High-CO2 Treatment Prolongs the Postharvest Shelf Life of Strawberry Fruits by Reducing Decay and Cell Wall Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, CO2 Treatment, and Storage
2.2. Analyses of Fruit Quality
2.3. Respiration-Rate Measurement
2.4. Total RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.5. Cell Wall Extraction
2.6. Size-Exclusion Chromatography
2.7. Polyuronide Assay
2.8. Botrytis Cinerea Inoculation
2.9. Statistical Analysis
3. Results
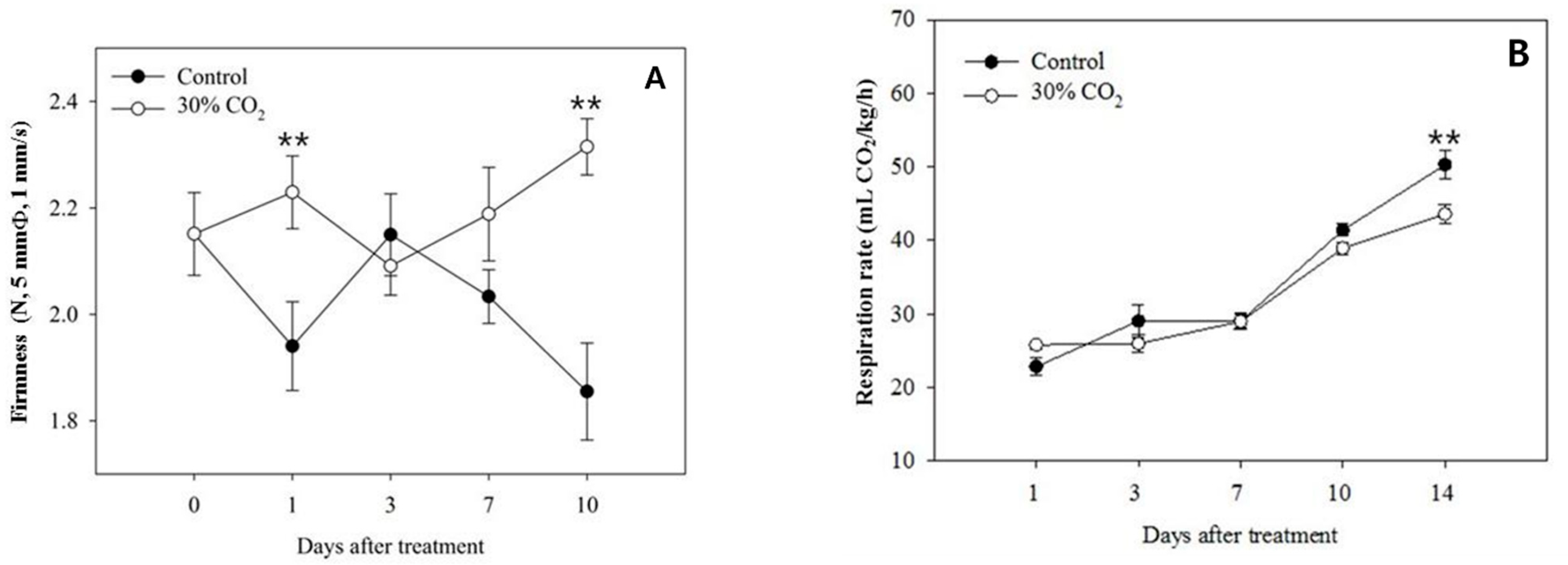
3.1. Fruit Quality and Respiration-Rate Measurements
3.2. Expression Levels of Genes Associated with Cell Wall Degradation
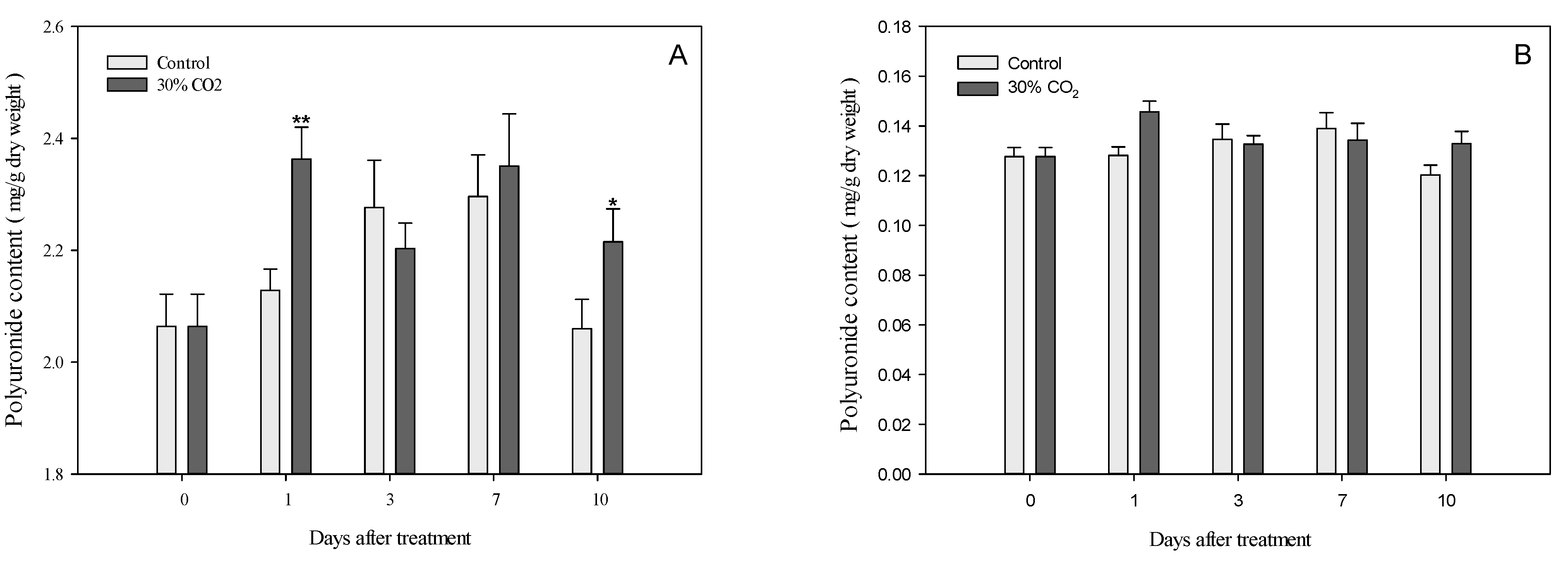
3.3. Polyuronides and Water-Soluble Pectin in Cell Wall Components
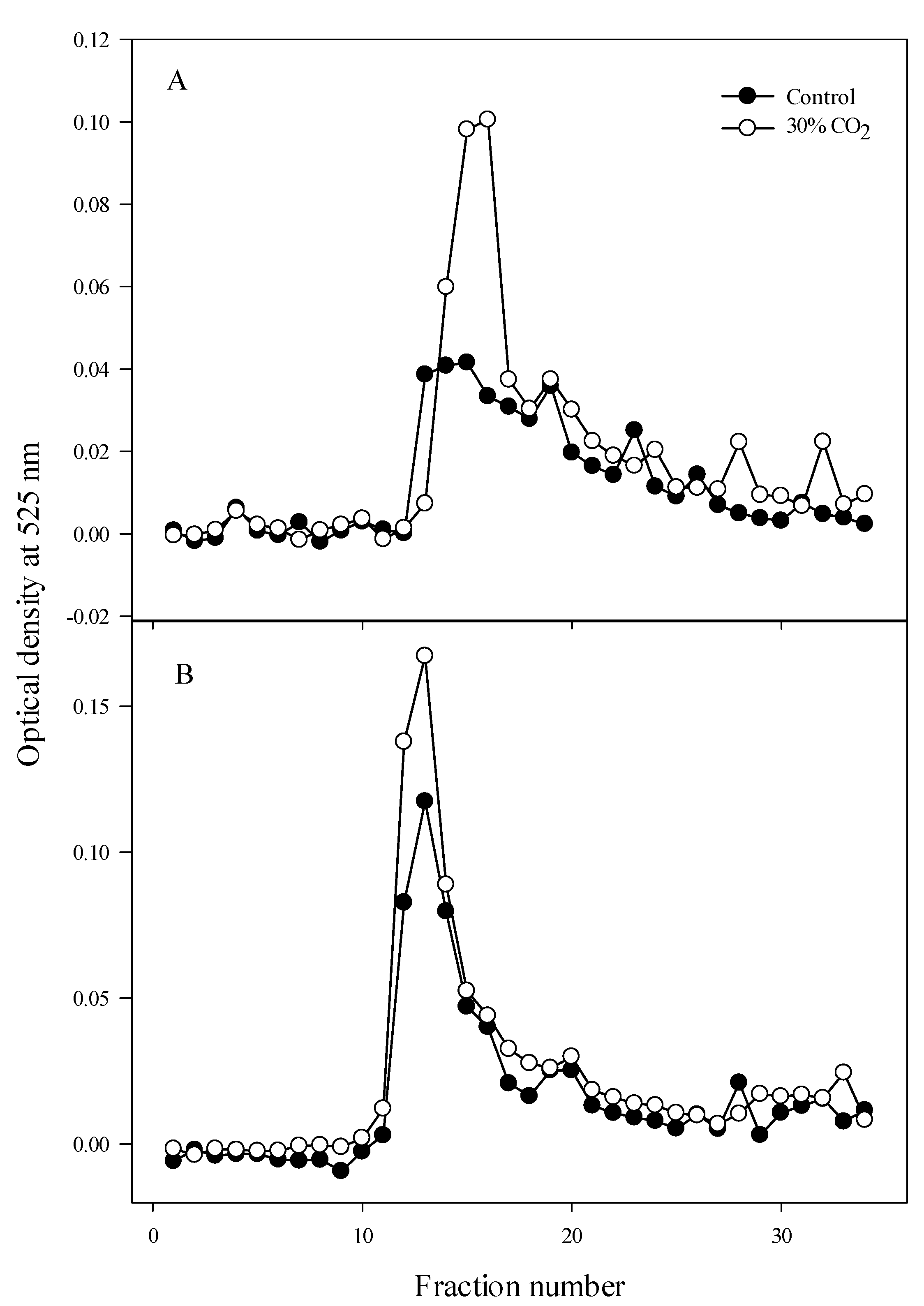
3.4. Size-Exclusion Chromatography
3.5. Botrytis Cinerea Inoculation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, J.-H.; Saftner, R.A.; Watada, A.E.; Lee, Y.S. Modified Atmosphere Maintains Quality of Fresh-Cut Cantaloupe (Cucumis melo L.). J. Food Sci. 2001, 66, 1207–1211. [Google Scholar] [CrossRef]
- Kerbel, E.L.; Kader, A.A.; Romani, R.J. Effects of Elevated CO2 Concentrations on Glycolysis in Intact ‘Bartlett’ Pear Fruit. Plant Physiol. 1988, 86, 1205–1209. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.H.; Kim, J.G.; Ahn, S.E.; Lee, A.Y.; Bae, T.M.; Kim, D.R.; Hwang, Y.S. Potential Role of Pectate Lyase and Ca2+ in the Increase in Strawberry Fruit Firmness Induced by Short-Term Treatment with High-Pressure CO2. J. Food Sci. 2014, 79, S685–S692. [Google Scholar] [CrossRef] [PubMed]
- Harker, F.; Elgar, H.; Watkins, C.; Jackson, P.J.; Hallett, I.C. Physical and mechanical changes in strawberry fruit after high carbon dioxide treatments. Postharvest Biol. Technol. 2000, 19, 139–146. [Google Scholar] [CrossRef]
- Watkins, C.B.; Manzano-Mendez, J.E.; Nock, J.F.; Zhang, J.; Maloney, K.E. Cultivar Variation in Response of Strawberry Fruit to High Carbon Dioxide Treatments. J. Sci. Food Agric. 1999, 79, 886–890. [Google Scholar] [CrossRef]
- Perkins-Veazie, P. Growth and Ripening of Strawberry Fruit. Hortic. Rev. 1995, 17, 267–297. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Cell Walls 2001, 47, 311–340. [Google Scholar] [CrossRef]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef]
- Jarvis, M.C. Plant cell walls: Supramolecular assembly, signalling and stress. Struct. Chem. 2009, 20, 245–253. [Google Scholar] [CrossRef]
- Harris, P.J.; Smith, B.G. Plant cell walls and cell-wall polysaccharides: Structures, properties and uses in food products. Int. J. Food Sci. Technol. 2006, 41, 129–143. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, S.; Wang, Y. Compositional Changes in Cell Wall Polyuronides and Enzyme Activities Associated with Melting/Mealy Textural Property During Ripening Following Long-Term Storage of ‘Comice’ and ‘d’Anjou’ Pears. Postharvest Biol. Technol. 2018, 135, 131–140. [Google Scholar] [CrossRef]
- Goto, T.; Goto, M.; Chachin, K.; Iwata, T. The Mechanism of the Increase of Firmness in Strawberry Fruit Treated with 100% CO2. Nippon. Shokuhin Kagaku Kogaku Kaishi 1996, 43, 1158–1162. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Min, J.H.; Kim, D.Y.; Kim, J.G.; Huber, D.J. Potential Mechanisms Associated with Strawberry Fruit Firm-ness Increases Mediated by Elevated pCO2. Hortic. Environ. Biotechnol. 2012, 53, 41–48. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hwang, Y.S.; Lee, C.H.; Huber, D.J. Changes in firmness and pectic polysaccharide solubility in three cultivars of strawberry fruit following short-term exposure to high PCO2. J. Food Qual. 2010, 33, 312–328. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Gambino, G.; Perrone, I.; Gribaudo, I. A Rapid and Effective Method for RNA Extraction from Different Tissues of Grape-vine and Other Woody Plants. Phytochem. Anal. 2008, 19, 520–525. [Google Scholar] [CrossRef]
- Chatkaew, A.; Kim, J. Correlations Among Disease Severity, Firmness, Minerals, and Cell Wall Composition in Radish (Raphanus sativus L.) and Baemoochae (×Brassicoraphanus) Roots in Relation to Tissue Maceration by Pectobacterium carotovorum. Hortic. Environ. Biotechnol. 2013, 54, 346–356. [Google Scholar] [CrossRef]
- An, H.J.; Lurie, S.; Greve, L.C.; Rosenquist, D.; Kirmiz, C.; Labavitch, J.M.; Lebrilla, C.B. Determination of Patho-gen-Related Enzyme Action by Mass Spectrometry Analysis of Pectin Breakdown Products of Plant Cell Walls. Anal. Biochem. 2005, 338, 71–82. [Google Scholar] [CrossRef]
- Mengiste, T.; Chen, X.; Salmeron, J.; Dietrich, R. The BOTRYTIS SUSCEPTIBLE1 Gene Encodes an R2R3MYB Transcription Factor Protein That Is Required for Biotic and Abiotic Stress Responses in Arabidopsis. Plant Cell 2003, 15, 2551–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benítez-Burraco, A.; Blanco-Portales, R.; Redondo-Nevado, J.; Bellido, M.L.; Moyano, E.; Caballero, J.L.; Muñoz-Blanco, J. Cloning and Characterization of Two Ripening-Related Strawberry (Fragaria × Ananassa Cv. Chandler) Pectate Lyase Genes. J. Exp. Bot. 2003, 54, 633–645. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, C.R.; Pimentel, P.; Gaete-Eastman, C.; Moya, M.; Herrera, R.; Caligari, P.D.S.; Moya-León, M.A. Softening Rate of the Chilean Strawberry (Fragaria chiloensis) Fruit Reflects the Expression of Polygalacturonase and Pectate Lyase Genes. Postharvest Biol. Technol. 2008, 49, 210–220. [Google Scholar] [CrossRef]
- Knox, J.; Knox, P. Revealing the structural and functional diversity of plant cell walls. Curr. Opin. Plant Biol. 2008, 11, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Englyst, K.N.; Liu, S. Nutritional characterization and measurement of dietary carbohydrates. Eur. J. Clin. Nutr. 2007, 61 (Suppl. 1), S19–S39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daas, P.J.H.; Meyer-Hansen, K.; Schols, H.A.; De Ruiter, G.A.; Voragen, A.G.J. Investigation of the Non-Esterified Galac-turonic Acid Distribution in Pectin with Endopolygalacturonase. Carbohydr. Res. 1999, 318, 135–145. [Google Scholar] [CrossRef]
- Cui, S.W.; Wang, Q. Cell wall polysaccharides in cereals: Chemical structures and functional properties. Struct. Chem. 2009, 20, 291–297. [Google Scholar] [CrossRef]
- Carpita, N.C.; Gibeaut, D.M. Structural Models of Primary Cell Walls in Flowering Plants: Consistency of Molecular Structure with the Physical Properties of the Walls During Growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.H.; Melton, L.D.; Newman, R.H. Solid-state 13C NMR characterization of cell walls of ripening strawberries. Can. J. Bot. 1997, 75, 1957–1964. [Google Scholar] [CrossRef]
- Rosli, H.; Civello, P.; Martínez, G. Changes in cell wall composition of three Fragaria x ananassa cultivars with different softening rate during ripening. Plant Physiol. Biochem. 2004, 42, 823–831. [Google Scholar] [CrossRef]
- Tavares, E.Q.P.; De Souza, A.; Buckeridge, M.S. How endogenous plant cell-wall degradation mechanisms can help achieve higher efficiency in saccharification of biomass. J. Exp. Bot. 2015, 66, 4133–4143. [Google Scholar] [CrossRef]
- Rajhi, I.; Yamauchi, T.; Takahashi, H.; Nishiuchi, S.; Shiono, K.; Watanabe, R.; Mliki, A.; Nagamura, Y.; Tsutsumi, N.; Nishizawa, N.K.; et al. Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol. 2010, 190, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Lim, S.; Yi, G.; Lee, J.G.; Lee, E.J. Integrated transcriptomic-metabolomic analysis reveals cellular responses of harvested strawberry fruit subjected to short-term exposure to high levels of carbon dioxide. Postharvest Biol. Technol. 2019, 148, 120–131. [Google Scholar] [CrossRef]
- Aspinall, G.O.; Craig, J.W.T.; Whyte, J.L. Lemon-Peel Pectin. Part I. Carbohydr. Res. 1968, 7, 442–452. [Google Scholar] [CrossRef]
- John, M.; Röhrig, H.; Schmidt, J.; Walden, R.; Schell, J. Cell signalling by oligosaccharides. Trends Plant Sci. 1997, 2, 111–115. [Google Scholar] [CrossRef]
- Nothnagel, E.A.; McNeil, M.; Albersheim, P.; Dell, A. Hosts Host-Pathogen Interactions: XXII. A Galacturonic Acid Oligo-saccharide from Plant Cell Walls Elicits Phytoalexins. Plant Physiol. 1983, 71, 916–926. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.R.; Darvill, A.G.; Albersheim, P. Several Biotic and Abiotic Elicitors Act Synergistically in the Induction of Phyto-alexin Accumulation in Soybean. Plant Mol. Biol. 1986, 6, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Galletti, R.; Denoux, C.; Gambetta, S.; Dewdney, J.; Ausubel, F.M.; De Lorenzo, G.; Ferrari, S. The AtrbohD-Mediated Oxida-tive Burst Elicited by Oligogalacturonides in Arabidopsis Is Dispensable for the Activation of Defense Responses Effective Against Botrytis cinerea. Plant Physiol. 2008, 148, 1695–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasul, S.; Dubreuil-Maurizi, C.; LaMotte, O.; Koen, E.; Poinssot, B.; Alcaraz, G.; Wendehenne, D.; Jeandroz, S. Nitric oxide production mediates oligogalacturonide-triggered immunity and resistance to Botrytis cinerea in Arabidopsis thaliana. Plant Cell Environ. 2012, 35, 1483–1499. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Storage Day | CIE L* | CIE a* | CIE b* | Chroma | Hue Angle |
|---|---|---|---|---|---|---|
| Control | 0 | 47.2 | 24.3 | 20.7 | 32.0 | 40.5 |
| 1 | 47.5 | 24.2 | 20.8 | 31.9 | 40.6 | |
| 3 | 42.0 | 30.6 | 23.7 | 38.7 | 37.8 | |
| 7 | 40.6 | 30.8 | 26.3 | 40.5 | 40.6 | |
| 10 | 40.2 | 29.1 | 21.2 | 36.0 | 36.1 | |
| 14 | 40.5 | 26.9 | 20.7 | 33.9 | 37.6 | |
| 30% CO2 | 0 | 47.2 | 24.3 | 20.7 | 32.0 | 40.5 |
| 1 | 53.2 | 19.5 | 17.6 | 26.3 | 42.1 | |
| 3 | 40.0 | 29.6 | 22.5 | 37.2 | 37.2 | |
| 7 | 40.2 | 29.3 | 22.3 | 36.8 | 37.2 | |
| 10 | 41.2 | 28.6 | 21.5 | 35.8 | 36.9 | |
| 14 | 39.7 | 26.8 | 19.8 | 33.3 | 36.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eum, H.-L.; Han, S.-H.; Lee, E.-J. High-CO2 Treatment Prolongs the Postharvest Shelf Life of Strawberry Fruits by Reducing Decay and Cell Wall Degradation. Foods 2021, 10, 1649. https://doi.org/10.3390/foods10071649
Eum H-L, Han S-H, Lee E-J. High-CO2 Treatment Prolongs the Postharvest Shelf Life of Strawberry Fruits by Reducing Decay and Cell Wall Degradation. Foods. 2021; 10(7):1649. https://doi.org/10.3390/foods10071649
Chicago/Turabian StyleEum, Hyang-Lan, Seung-Hyun Han, and Eun-Jin Lee. 2021. "High-CO2 Treatment Prolongs the Postharvest Shelf Life of Strawberry Fruits by Reducing Decay and Cell Wall Degradation" Foods 10, no. 7: 1649. https://doi.org/10.3390/foods10071649
APA StyleEum, H.-L., Han, S.-H., & Lee, E.-J. (2021). High-CO2 Treatment Prolongs the Postharvest Shelf Life of Strawberry Fruits by Reducing Decay and Cell Wall Degradation. Foods, 10(7), 1649. https://doi.org/10.3390/foods10071649