Bioaccessibility and Bioactivity of Cereal Polyphenols: A Review
Abstract
:1. Introduction
2. Cereal Polyphenols
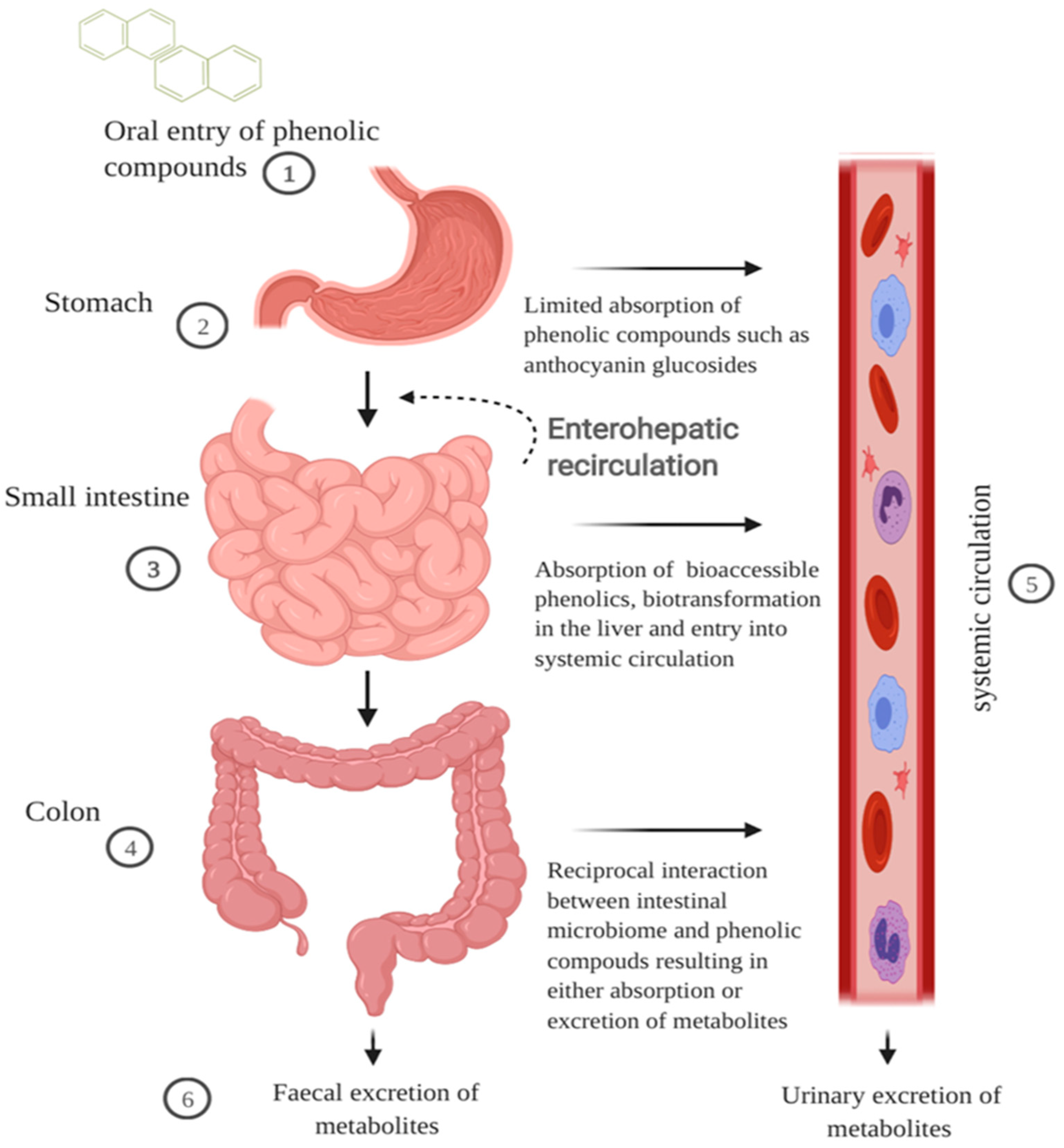
2.1. GI Digestion of Cereal Polyphenols
2.1.1. Oral and Gastric Digestion
2.1.2. Small Intestinal Metabolism and Absorption
2.1.3. Colonic Metabolism
2.1.4. Extended Metabolism and Elimination
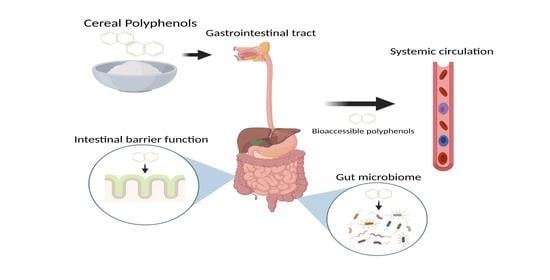
3. Polyphenol Bioaccessibility in the GI Tract
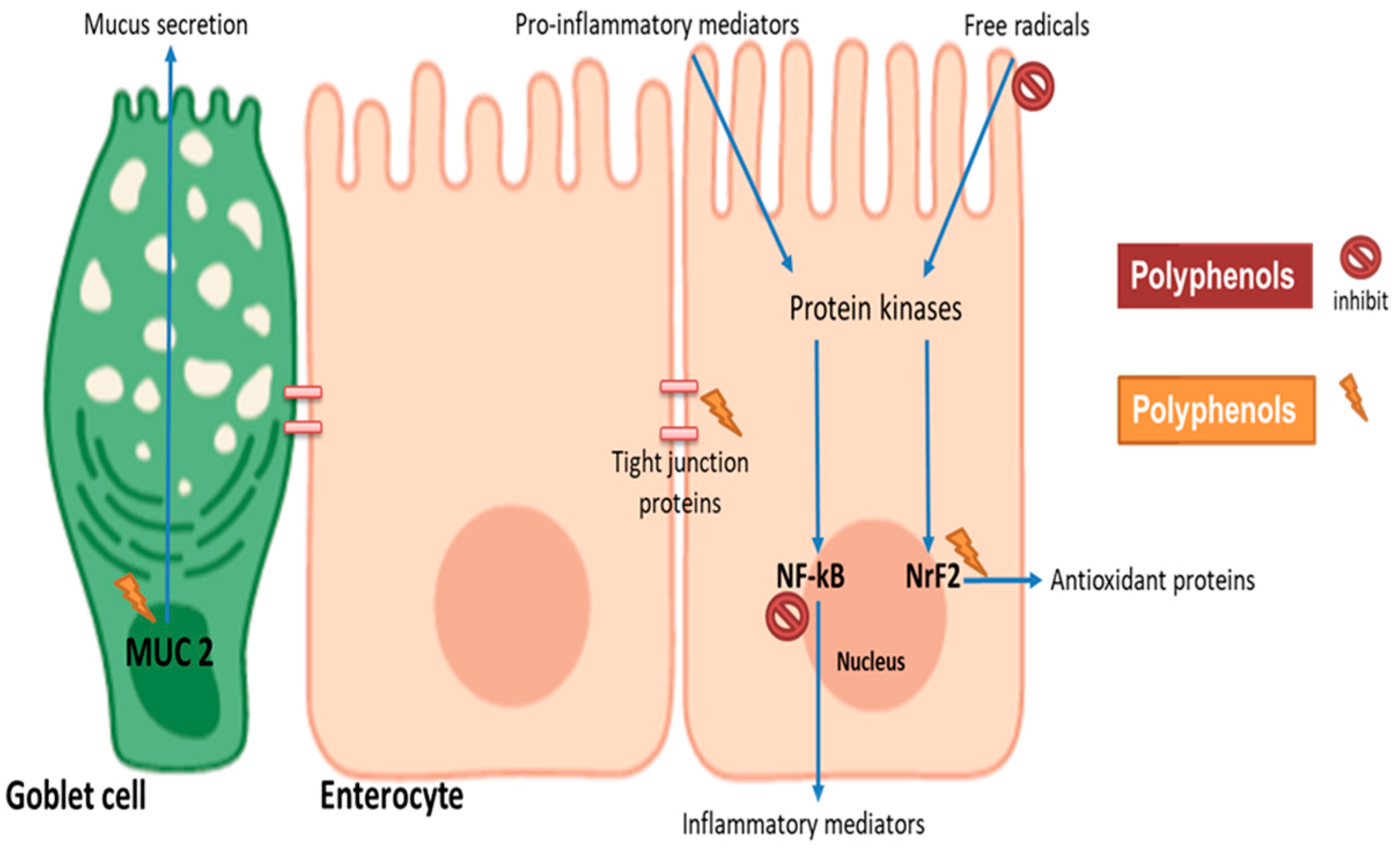
4. Impact of Polyphenols on Intestinal Barrier Function
5. Cereal Polyphenol Impact on the Gut Microbiome and Plasma Anti-Inflammatory Status
6. Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McKevith, B. Nutritional aspects of cereals. Nutr. Bull. 2004, 29, 111–142. [Google Scholar] [CrossRef]
- Kushi, L.H.; Meyer, K.A.; Jacobs, D.R., Jr. Cereals, legumes, and chronic disease risk reduction: Evidence from epidemiologic studies. Am. J. Clin. Nutr. 1999, 70, 451s–458s. [Google Scholar] [CrossRef] [Green Version]
- Stainer, A.R.; Sasikumar, P.; Bye, A.P.; Unsworth, A.J.; Holbrook, L.M.; Tindall, M.; Lovegrove, J.A.; Gibbins, J.M. The Metabolites of the Dietary Flavonoid Quercetin Possess Potent Antithrombotic Activity, and Interact with Aspirin to Enhance Antiplatelet Effects. TH Open 2019, 3, e244–e258. [Google Scholar] [CrossRef]
- Francis, N.; Rao, S.; Blanchard, C.; Santhakumar, A. Black Sorghum Phenolic Extract Regulates Expression of Genes Associated with Oxidative Stress and Inflammation in Human Endothelial Cells. Molecules 2019, 24, 3321. [Google Scholar] [CrossRef] [Green Version]
- Santhakumar, A.B.; Bulmer, A.C.; Singh, I. A review of the mechanisms and effectiveness of dietary polyphenols in reducing oxidative stress and thrombotic risk. J. Hum. Nutr. Diet. 2014, 27, 1–21. [Google Scholar] [CrossRef]
- Thompson, K.; Hosking, H.; Pederick, W.; Singh, I.; Santhakumar, A. The effect of anthocyanin supplementation in modulating platelet function in sedentary population: A randomised, double-blind, placebo-controlled, cross-over trial. Br. J. Nutr. 2017, 118, 368–374. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 113, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Z.; Zhu, C.-C.; Hu, M.; Wu, A.-Z.; Bairu, Z.-D.; Kangsa, S.-Q. Structure-activity relationships of antioxidant activity in vitro about flavonoids isolated from Pyrethrum tatsienense. J. Intercult. Ethnopharmacol. 2014, 3, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Wu, G.; Johnson, S.K.; Blanchard, C.L. Characterization of phenolic compounds and antioxidant activity in sorghum grains. J. Cereal Sci. 2018, 84, 103–111. [Google Scholar] [CrossRef]
- Rao, S.; Schwarz, L.J.; Santhakumar, A.B.; Chinkwo, K.A.; Blanchard, C.L. Cereal phenolic contents as affected by variety and environment. Cereal Chem. 2018, 95, 589–602. [Google Scholar] [CrossRef]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Fang, Z. Changes in whole grain polyphenols and antioxidant activity of six sorghum genotypes under different irrigation treatments. Food Chem. 2017, 214, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callcott, E.T.; Blanchard, C.L.; Snell, P.; Santhakumar, A.B. The anti-inflammatory and antioxidant effects of pigmented rice consumption in an obese cohort. Food Funct. 2019, 10, 8016–8025. [Google Scholar] [CrossRef] [PubMed]
- Callcott, E.T.; Santhakumar, A.B.; Strappe, P.; Luo, J.; Blanchard, C.L. Polyphenols from Australian-grown pigmented red and purple rice inhibit adipocyte differentiation. J. Cereal Sci. 2018, 81, 140–146. [Google Scholar] [CrossRef]
- Fernandes, I.; de Freitas, V.; Mateus, N. Anthocyanins and human health: How gastric absorption may influence acute human physiology. Nutr. Aging 2014, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.-L.; Rémésy, C. Anthocyanins Are Efficiently Absorbed from the Stomach in Anesthetized Rats. J. Nutr. 2003, 133, 4178–4182. [Google Scholar] [CrossRef]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.L.; Rémésy, C. Anthocyanins are efficiently absorbed from the small intestine in rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Bianchi, M.G.; Chiu, M.; Taurino, G.; Brighenti, F.; Del Rio, D.; Mena, P.; Bussolati, O. Catechin and Procyanidin B(2) Modulate the Expression of Tight Junction Proteins but Do Not Protect from Inflammation-Induced Changes in Permeability in Human Intestinal Cell Monolayers. Nutrients 2019, 11, 2271. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Jin, X.; Chen, Y.; Song, Z.; Jiang, X.; Hu, F.; Conlon, M.A.; Topping, D.L. Polyphenol-Rich Propolis Extracts Strengthen Intestinal Barrier Function by Activating AMPK and ERK Signaling. Nutrients 2016, 8, 272. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Li, S.; Chang, H.; Anderson, R.; Chopra, S.; Reddivari, L. Maize Flavan-4-ols and Anthocyanins Alleviated Dextran Sulfate Sodium-Induced Colitis in Mice via Intestinal Barrier Function Restoration. Curr. Dev. Nutr. 2020, 4, 488. [Google Scholar] [CrossRef]
- Biasi, F.; Guina, T.; Maina, M.; Cabboi, B.; Deiana, M.; Tuberoso, C.I.; Calfapietra, S.; Chiarpotto, E.; Sottero, B.; Gamba, P.; et al. Phenolic compounds present in Sardinian wine extracts protect against the production of inflammatory cytokines induced by oxysterols in CaCo-2 human enterocyte-like cells. Biochem. Pharmacol. 2013, 86, 138–145. [Google Scholar] [CrossRef]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Blanchard, C.L. Characterization of phenolic compound antioxidant activity in oat varieties using UHPLC–online ABTS and LC Q-TOF. Cereal Chem. 2019, 96, 958–966. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C. Benzoic and Cinnamic Acid Derivatives as Antioxidants: Structure−Activity Relation. J. Agric. Food Chem. 1999, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Simić, A.; Manojlović, D.; Segan, D.; Todorović, M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woong, K.; Beomgi, L.; Jaeyoung, P.; Ho-Joong, K.; Hyeonsook, C. Comparative Antioxidant Activity and Structural Feature of Protocatechuic Acid and Phenolic Acid Derivatives by DPPH and Intracellular ROS. Lett. Drug Des. Discov. 2018, 15, 612–620. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awika, J.M.; Rose, D.J.; Simsek, S. Complementary effects of cereal and pulse polyphenols and dietary fiber on chronic inflammation and gut health. Food Funct. 2018, 9, 1389–1409. [Google Scholar] [CrossRef]
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Bratt, K.; Sunnerheim, K.; Bryngelsson, S.; Fagerlund, A.; Engman, L.; Andersson, R.E.; Dimberg, L.H. Avenanthramides in oats (Avena sativa L.) and structure-antioxidant activity relationships. J. Agric. Food Chem. 2003, 51, 594–600. [Google Scholar] [CrossRef]
- Chen, C.Y.; Milbury, P.E.; Collins, F.W.; Blumberg, J.B. Avenanthramides are bioavailable and have antioxidant activity in humans after acute consumption of an enriched mixture from oats. J. Nutr. 2007, 137, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- Peterson, D.M.; Hahn, M.J.; Emmons, C.L. Oat avenanthramides exhibit antioxidant activities in vitro. Food Chem. 2002, 79, 473–478. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Zhu, Y.; McBride, J.; Fu, J.; Sang, S. Oat Avenanthramide-C (2c) Is Biotransformed by Mice and the Human Microbiota into Bioactive Metabolites. J. Nutr. 2014, 145, 239–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alminger, M.; Aura, A.-M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In Vitro Models for Studying Secondary Plant Metabolite Digestion and Bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joye, I. Protein Digestibility of Cereal Products. Foods 2019, 8, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols-A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Spencer, J.P.; Chaudry, F.; Pannala, A.S.; Srai, S.K.; Debnam, E.; Rice-Evans, C. Decomposition of cocoa procyanidins in the gastric milieu. Biochem. Biophys. Res. Commun. 2000, 272, 236–241. [Google Scholar] [CrossRef]
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The interaction of anthocyanins with bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636. [Google Scholar] [CrossRef]
- Ashley, D.; Marasini, D.; Brownmiller, C.; Lee, J.A.; Carbonero, F.; Lee, S.-O. Impact of Grain Sorghum Polyphenols on Microbiota of Normal Weight and Overweight/Obese Subjects during In Vitro Fecal Fermentation. Nutrients 2019, 11, 217. [Google Scholar] [CrossRef] [Green Version]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing Potential Bioavailability of Raspberry Anthocyanins Using an in Vitro Digestion System. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vicente, A.; Gil-Izquierdo, A.; García-Viguera, C. In Vitro Gastrointestinal Digestion Study of Pomegranate Juice Phenolic Compounds, Anthocyanins, and Vitamin C. J. Agric. Food Chem. 2002, 50, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Thomasset, S.C.; Berry, D.P.; Garcea, G.; Brown, K.; Steward, W.P.; Gescher, A.J. Determination of anthocyanins in the urine of patients with colorectal liver metastases after administration of bilberry extract. Biomed. Chromatogr. 2011, 25, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Gil-Izquierdo, A.; Lamaison, J.L.; Rémésy, C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J. Agric. Food Chem. 2005, 53, 3902–3908. [Google Scholar] [CrossRef]
- Ader, P.; Grenacher, B.; Langguth, P.; Scharrer, E.; Wolffram, S. Cinnamate uptake by rat small intestine: Transport kinetics and transepithelial transfer. Exp. Physiol. 1996, 81, 943–955. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolffram, S.; Blöck, M.; Ader, P. Quercetin-3-Glucoside Is Transported by the Glucose Carrier SGLT1 across the Brush Border Membrane of Rat Small Intestine. J. Nutr. 2002, 132, 630–635. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liu, J.; Li, Z.; Wang, L.; Gao, W.; Zhang, Z.; Guo, C. Sodium-dependent glucose transporter 1 and glucose transporter 2 mediate intestinal transport of quercetrin in Caco-2 cells. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]
- Day, A.J.; Cañada, F.J.; Díaz, J.C.; Kroon, P.A.; Mclauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.A.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Walton, M.C.; McGhie, T.K.; Reynolds, G.W.; Hendriks, W.H. The flavonol quercetin-3-glucoside inhibits cyanidin-3-glucoside absorption in vitro. J. Agric. Food Chem. 2006, 54, 4913–4920. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Tomas, M.; Ozdal, T.; Capanoglu, E. Effect of food matrix on the content and bioavailability of flavonoids. Trends Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Rémésy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [CrossRef] [Green Version]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo Anson, N.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioavailability of ferulic acid is determined by its bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Boersma, M.G.; van der Woude, H.; Bogaards, J.; Boeren, S.; Vervoort, J.; Cnubben, N.H.; van Iersel, M.L.; van Bladeren, P.J.; Rietjens, I.M. Regioselectivity of phase II metabolism of luteolin and quercetin by UDP-glucuronosyl transferases. Chem. Res. Toxicol. 2002, 15, 662–670. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazzon, A.; Vrhovsek, U.; Masuero, D.; Mattivi, F.; Mandoj, F.; Nardini, M. Antioxidant Activity of Phenolic Acids and Their Metabolites: Synthesis and Antioxidant Properties of the Sulfate Derivatives of Ferulic and Caffeic Acids and of the Acyl Glucuronide of Ferulic Acid. J. Agric. Food Chem. 2012, 60, 12312–12323. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Ulrih, N.P.; Sengupta, P.K.; Xiao, J. Plasma protein binding of dietary polyphenols to human serum albumin: A high performance affinity chromatography approach. Food Chem. 2019, 270, 257–263. [Google Scholar] [CrossRef]
- Price, R.K.; Welch, R.W.; Lee-Manion, A.M.; Bradbury, I.; Strain, J.J. Total Phenolics and Antioxidant Potential in Plasma and Urine of Humans after Consumption of Wheat Bran. Cereal Chem. 2008, 85, 152–157. [Google Scholar] [CrossRef]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A.A.; Giacco, R.; Ercolini, D.; Gibbons, S.M.; La Storia, A.; Gilbert, J.A.; Jonnalagadda, S.; et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: Role of polyphenols bound to cereal dietary fiber. Am. J. Clin. Nutr. 2015, 101, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Anhê, F.F.; Varin, T.V.; Le Barz, M.; Pilon, G.; Dudonné, S.; Trottier, J.; St-Pierre, P.; Harris, C.S.; Lucas, M.; Lemire, M.; et al. Arctic berry extracts target the gut-liver axis to alleviate metabolic endotoxaemia, insulin resistance and hepatic steatosis in diet-induced obese mice. Diabetologia 2018, 61, 919–931. [Google Scholar] [CrossRef] [Green Version]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Hilary, S.; Tomás-Barberán, F.A.; Martinez-Blazquez, J.A.; Kizhakkayil, J.; Souka, U.; Al-Hammadi, S.; Habib, H.; Ibrahim, W.; Platat, C. Polyphenol characterisation of Phoenix dactylifera L. (date) seeds using HPLC-mass spectrometry and its bioaccessibility using simulated in-vitro digestion/Caco-2 culture model. Food Chem. 2020, 311, 125969. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Shahidi, F. Bioaccessibility and antioxidant potential of millet grain phenolics as affected by simulated in vitro digestion and microbial fermentation. J. Funct. Foods 2012, 4, 226–237. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Platat, C.; Hillary, S.; Tomas-Barberan, F.A.; Martinez-Blazquez, J.A.; Al-Meqbali, F.; Souka, U.; Al-Hammadi, S.; Ibrahim, W. Urine Metabolites and Antioxidant Effect after Oral Intake of Date (Phoenix dactylifera L.) Seeds-Based Products (Powder, Bread and Extract) by Human. Nutrients 2019, 11, 2489. [Google Scholar] [CrossRef] [Green Version]
- Lila, M.A.; Ribnicky, D.M.; Rojo, L.E.; Rojas-Silva, P.; Oren, A.; Havenaar, R.; Janle, E.M.; Raskin, I.; Yousef, G.G.; Grace, M.H. Complementary Approaches To Gauge the Bioavailability and Distribution of Ingested Berry Polyphenolics. J. Agric. Food Chem. 2012, 60, 5763–5771. [Google Scholar] [CrossRef] [Green Version]
- Chitindingu, K.; Benhura, M.A.N.; Muchuweti, M. In vitro bioaccessibility assessment of phenolic compounds from selected cereal grains: A prediction tool of nutritional efficiency. LWT Food Sci. Technol. 2015, 63, 575–581. [Google Scholar] [CrossRef]
- Hole, A.S.; Kjos, N.P.; Grimmer, S.; Kohler, A.; Lea, P.; Rasmussen, B.; Lima, L.R.; Narvhus, J.; Sahlstrøm, S. Extrusion of Barley and Oat Improves the Bioaccessibility of Dietary Phenolic Acids in Growing Pigs. J. Agric. Food Chem. 2013, 61, 2739–2747. [Google Scholar] [CrossRef]
- Ninfali, P.; Mari, M.; Meli, M.A.; Roselli, C.; Antonini, E. In vitro bioaccessibility of avenanthramides in cookies made with malted oat flours. Int. J. Food Sci. Technol. 2019, 54, 1558–1565. [Google Scholar] [CrossRef]
- Chen, W.; Su, H.; Xu, Y.; Jin, C. In vitro gastrointestinal digestion promotes the protective effect of blackberry extract against acrylamide-induced oxidative stress. Sci. Rep. 2017, 7, 40514. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Williams, B.A.; Ferruzzi, M.G.; D’Arcy, B.R. Microbial metabolites, but not other phenolics derived from grape seed phenolic extract, are transported through differentiated Caco-2 cell monolayers. Food Chem. 2013, 138, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liu, C.; Luo, S.; Chen, J.; Gong, E. The Profile and Bioaccessibility of Phenolic Compounds in Cereals Influenced by Improved Extrusion Cooking Treatment. PLoS ONE 2016, 11, e0161086. [Google Scholar] [CrossRef] [PubMed]
- Anson, N.M.; Selinheimo, E.; Havenaar, R.; Aura, A.M.; Mattila, I.; Lehtinen, P.; Bast, A.; Poutanen, K.; Haenen, G.R. Bioprocessing of wheat bran improves in vitro bioaccessibility and colonic metabolism of phenolic compounds. J. Agric. Food Chem. 2009, 57, 6148–6155. [Google Scholar] [CrossRef]
- Li, M.; Koecher, K.; Hansen, L.; Ferruzzi, M.G. Phenolic recovery and bioaccessibility from milled and finished whole grain oat products. Food Funct. 2016, 7, 3370–3381. [Google Scholar] [CrossRef]
- Tran, C.D.; Grice, D.M.; Wade, B.; Kerr, C.A.; Bauer, D.C.; Li, D.; Hannan, G.N. Gut permeability, its interaction with gut microflora and effects on metabolic health are mediated by the lymphatics system, liver and bile acid. Future Microbiol. 2015, 10, 1339–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.L.; Gao, Y. Effects of methanolic extract form Fuzhuan brick-tea on hydrogen peroxide-induced oxidative stress in human intestinal epithelial adenocarcinoma Caco-2 cells. Mol. Med. Rep. 2014, 9, 1061–1067. [Google Scholar] [CrossRef]
- Van Buiten, C.B.; Lambert, J.D.; Elias, R.J. Green Tea Polyphenols Mitigate Gliadin-Mediated Inflammation and Permeability in Vitro. Mol. Nutr. Food Res. 2018, 62, e1700879. [Google Scholar] [CrossRef]
- Volstatova, T.; Marchica, A.; Hroncova, Z.; Bernardi, R.; Doskocil, I.; Havlik, J. Effects of chlorogenic acid, epicatechin gallate, and quercetin on mucin expression and secretion in the Caco-2/HT29-MTX cell model. Food Sci. Nutr. 2019, 7, 492–498. [Google Scholar] [CrossRef]
- Valdez, J.C.; Cho, J.; Bolling, B.W. Aronia berry inhibits disruption of Caco-2 intestinal barrier function. Arch. Biochem. Biophys. 2020, 688, 108409. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases-Promises, Perspectives, and Pitfalls. Oxidative Med. Cell. Longev. 2016, 2016, 9346470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, F.-Y.; Sang, L.-X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [Green Version]
- Romier, B.; Van De Walle, J.; During, A.; Larondelle, Y.; Schneider, Y.J. Modulation of signalling nuclear factor-kappaB activation pathway by polyphenols in human intestinal Caco-2 cells. Br. J. Nutr. 2008, 100, 542–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiano, S.; Sasso, A.; De Felice, B.; Di Gregorio, I.; La Rosa, G.; Lupoli, G.A.; Belfiore, A.; Mondola, P.; Santillo, M. Quercetin Increases MUC2 and MUC5AC Gene Expression and Secretion in Intestinal Goblet Cell-Like LS174T via PLC/PKCα/ERK1-2 Pathway. Front. Physiol. 2018, 9, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Zhang, R.; Wu, Y.; Wu, C.; Jia, X.; Dong, L.; Liu, L.; Chen, Y.; Bai, Y.; Zhang, M. Rice Bran Phenolic Extract Protects against Alcoholic Liver Injury in Mice by Alleviating Intestinal Microbiota Dysbiosis, Barrier Dysfunction, and Liver Inflammation Mediated by the Endotoxin-TLR4-NF-κB Pathway. J. Agric. Food Chem. 2020, 68, 1237–1247. [Google Scholar] [CrossRef]
- Wu, B.; Bhatnagar, R.; Indukuri, V.V.; Chopra, S.; March, K.; Cordero, N.; Chopra, S.; Reddivari, L. Intestinal Mucosal Barrier Function Restoration in Mice by Maize Diet Containing Enriched Flavan-4-Ols. Nutrients 2020, 12, 896. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, D.; Cimino, F.; Fratantonio, D.; Molonia, M.S.; Bashllari, R.; Busa, R.; Saija, A.; Speciale, A. Cyanidin-3-O-Glucoside Modulates the In Vitro Inflammatory Crosstalk between Intestinal Epithelial and Endothelial Cells. Mediat. Inflamm. 2017, 2017, 3454023. [Google Scholar] [CrossRef]
- He, S.; Guo, Y.; Zhao, J.; Xu, X.; Wang, N.; Liu, Q. Ferulic Acid Ameliorates Lipopolysaccharide-Induced Barrier Dysfunction via MicroRNA-200c-3p-Mediated Activation of PI3K/AKT Pathway in Caco-2 Cells. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, H.; Chen, P.; Xie, H.; Tao, Y. Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct. Target. Ther. 2019, 4, 41. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [Green Version]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, K.; Mahbub, R.; Fox, J.G. Xenobiotics: Interaction with the Intestinal Microflora. ILAR J. 2015, 56, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef] [Green Version]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Chen, J.; Meng, Y.; Yang, J.; Cui, Q.; Zhou, Y. Metformin Alters Gut Microbiota of Healthy Mice: Implication for Its Potential Role in Gut Microbiota Homeostasis. Front. Microbiol. 2018, 9, 1336. [Google Scholar] [CrossRef] [Green Version]
- Kristek, A.; Wiese, M.; Heuer, P.; Kosik, O.; Schär, M.Y.; Soycan, G.; Alsharif, S.; Kuhnle, G.G.C.; Walton, G.; Spencer, J.P.E. Oat bran, but not its isolated bioactive β-glucans or polyphenols, have a bifidogenic effect in an in vitro fermentation model of the gut microbiota. Br. J. Nutr. 2019, 121, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Teoh, K.T.; Savary, B.J.; Chen, M.-H.; McClung, A.; Lee, S.-O. In Vitro Fermentation Patterns of Rice Bran Components by Human Gut Microbiota. Nutrients 2017, 9, 1237. [Google Scholar] [CrossRef] [Green Version]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the Mechanism of the Health Benefits of Proanthocyanidins: Absorption, Metabolism, and Interaction with Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 971–985. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Romero, S.; Hereu, M.; Molinar-Toribio, E.; Almajano, M.P.; Méndez, L.; Medina, I.; Taltavull, N.; Romeu, M.; Nogués, M.R.; Torres, J.L. Effects of the combination of ω-3 PUFAs and proanthocyanidins on the gut microbiota of healthy rats. Food Res. Int. 2017, 97, 364–371. [Google Scholar] [CrossRef]
- Déprez, S.p.; Brezillon, C.; Rabot, S.; Philippe, C.; Mila, I.; Lapierre, C.; Scalbert, A. Polymeric Proanthocyanidins Are Catabolized by Human Colonic Microflora into Low-Molecular-Weight Phenolic Acids. J. Nutr. 2000, 130, 2733–2738. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, P.; Kalariya, H.M.; Poulev, A.; Ribnicky, D.M.; Jaja-Chimedza, A.; Roopchand, D.E.; Raskin, I. Grape polyphenols reduce gut-localized reactive oxygen species associated with the development of metabolic syndrome in mice. PLoS ONE 2018, 13, e0198716. [Google Scholar] [CrossRef] [Green Version]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83. [Google Scholar] [CrossRef] [Green Version]
- Kopf, J.C.; Suhr, M.J.; Clarke, J.; Eyun, S.I.; Riethoven, J.M.; Ramer-Tait, A.E.; Rose, D.J. Role of whole grains versus fruits and vegetables in reducing subclinical inflammation and promoting gastrointestinal health in individuals affected by overweight and obesity: A randomized controlled trial. Nutr. J. 2018, 17, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanegas, S.M.; Meydani, M.; Barnett, J.B.; Goldin, B.; Kane, A.; Rasmussen, H.; Brown, C.; Vangay, P.; Knights, D.; Jonnalagadda, S.; et al. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am. J. Clin. Nutr. 2017, 105, 635–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamel, T.H.; Abdel-Aal, E.-S.M.; Tucker, A.J.; Pare, S.M.; Faughnan, K.; O’Brien, C.D.; Dykun, A.; Rabalski, I.; Pickard, M.; Wright, A.J. Consumption of whole purple and regular wheat modestly improves metabolic markers in adults with elevated high-sensitivity C-reactive protein: A randomised, single-blind parallel-arm study. Br. J. Nutr. 2020, 124, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, P.; Zhang, M.; Xia, M.; Zhu, H.; Ma, J.; Hou, M.; Tang, Z.; Ling, W. Supplementation of black rice pigment fraction improves antioxidant and anti-inflammatory status in patients with coronary heart disease. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. 1), 295–301. [Google Scholar] [PubMed]
| Hydroxybenzoic acid | Hydroxycinnamic acid |
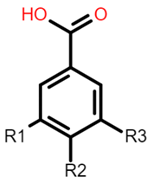 Gallic acid: R1 = OH; R2 = OH; R3 = OH Protocatechuic acid: R1 = OH; R2 = OH; R3 = H Vanillic acid: R1 = OH; R2 = OCH3; R3 = H Hydrobenzoic acid: R1 = H; R2 = OH; R3 = H |  Caffeic acid: R1 = OH; R2 = OH Ferulic acid: R1 = OCH3; R2 = OH p-coumaric acid: R1 = H; R2 = OH |
| Flavonoid backbone | Flavonol |
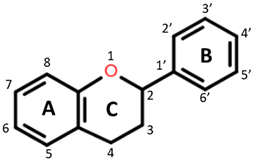 Flavanones: C4 keto group Flavanols: C4 keto group, C3 hydroxyl group Flavones: C2–C3 double bond, C4 keto group Flavan-3-ols: C3-hydroxyl group |  Quercetin: R1 = OH Rutin (quercetin 3-O glucoside): R1 = O-β-D-rutinoside |
| Anthocyanin | 3-Deoxyanthocyanidin |
 Cyanidin 3-glucoside: R1 = OH; R2 = OH; R3 = H; R4 = O-β-D-glucoside Delphinidin 3-glucoside: R1 = OH; R2 = OH; R3 = OH; R4 = O-β-D-glucoside Peonidin 3-glucoside: R1 = OCH3; R2 = OH; R3 = H; R4 = O-β-D-glucoside Petunidin 3-glucoside: R1 = OH; R2 = OH; R3 = OCH3; R4 = O-β-D-glucoside Malvidin 3-glucoside: R1 = OCH3; R2 = OH; R3 = OCH3; R4 = O-β-D-glucoside Pelargonidin 3-glucoside: R1 = H; R2 = OH; R3 = H; R4 = O-β-D-glucoside | 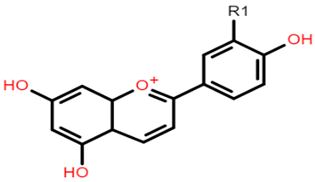 Apigeninidin: R1 = H Luteolinidin: R1 = OH |
| Avenanthramide | Tannin repeating unit |
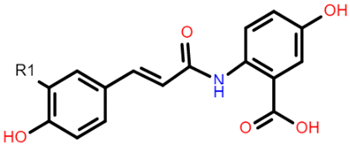 AVN A: R1 = H AVN B: R1 = OCH3 AVN C: R1 = OH | 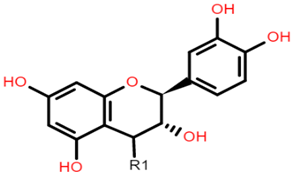 Catechin (or epicatechin) basic repeating unit: R1 = H Proanthocyanidin (or Procyanidin): R1 = Catechin |
| Food Source | Study Design | Bioaccessibility Model | Findings | Ref. |
|---|---|---|---|---|
| Four Luxembourgish apple varieties | In vitro | Gastric and intestinal phase digestion combined with dialysis to assess recovery |
| [66] |
| Grape seed phenolic extracts | In vitro | Gastric, intestinal digestion, and in vitro ileal and faecal fermentation combined with a Caco-2 cell transport |
| [73] |
| Phoenix dactylifera L. (date) seeds; Date seed powder (DSP), date seed pita bread (DSB), date seed extract (DSE) | In vitro | Gastric and intestinal phase digestion combined with Caco-2 cell transport |
| [64] |
| Zimbabwean cereal grains | In vitro | Gastric, intestinal digestion, and colonic fermentation combined with dialysis |
| [69] |
| Wheat, brown rice, oat grains | In vitro | Gastric and intestinal digestion combined with dialysis |
| [74] |
| Cookies from malted oat flours (avenanthramides) | In vitro | Single bioreactor (oral, gastric and intestinal phases of digestion) |
| [71] |
| Wheat bran | In vitro | TIM-1 (upper GI digestion) and TIM-2 (human colon fermentation) |
| [75] |
| Extruded Barley and Oats | In vivo (pigs) | N/A—blood and faecal samples collected at the start and during the trial |
| [70] |
| Whole grain oat products | In vitro | Gastrointestinal digestion (oral, gastric and intestinal) coupled with Caco-2 cell uptake |
| [76] |
| Cooked millet grains (kodo, finger, proso, foxtail and pearl) | In vitro | Gastric, intestinal digestion, and colonic fermentation combined with dialysis |
| [65] |
| Polyphenol/Food Source | Significant Dose | Study Design | Agonist | Cell Culture Model | Biomarkers | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| Cyanidin-3-glucoside (C3G) | 20 μM | In vitro | TNF-α | Caco-2/HUVEC/ leucocytes | E-selectin, VCAM-1, NF-κB and TNF-α and IL-8 |
| [89] |
| Chlorogenic acid and epicatechin gallate | 10 μM | In vitro | N/A | Caco-2 and HT29-MTX (mucus-secreting goblet cells) | Mucin protein and gene expression |
| [80] |
| Ferulic acid | 100 μM | In vitro | LPS | Caco-2 cells | microRNA expression, epithelial permeability, TJ proteins., P13K and AKT protein |
| [90] |
| Polyphenol-rich propolis extract (PPE) | 50 µg/mL | In vivo & in vitro (18 male Sprague-Dawley rats; 300–320 g) | N/A | Caco-2 cells | TEER, LY flux, TJ proteins (Zonulin and occludin tight junction protein gene expression), AMPK and ERK expression |
| [20] |
| Sardinian wine extracts (red Cannonau and white Vermentino) | 25 μg/mL | In vitro | Oxysterol | Caco-2 cells | NOX1, IL6 and IL-8 |
| [22] |
| Fuzhuan brick-tea extract | 25, 100 and 200 μg/mL | In vitro | Hydrogen peroxide | Caco-2 cells | GSH, IL-8 and MDA |
| [78] |
| Green tea polyphenols | 1 mg/mL | In vitro | Gliadin | Caco-2 cells | Intestinal permeability, IL-6 and IL-8 |
| [79] |
| Flavan-4-ols enriched maize | 15% or 25% (w/w) | In vivo (C57BL6 mice for 6 weeks) | Carboxymethylcellulose (CMC) | N/A | IL-6 and intestinal permeability |
| [88] |
| Flavan-4-ols- and anthocyanin- enriched maize | 25% (w/w) | In vivo (C57BL6 mice) | Dextran sulfate sodium (DSS) | N/A | Intestinal permeability, IL-1β and IL-6 |
| [21] |
| Rice bran phenolic extract (RBPE) | 100 and 200 mg/kg | In vivo (C57BL6 mice) | Ethanol | N/A | TJ proteins (ZO-1, Claudin), TNF-α, IL-1β, IL-6, IFN-γ, and MCP-1 |
| [87] |
| Polyphenol Rich-Cereal(s) | Dose | Population | Study Design | Duration | Gut Microbiome | Microbial Metabolites | Inflammatory Markers | Ref. |
|---|---|---|---|---|---|---|---|---|
| Oat bran and matched concentrations of β-glucan extract or polyphenol mix | 1 and 3% w/v | 3 female donors | In vitro batch fermentation | 24 h | Increases in Bifidobacterium, and bacteria from the phyla of Proteobacteria and Bacteriodetes. | Increase in SCFA. | N/A | [101] |
| Black sorghum bran extract, sumac sorghum bran extract, fructooligosaccharides (FOS) | 5 g/L | 11 each of Obese and Healthy weight | In vitro batch fermentation | 24 h | Sorghum bran polyphenols worked with FOS to enhance Bifidobacterium and Lactobacillus, and independently stimulated Roseburia and Prevotella. | No significant differences in total and individual SCFA production were observed between obese and healthy weight subjects. | N/A | [40] |
| Soluble feruloylated arabinoxylan oligosaccharides, rice bran polyphenols | 50 mg | 15 males and 17 females (21–45 years of age) | In vitro batch fermentation | 24 h | Increased butyrogenic bacteria, Coprococcus and Roseburia. | No significant increase in SCFA. | N/A | [102] |
| Whole grain (WG) vs. refined grain (RG) foods | 8 g (RG) 16 g (WG) per day | 49 men, 32 women (ages 40–65 years) | Randomised, controlled, parallel-design human trial | 6 weeks (after 2 weeks run-in Western-style diet) | WG group also showed more increase in stool weight, stool frequency and SCFA bacteria (Lachnospira) but decreased proinflammatory bacteria (Enterobacteriaceae). | N/A | No effect on salivary IgA concentration or stool IgA or stool cytokines concentrations. A higher percentage of terminal effector memory T cells and LPS-stimulated ex vivo production of TNF-α in WG than RG (p = 0.004). | [109] |
| Whole Grain Wheat (Whole vs. Refined) | 70 g (WG) and 60 g (RW) per day | 80 healthy overweight/obese subjects | A placebo-controlled, parallel-group randomised trial | 8 weeks with measurements taken at baseline and every 4 weeks. | TNF-α reduction with WG consumption correlated with increased abundance of Bacteroides and Lactobacillus increases. | WG consumption increased serum dihydroferulic acid and faecal ferulic acid. | TNF-α and IL-10 reduced significantly | [60] |
| Whole grain (WG) versus Fruits and vegetables (FV) | 3 servings/day | 49 obese subjects | Randomised parallel-arm trial | 6 weeks | No significant microbiota changes between groups were detected. | N/A | There was a significant decrease in LBP for participants on WG and FV diets with no change on the control diet. FV diet-induced significant change in IL-6 but no significant change in the other diets. WG diet resulted in a significant decrease in TNF-α whereas no significant effects by the other diets. | [108] |
| WG and RG | 179 g/day (WG) and 13 g/day (RG) | 60 Danish (20–65 year old with BMI 25–35 kg/m2) | A randomised, controlled crossover | Two 8-week intervention | Compared to RG, WG did not significantly induce major changes in faecal microbiome. | Plasma SCFA was not affected. | WG diet decreased bodyweight, serum inflammatory markers, IL-6 and CRP. | [107] |
| Purple wheat (PW) and regular wheat (RW) | 4 servings/day (160 g total) | 29 overweight and obese subjects | Randomised Single-blind parallel-arm study | 8 weeks | N/A | No anthocyanins detected after 4 and 8 weeks. Ferulic acid and hippuric acid detected. No difference between PW and RW groups in terms of total phenolic acids. | IL-6 and adiponectin were reduced significantly with PW. TNF-α reduced in both groups. Plasma GSH level increased significantly in pooled data. | [110] |
| Black rice pigmented fraction (BFR) vs. white rice pigment fraction (WRF) | 10 g | 60 Coronary heart disease patients (45–75) | Randomised single-blind parallel-arm | 6 months | N/A | N/A | No changes in plasma total superoxide dismutase activity and lipid levels. Reduced levels of vascular cell adhesion molecule-1(VCAM-1), high sensitive C-reactive protein (hs-CRP), soluble CD40 ligand. | [111] |
| Purple rice, red rice and brown rice | 1 serving | 22 obese participants | Crossover intervention trial | Over 4 h (30 min, 1 h, 2 h and 4 h time point) | N/A | N/A | A significant reduction in MDA levels with red rice and purple rice. IL-10 significantly reduced with purple rice (30 min). Red rice reduced IL-6 at 30 min and 1 h. Both purple and red reduced IL-12 at 10 min by 13.6% and 11.0% respectively. Brown rice did not show any effects on biomarkers. | [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ed Nignpense, B.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Bioaccessibility and Bioactivity of Cereal Polyphenols: A Review. Foods 2021, 10, 1595. https://doi.org/10.3390/foods10071595
Ed Nignpense B, Francis N, Blanchard C, Santhakumar AB. Bioaccessibility and Bioactivity of Cereal Polyphenols: A Review. Foods. 2021; 10(7):1595. https://doi.org/10.3390/foods10071595
Chicago/Turabian StyleEd Nignpense, Borkwei, Nidhish Francis, Christopher Blanchard, and Abishek Bommannan Santhakumar. 2021. "Bioaccessibility and Bioactivity of Cereal Polyphenols: A Review" Foods 10, no. 7: 1595. https://doi.org/10.3390/foods10071595
APA StyleEd Nignpense, B., Francis, N., Blanchard, C., & Santhakumar, A. B. (2021). Bioaccessibility and Bioactivity of Cereal Polyphenols: A Review. Foods, 10(7), 1595. https://doi.org/10.3390/foods10071595