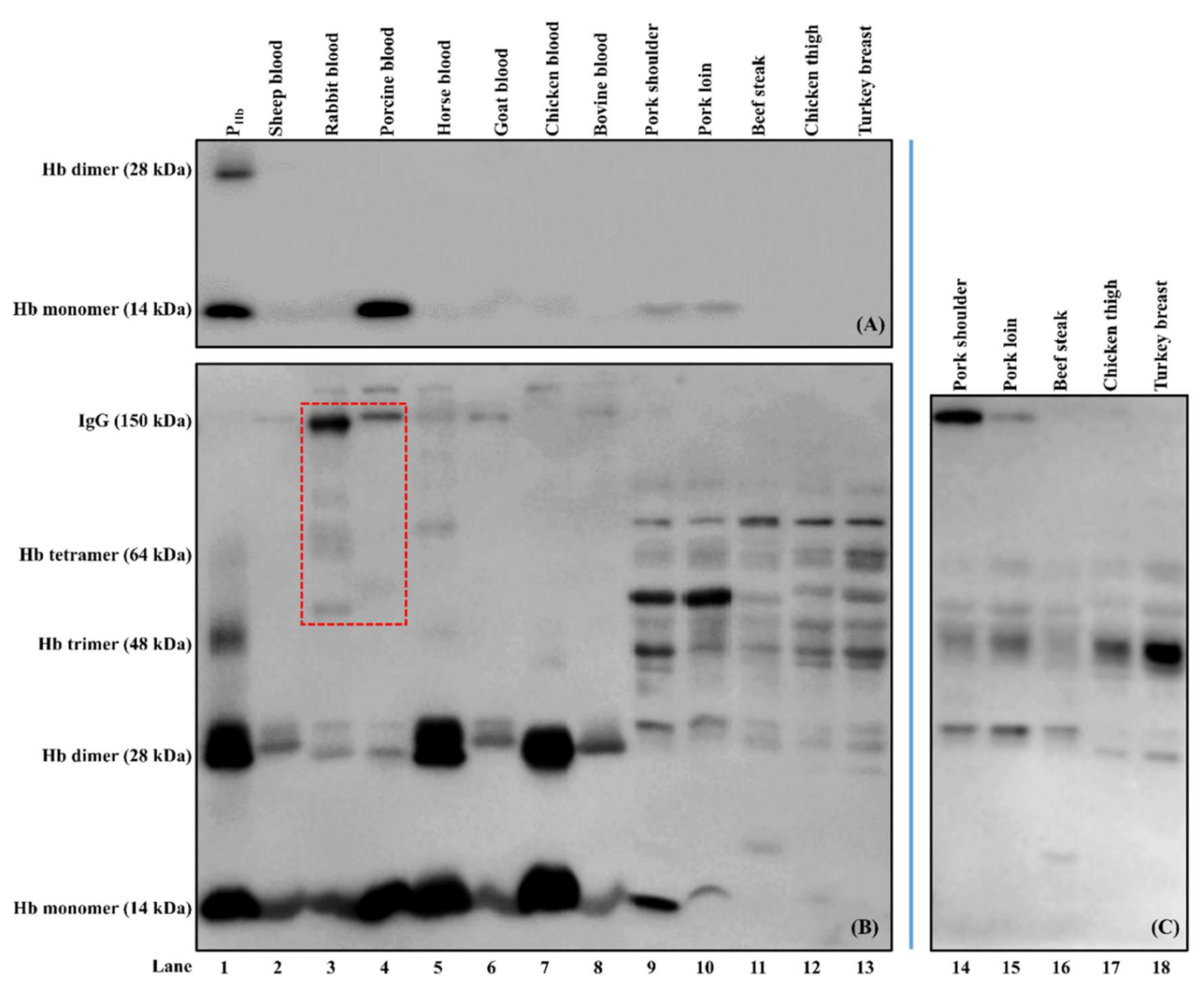
2.4. Western Blot
2.5. Statistical Analysis
3. Results and Discussion
3.1. Antibody Characterization
3.2. Non-Specific Binding (NSB)
3.2.1. Effect of Tween-20
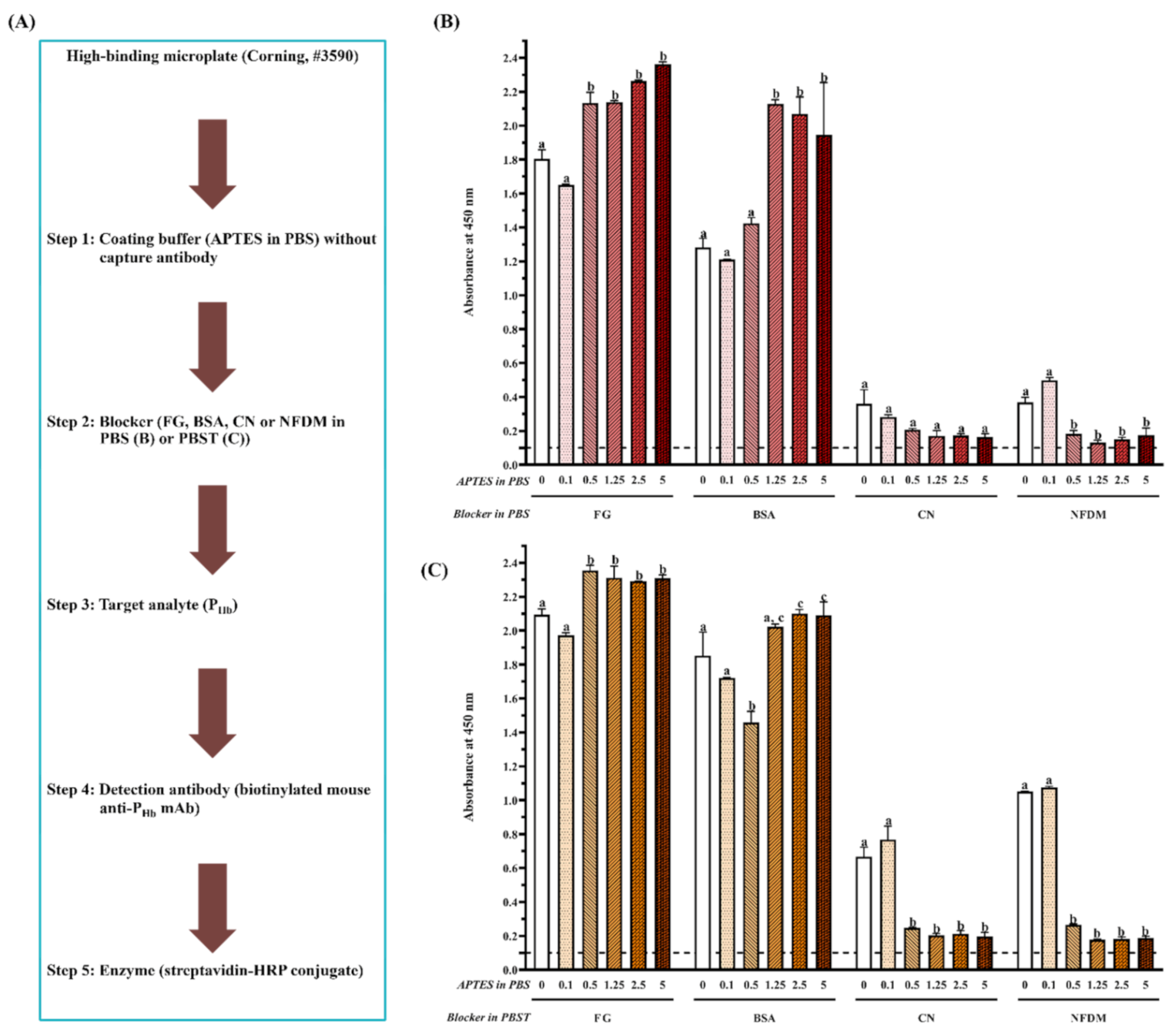
3.2.2. Effect of Protein and Non-Protein Blockers
3.2.3. Effect of Microplate Type
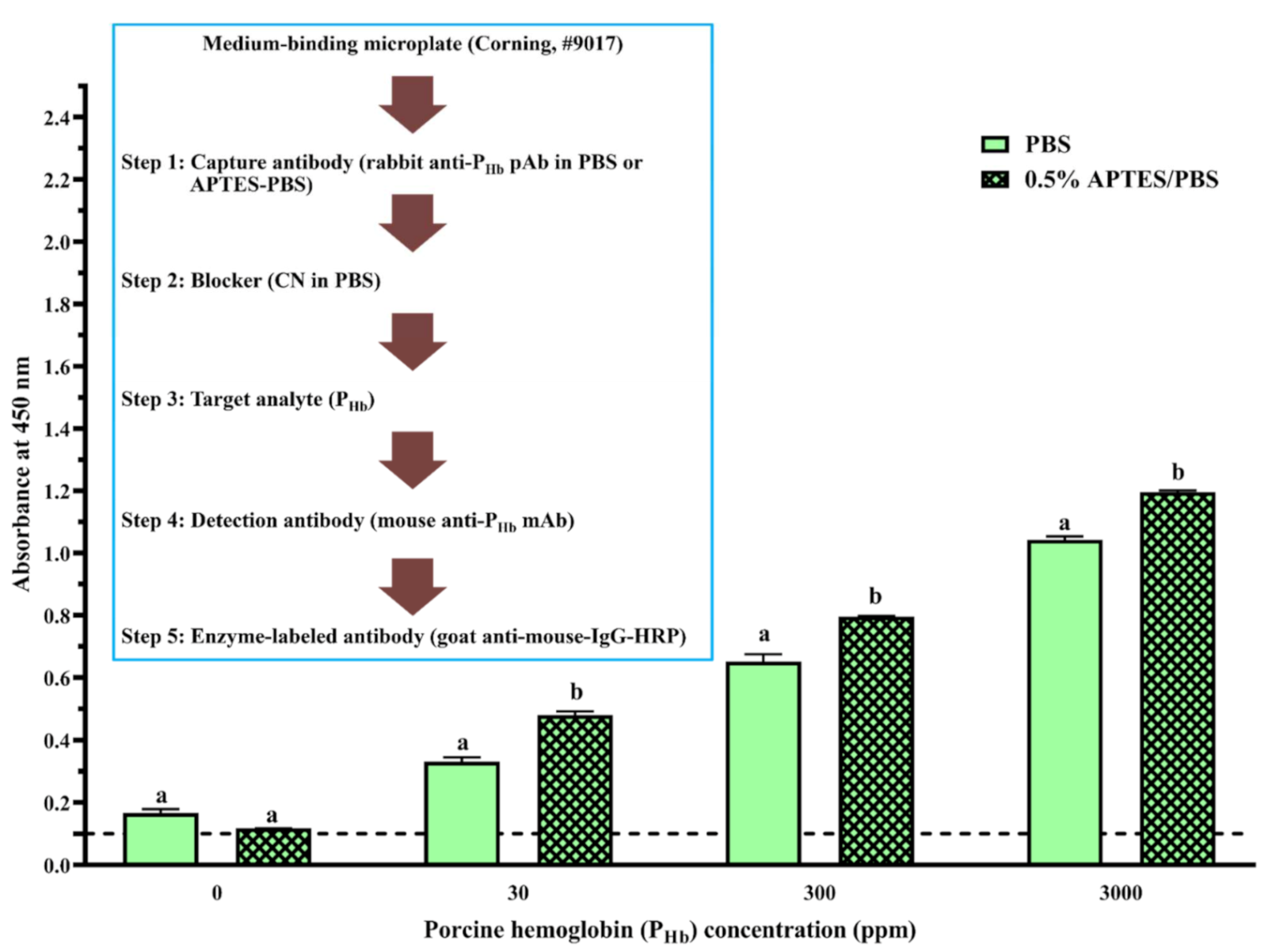
3.2.4. Effect of APTES during Coating
3.3. Cross-Reaction
3.3.1. Cross-Reaction of the Enzyme-Labeled Secondary Antibody and Unintended IgGs
3.3.2. Cross-Reaction among Capture Antibody, Non-PHb Proteins, and Enzyme-Labeled Secondary Antibody
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spink, J.; Moyer, D.C. Defining the public health threat of food fraud. J. Food Sci. 2011, 76, R157–R163. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.C.; Spink, J.; Lipp, M. Development and application of a database of food ingredient fraud and economically motivated adulteration from 1980 to 2010. J. Food Sci. 2012, 77, R118–R126. [Google Scholar] [CrossRef]
- Everstine, K.; Spink, J.; Kennedy, S. Economically motivated adulteration (EMA) of food: Common characteristics of EMA incidents. J. Food Protect. 2013, 76, 723–735. [Google Scholar] [CrossRef]
- Johnson, R. Food Fraud and “Economically Motivated Adulteration” of Food and Food Ingredients. Available online: https://fas.org/sgp/crs/misc/R43358.pdf (accessed on 12 July 2021).
- Points, J.; Mannings, L. Facing up to food fraud in a pandemic. Food Sci. Technol. 2020, 34, 16–20. [Google Scholar] [CrossRef]
- Knorr, D.; Khoo, C.S.H. COVID-19 and food: Challenges and research needs. Front. Nutr. 2020, 7, 598913. [Google Scholar] [CrossRef] [PubMed]
- Van Ruth, S.M. Impact of the COVID-19 Pandemic on Food Fraud Vulnerability in Food Supply Networks. Available online: https://research.wur.nl/en/publications/impact-of-the-covid-19-pandemic-on-food-fraud-vulnerability-in-fo (accessed on 12 July 2021).
- Asensio, L.; Gonzalez, I.; Garcia, T.; Martin, R. Determination of food authenticity by enzyme-linked immunosorbent assay (ELISA). Food Control 2008, 19, 1–8. [Google Scholar] [CrossRef]
- BCC Research. Global Markets and Technologies for Food Safety Testing. Available online: https://academic.bccresearch.com/market-research/food-and-beverage/food-safety-testing-technologies-markets-report.html (accessed on 12 July 2021).
- Grand View Research. Immunoassay Market Size, Share & Trends Analysis Report by Product (Reagent & Kits, Analyzers/Instruments, Software & Services), by Technology (RIA, ELISA, Rapid Test), by Application, by End Use, by Region, and Segment Forecasts, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/immunoassay-market (accessed on 12 July 2021).
- BCC Research. Immunoassays: Technologies and Global Markets—Focus on Enzyme Immunoassays. Available online: https://www.bccresearch.com/market-research/biotechnology/enzyme-immunoassays-bio120a.html (accessed on 12 July 2021).
- Engvall, E. The ELISA, enzyme-linked immunosorbent assay. Clin. Chem. 2010, 56, 319–320. [Google Scholar] [CrossRef]
- Shah, K.; Maghsoudlou, P. Enzyme-linked immunosorbent assay (ELISA): The basics. Brit. J. Hosp. Med. 2016, 77, C98–C101. [Google Scholar] [CrossRef]
- Ciaurriz, P.; Fernandez, F.; Tellechea, E.; Moran, J.F.; Asensio, A.C. Comparison of four functionalization methods of gold nanoparticles for enhancing the enzyme-linked immunosorbent assay (ELISA). Beilstein J. Nanotech. 2017, 8, 244–253. [Google Scholar] [CrossRef]
- Aaby, P.; Aftahi, N.; Aldrich, C.; Alzari, P.M.; Bevan, M.M. Chapter 4 Specificity and Cross-Reactivity. In Immunology and Evolution of Infectious Disease; Frank, S.A., Ed.; Princeton University Press: Princeton, NJ, USA, 2002; pp. 34–56. [Google Scholar]
- USFDA. Guidance for Industry Bioanalytical Method Validation. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf (accessed on 12 July 2021).
- Crowther, J.R. Chapter 3 Stages in ELISA. In The ELISA Guidebook; Walker, J.M., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2009; pp. 43–78. [Google Scholar]
- Jørgensen, C.S.; Hansen, K.B.; Jacobsen, S.; Halberg, P.; Ullman, S.; Hansen, D.; Mikkelsen, T.L.; Weile, B.; Madsen, M.H.; Wiik, A.; et al. Absence of high-affinity calreticulin autoantibodies in patients with systemic rheumatic diseases and coeliac disease. Scand. J. Clin. Lab. Inv. 2005, 65, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Péterfi, Z.; Kocsis, B. Comparison of blocking agents for an ELISA for LPS. J. Immunoass. 2000, 21, 341–354. [Google Scholar] [CrossRef]
- Güven, E.; Duus, K.; Lydolph, M.C.; Jørgensen, C.S.; Laursen, I.; Houen, G. Non-specific binding in solid phase immunoassays for autoantibodies correlates with inflammation markers. J. Immunol. Methods 2014, 403, 26–36. [Google Scholar] [CrossRef]
- Konishi, E.; Kitai, Y.; Nishimura, K.; Harada, S. Antibodies to bovine serum albumin in human sera: Problems and solutions with casein-based ELISA in the detection of natural Japanese encephalitis virus infections. Jpn. J. Infect. Dis. 2010, 63, 296–298. [Google Scholar]
- Xiao, Y.; Isaacs, S.N. Enzyme-linked immunosorbent assay (ELISA) and blocking with bovine serum albumin (BSA)—Not all BSAs are alike. J. Immunol. Methods 2012, 384, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Chart, H.; Evans, J.; Chalmers, R.M.; Salmon, R.L. Escherichia coli O157 serology: False-positive ELISA results caused by human antibodies binding to bovine serum albumin. Lett. Appl. Microbiol. 1998, 27, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Y.; Wu, M.; Dong, W.Y.; Rao, Q.C.; Huo, H.L.; Han, Q.G. Monoclonal antibody-based sandwich enzyme-linked immunosorbent assay for porcine hemoglobin quantification. Food Chem. 2020, 324, 126880. [Google Scholar] [CrossRef]
- Corning Inc. Corning and Falcon Microplates Selection Guide: For Assays and Drug Discovery. Available online: https://www.corning.com/media/worldwide/cls/documents/CLS-C-DL-MP-014REV9.pdf (accessed on 12 July 2021).
- Ofori, J.A.; Hsieh, Y.-H.P. Monoclonal antibodies as probes for the detection of porcine blood derived food ingredients. J. Agr. Food Chem. 2016, 64, 3705–3711. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Fuller, D.; Hsieh, Y.-H.P.; Rao, Q.C. Monoclonal antibody-based ELISA for the quantification of porcine hemoglobin in meat products. Food Chem. 2018, 250, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Prusaksochaczewski, E.; Luong, J.H.T. An improved ELISA method for the detection of Salmonella typhimurium. J. Appl. Bacteriol. 1989, 66, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Steinitz, M. Quantitation of the blocking effect of Tween 20 and bovine serum albumin in ELISA microwells. Anal. Biochem. 2000, 282, 232–238. [Google Scholar] [CrossRef]
- Hakami, A.R.; Ball, J.K.; Tarr, A.W. Non-ionic detergents facilitate non-specific binding of M13 bacteriophage to polystyrene surfaces. J. Virol. Methods 2015, 221, 1–8. [Google Scholar] [CrossRef]
- Julian, E.; Cama, M.; Martinez, P.; Luquin, M. An ELISA for five glycolipids from the cell wall of Mycobacterium tuberculosis: Tween 20 interference in the assay. J. Immunol. Methods 2001, 251, 21–30. [Google Scholar] [CrossRef]
- Bird, C.R.; Gearing, A.J.H.; Thorpe, R. The use of Tween-20 alone as a blocking-agent for immunoblotting can cause artefactual results. J. Immunol. Methods 1988, 106, 175–179. [Google Scholar] [CrossRef]
- Waritani, T.; Chang, J.; McKinney, B.; Terato, K. An ELISA protocol to improve the accuracy and reliability of serological antibody assays. MethodsX 2017, 4, 153–165. [Google Scholar] [CrossRef]
- Mohammad, K.; Esen, A. A blocking agent and a blocking step are not needed in ELISA, immunostaining dot-blots and Western blots. J. Immunol. Methods 1989, 117, 141–145. [Google Scholar] [CrossRef]
- Galva, C.; Gatto, C.; Milanick, M. Soymilk: An effective and inexpensive blocking agent for immunoblotting. Anal. Biochem. 2012, 426, 22–23. [Google Scholar] [CrossRef][Green Version]
- Gibbs, J. Effective Blocking Procedures: ELISA Technical Bulletin—No. 3. Available online: http://www.labcluster.com/news4_3/Corning_elisa3.pdf (accessed on 12 July 2021).
- Vogt, R.F.; Phillips, D.L.; Henderson, L.O.; Whitfield, W.; Spierto, F.W. Quantitative differences among various proteins as blocking agents for ELISA microtiter plates. J. Immunol. Methods 1987, 101, 43–50. [Google Scholar] [CrossRef]
- Gravel, P. Protein blotting by the semidry method. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 621–629. [Google Scholar]
- Yu, F.Y.; Vdovenko, M.M.; Wang, J.J.; Sakharov, I.Y. Comparison of enzyme-linked immunosorbent assays with chemiluminescent and colorimetric detection for the determination of ochratoxin A in food. J. Agr. Food Chem. 2011, 59, 809–813. [Google Scholar] [CrossRef] [PubMed]
- He, S.F.; Li, X.; Gao, J.Y.; Tong, P.; Chen, H.B. Development of sandwich ELISA for testing bovine beta-lactoglobulin allergenic residues by specific polyclonal antibody against human IgE binding epitopes. Food Chem. 2017, 227, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Zhang, Q.; Chen, D.F.; Liu, Y.; Li, C.Y.; Hu, B.S.; Du, D.; Liu, F.Q. Development of a specific enzyme-linked immunosorbent assay (ELISA) for the analysis of the organophosphorous pesticide fenthion in real samples based on monoclonal antibody. Anal. Lett. 2011, 44, 1591–1601. [Google Scholar] [CrossRef]
- Rajasekariah, G.H.R.; Ryan, J.R.; Hillier, S.R.; Yi, L.P.; Stiteler, J.M.; Cui, L.W.; Smithyman, A.M.; Martin, S.K. Optimisation of an ELISA for the serodiagnosis of visceral leishmaniasis using in vitro derived promastigote antigens. J. Immunol. Methods 2001, 252, 105–119. [Google Scholar] [CrossRef]
- Farajollahi, M.M.; Cook, D.B.; Hamzehlou, S.; Self, C.H. Reduction of non-specific binding in immunoassays requiring long incubations. Scand. J. Clin. Lab. Inv. 2012, 72, 531–539. [Google Scholar] [CrossRef]
- Ahirwar, R.; Bariar, S.; Balakrishnan, A.; Nahar, P. BSA blocking in enzyme-linked immunosorbent assays is a non-mandatory step: A perspective study on mechanism of BSA blocking in common ELISA protocols. RSC Adv. 2015, 5, 100077–100083. [Google Scholar] [CrossRef]
- Farrell, H.M.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the proteins of cows’ milk—sixth revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Pratt, R.P.; Roser, B. Comparison of Blocking Agents for ELISA. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/Application-Notes/D19561.pdf (accessed on 12 July 2021).
- Grogan, C.; Raiteri, R.; O’Connor, G.M.; Glynn, T.J.; Cunningham, V.; Kane, M.; Charlton, M.; Leech, D. Characterisation of an antibody coated microcantilever as a potential immuno-based biosensor. Biosens. Bioelectron. 2002, 17, 201–207. [Google Scholar] [CrossRef]
- Kenna, J.G.; Major, G.N.; Williams, R.S. Methods for reducing non-specific antibody binding in enzyme-linked immunosorbent assays. J. Immunol. Methods 1985, 85, 409–419. [Google Scholar] [CrossRef]
- Sentandreu, M.A.; Aubry, L.; Toldra, F.; Ouali, A. Blocking agents for ELISA quantification of compounds coming from bovine muscle crude extracts. Eur. Food Res. Technol. 2007, 224, 623–628. [Google Scholar] [CrossRef]
- Douglas, J.T.; Wu, Q.X.; Agustin, G.P.; Madarang, M.G. Evaluation of inexpensive blocking agents for ELISA in the detection of antibody in leprosy. Leprosy Rev. 1988, 59, 37–43. [Google Scholar] [CrossRef]
- Akerstedt, J. An indirect ELISA for detection of Encephalitozoon cuniculi infection in farmed blue foxes (Alopex lagopus). Acta Vet. Scand. 2002, 43, 211–220. [Google Scholar] [CrossRef]
- Huber, D.; Rudolf, J.; Ansari, P.; Galler, B.; Fuhrer, M.; Hasenhindl, C.; Baumgartner, S. Effectiveness of natural and synthetic blocking reagents and their application for detecting food allergens in enzyme-linked immunosorbent assays. Anal. Bioanal. Chem. 2009, 394, 539–548. [Google Scholar] [CrossRef]
- Hoffman, W.L.; Jump, A.A. Inhibition of the streptavidin-biotin interaction by milk. Anal. Biochem. 1989, 181, 318–320. [Google Scholar] [CrossRef]
- Leite, M.C.; Galland, F.; Brolese, G.; Guerra, M.C.; Bortolotto, J.W.; Freitas, R.; de Almeida, L.M.V.; Gottfried, C.; Goncalves, C.A. A simple, sensitive and widely applicable ELISA for S100B: Methodological features of the measurement of this glial protein. J. Neurosci. Methods 2008, 169, 93–99. [Google Scholar] [CrossRef]
- Tang, R.H.; Yang, H.; Choi, J.R.; Gong, Y.; Hu, J.; Feng, S.S.; Pingguan-Murphy, B.; Mei, Q.B.; Xu, F. Improved sensitivity of lateral flow assay using paper-based sample concentration technique. Talanta 2016, 152, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.X.; He, R.; Ju, X.R.; Zhang, J.; Wang, M.J.; Xing, C.R.; Yuan, J. Development and optimization of an immunoassay for the detection of Hg(II) in lake water. Food Sci. Nutr. 2019, 7, 1615–1622. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Immunoassay Plate Guide. Available online: http://assets.thermofisher.com/TFS-Assets/LCD/Scientific-Resources/Immunoassay_Plate_Guide.pdf (accessed on 12 July 2021).
- Jones, E.; Michael, S.; Sittampalam, G. Basics of assay equipment and instrumentation for high throughput screening. In Assay Guidance Manual; Sittampalam, G.S., Coussens, N.P., Brimacombe, K., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Bejcek, B., Caaveiro, J.M.M., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2012. [Google Scholar]
- Bergeron, A.B.; Bortz, C.J.; Rossi, A. Corning Medium and High Binding ELISA Microplates for Select Target Size Binding Assays Application Note. Available online: https://www.corning.com/media/worldwide/cls/documents/applications/CLS-AN-497%20DL.pdf (accessed on 12 July 2021).
- Gibbs, J.; Vessels, M.; Rothenberg, M. Immobilization Principles—Selecting the Surface. Available online: http://www.labcluster.com/news4_2/Corning_elisa1_2.pdf (accessed on 12 July 2021).
- The UniProt Consortium. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017, 45, D158–D169. [Google Scholar] [CrossRef]
- Kim, J.Y.; Seidler, P.; Wan, L.S.; Fill, C. Formation, structure, and reactivity of amino-terminated organic films on silicon substrates. J. Colloid Interface Sci. 2009, 329, 114–119. [Google Scholar] [CrossRef]
- Dixit, C.K.; Vashist, S.K.; MacCraith, B.D.; O’Kennedy, R. Multisubstrate-compatible ELISA procedures for rapid and high-sensitivity immunoassays. Nat. Protoc. 2011, 6, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Jin, G. Silicon surface modification with a mixed silanes layer to immobilize proteins for biosensor with imaging ellipsometry. Colloid Surf. B 2004, 34, 173–177. [Google Scholar] [CrossRef]
- Cunliffe, D.; Smart, C.A.; Alexander, C.; Vulfson, E.N. Bacterial adhesion at synthetic surfaces. Appl. Environ. Microb. 1999, 65, 4995–5002. [Google Scholar] [CrossRef]
- Vashist, S.K.; Marion Schneider, E.; Lam, E.; Hrapovic, S.; Luong, J.H.T. One-step antibody immobilization-based rapid and highly-sensitive sandwich ELISA procedure for potential in vitro diagnostics. Sci. Rep. 2014, 4, 4407. [Google Scholar] [CrossRef]
- Dixit, C.K.; Vashist, S.K.; O’Neill, F.T.; O’Reilly, B.; MacCraith, B.D.; O’Kennedy, R. Development of a high sensitivity rapid sandwich ELISA procedure and its comparison with the conventional approach. Anal. Chem. 2010, 82, 7049–7052. [Google Scholar] [CrossRef]
- David, C.; Stålberg, J.; Larsson, A. Chicken antibodies: A clinical chemistry perspective. Ups. J. Med. Sci. 1999, 104, 179–189. [Google Scholar] [CrossRef]
- Boscato, L.M.; Stuart, M.C. Incidence and specificity of interference in two-site immunoassays. Clin. Chem. 1986, 32, 1491–1495. [Google Scholar] [CrossRef]
- Tate, J.; Ward, G. Interferences in immunoassay. Clin. Biochem. Rev. 2004, 25, 105–120. [Google Scholar] [PubMed]
- R&D Systems. Goat Anti-Mouse IgG Biotinylated Antibody. Available online: https://www.rndsystems.com/products/goat-anti-mouse-igg-biotinylated-antibody_baf007 (accessed on 12 July 2021).
- Thermo Fisher Scientific. Secondary Antibody Cross-Adsorption and Cross-Reactivity. Available online: https://www.thermofisher.com/us/en/home/life-science/antibodies/antibodies-learning-center/antibodies-resource-library/antibody-methods/secondary-antibody-cross-adsorption-cross-reactivity.html#:~:text=Cross%2Dadsorbed%20secondary%20antibodies%20are,cross%2Dreactivity%20and%20increases%20specificity (accessed on 12 July 2021).
- Abcam. Pre-Adsorbed Secondary Antibodies. Available online: https://www.abcam.com/secondary-antibodies/pre-adsorbed-secondary-antibodies (accessed on 12 July 2021).
- Sigma-Aldrich. Anti-Mouse IgG (H + L), Highly Cross Adsorbed Antibody Produced in Goat. Available online: https://www.sigmaaldrich.com/catalog/product/sigma/sab3701071?lang=en®ion=US (accessed on 12 July 2021).
- Jackson ImmunoResearch Laboratories Inc. Cross-Adsorbed (Min X) Secondary Antibodies and Cross-Reactivity. Available online: https://www.jacksonimmuno.com/technical/products/cross-adsorbed-secondary-antibodies (accessed on 12 July 2021).
- Lipman, N.S.; Jackson, L.R.; Trudel, L.J.; Weis-Garcia, F. Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications, and information resources. ILAR J. 2005, 46, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, Y.; Li, Z.; Pramod, S.N.; Zhang, L.; Lin, H. Development of ELISA method for detecting crustacean major allergen tropomyosin in processed food samples. Food Anal. Methods 2019, 12, 2719–2729. [Google Scholar] [CrossRef]
- Bordeaux, J.; Welsh, A.W.; Agarwal, S.; Killiam, E.; Baquero, M.T.; Hanna, J.A.; Anagnostou, V.K.; Rimm, D.L. Antibody validation. BioTechniques 2010, 48, 197–209. [Google Scholar] [CrossRef]
- Weller, M.G. Ten basic rules of antibody validation. Anal. Chem. Insights 2018, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Bandrowski, A.; Carr, S.; Edwards, A.; Ellenberg, J.; Lundberg, E.; Rimm, D.L.; Rodriguez, H.; Hiltke, T.; Snyder, M.; et al. A proposal for validation of antibodies. Nat. Methods 2016, 13, 823–828. [Google Scholar] [CrossRef]
- Staggs, C.G.; Sealey, W.M.; McCabe, B.J.; Teague, A.M.; Mock, D.M. Determination of the biotin content of select foods using accurate and sensitive HPLC/avidin binding. J. Food Compost. Anal. 2004, 17, 767–776. [Google Scholar] [CrossRef] [PubMed]
- USFDA. Biotin Interference with Troponin Lab Tests—Assays Subject to Biotin Interference. Available online: https://www.fda.gov/medical-devices/in-vitro-diagnostics/biotin-interference-troponin-lab-tests-assays-subject-biotin-interference (accessed on 12 July 2021).
- Holmes, E.W.; Samarasinghe, S.; Emanuele, M.A.; Meah, F. Biotin interference in clinical immunoassays: A cause for concern. Arch. Pathol. Lab. Med. 2017, 141, 1459–1460. [Google Scholar] [CrossRef] [PubMed]
- Colon, P.J.; Greene, D.N. Biotin interference in clinical immunoassays. J. Appl. Lab. Med. 2018, 2, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Luong, J.H.T.; Vashist, S.K. Chemistry of biotin–streptavidin and the growing concern of an emerging biotin interference in clinical immunoassays. ACS Omega 2020, 5, 10–18. [Google Scholar] [CrossRef]
- Gifford, J.L.; de Koning, L.; Sadrzadeh, S.M.H. Strategies for mitigating risk posed by biotin interference on clinical immunoassays. Clin. Biochem. 2019, 65, 61–63. [Google Scholar] [CrossRef] [PubMed]
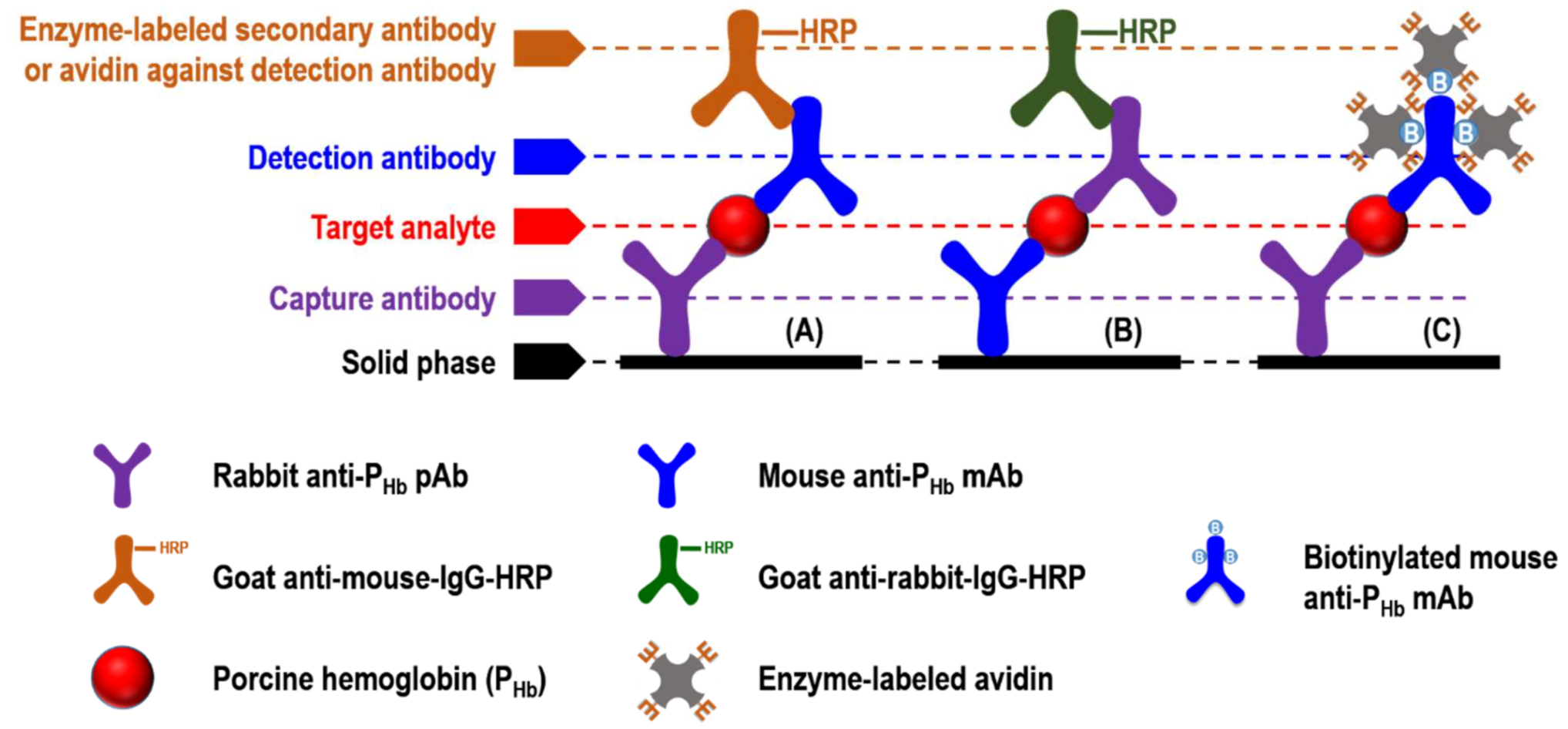


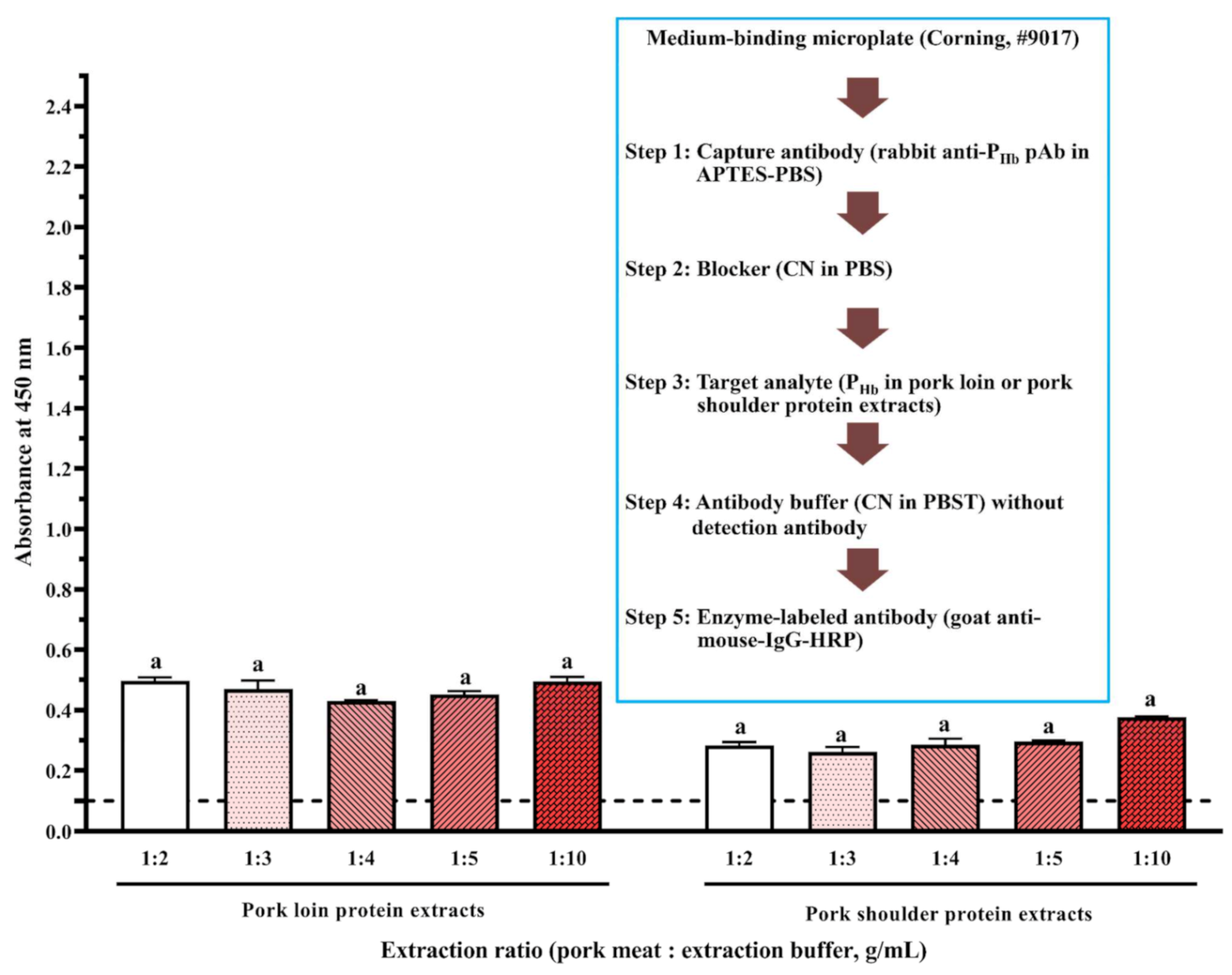

| Sample Preparation Steps | Sample Model 1 | Sample Model 2 | Sample Model 3 |
|---|---|---|---|
| Meat Protein Extracts | Whole Blood Protein Extracts | PHb (H4131, Sigma-Aldrich) | |
| 1. Extraction/dissolving (12.5 mM NaHCO3 and 25 mM NaCl, pH 8.3) | 1:2, 1:3, 1:4, 1:5, 1:10 (g/mL) * | 1:100 (mL/mL) | 3 mg/mL |
| 2. Homogenization (11,000 rpm, 2 min) | Yes | No | No |
| 3. Sonication (130 W, 15 min) | Yes | Yes | No |
| 4. End-over-end rotation (1 h, 4 °C) | Yes | Yes | No |
| 5. Centrifugation (20,000× g, 15 min, 4 °C) | Yes | Yes | No |
| 6. Protein concentration determination (BCA assay) | Yes | Yes | No |
| 7. Relevant figures | 6 and 7 | 7 and 8 | 2, 3, 4 and 5 |
| Blockers | Component I | Concentration (%, g/mL) | Component II | Concentration (%, g/mL) |
|---|---|---|---|---|
| Protein-based | ||||
| BSA | Bovine serum albumin | 5 | ||
| CN | Casein | 1 | ||
| NFDM | Non-fat dry milk | 5 | ||
| FG | Fish gelatin | 1 | ||
| Non-protein-based | ||||
| PEG + CN | Polyethylene glycol | 5 | Casein | 1 |
| PVP + CN | Polyvinylpyrrolidone | 5 | Casein | 1 |
| Experiment No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Schematics | Figure 1B | Figure 1B | Figure 1C | Figure 1A | Figure 1B |
| Objectives | Non-specific binding | Cross-reaction | |||
| Step 1: Capture antibody in PBS | None | Rabbit anti-PHb pAb | Mouse anti-PHb mAb | ||
| Step 2: Blocker | CN in PBS | ||||
| Step 3: Target analyte (PHb) | None | ||||
| Step 4: Detection antibody in antibody buffer (CN in PBST) | None | Mouse anti-PHb mAb | Biotinylated mouse anti-PHb mAb | None | None |
| Step 5: Enzyme-labeled antibody against detection antibody in antibody buffer | Goat anti-mouse-IgG-HRP (RRID: AB_258008) | None | Goat anti-mouse-IgG-HRP (RRID: AB_258008) | Goat anti-rabbit-IgG-HRP (RRID: AB_257896) | |
| Streptavidin-HRP conjugate in PBST | |||||
| A450 (mean ± SEM) | 0.044 ± 0 | 0.050 ± 0.003 | 0.050 ± 0.003 | 0.176 ± 0.007 | 0.644 ± 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Wu, M.; Albo, J.; Rao, Q. Non-Specific Binding and Cross-Reaction of ELISA: A Case Study of Porcine Hemoglobin Detection. Foods 2021, 10, 1708. https://doi.org/10.3390/foods10081708
Jiang X, Wu M, Albo J, Rao Q. Non-Specific Binding and Cross-Reaction of ELISA: A Case Study of Porcine Hemoglobin Detection. Foods. 2021; 10(8):1708. https://doi.org/10.3390/foods10081708
Chicago/Turabian StyleJiang, Xingyi, Meng Wu, Jonathan Albo, and Qinchun Rao. 2021. "Non-Specific Binding and Cross-Reaction of ELISA: A Case Study of Porcine Hemoglobin Detection" Foods 10, no. 8: 1708. https://doi.org/10.3390/foods10081708
APA StyleJiang, X., Wu, M., Albo, J., & Rao, Q. (2021). Non-Specific Binding and Cross-Reaction of ELISA: A Case Study of Porcine Hemoglobin Detection. Foods, 10(8), 1708. https://doi.org/10.3390/foods10081708







