Preliminary Investigations into the Use of Amylases and Lactic Acid Bacteria to Obtain Fermented Vegetable Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Proximate Composition of Legume Flours
2.2. Production of the Vegetable Milk
2.3. Enzymatic Hydrolysis
2.4. Lactic Acid Fermentation
2.5. Antioxidant Activity Determination
2.5.1. DPPH Radical Scavenging Ability
2.5.2. Total Phenolics Content
2.6. Titratable Acidity Determination
2.7. Syneresis Determination
2.8. Color Determination
2.9. Sensory Analysis
2.10. Rheological Measurements
2.11. Statistical Analysis
3. Results and Discussion
3.1. Influence of Amylase Assisted Hydrolysis on the Antioxidant Activity of Vegetable Milk and Fermented Products
3.2. Physicochemical Characteristics of the Fermented Products
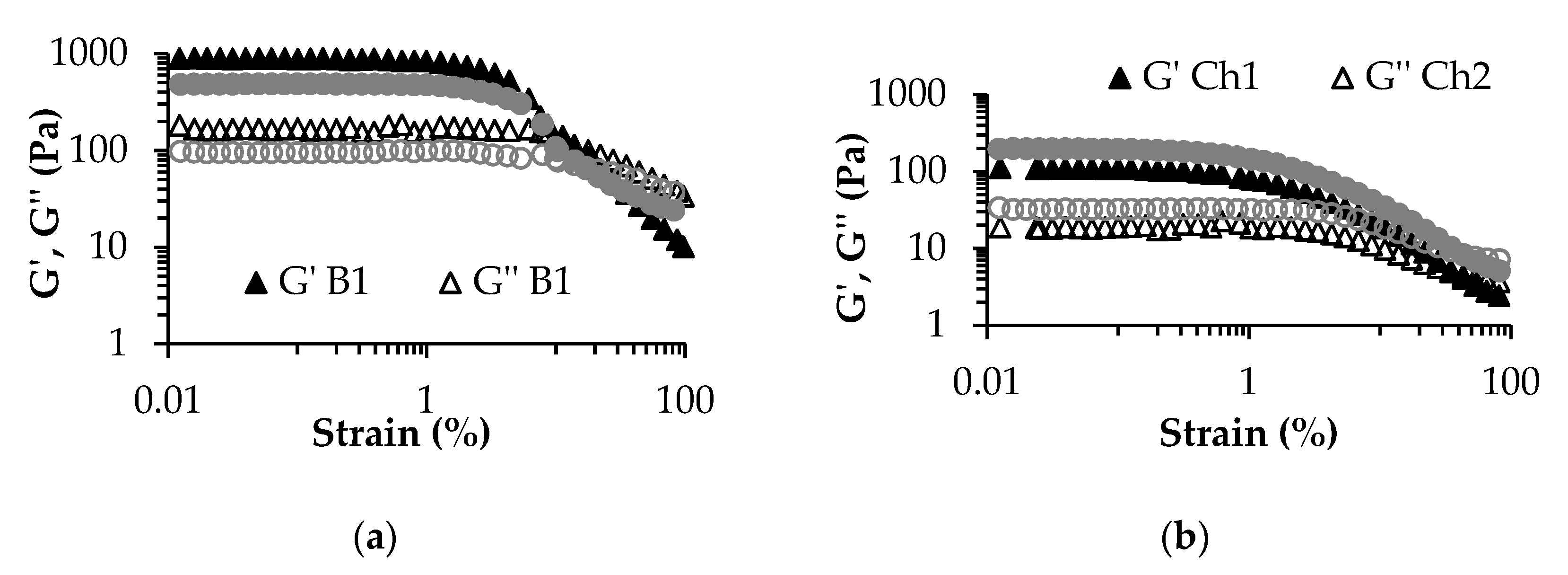
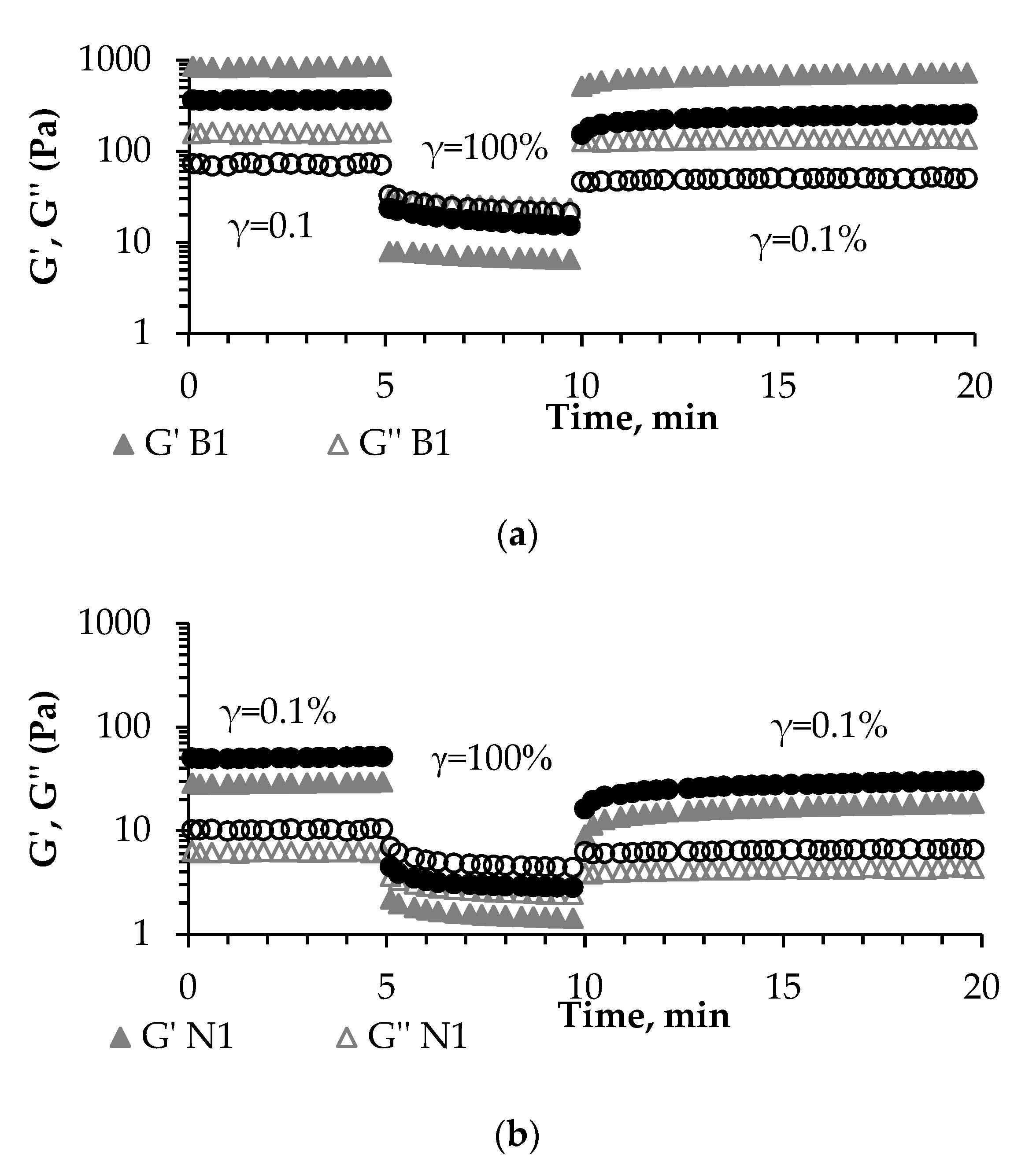
3.3. Rheological Characteristics of the Fermented Products
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rincon, L.; Braz Assunção Botelho, R.; de Alencar, E.R. Development of novel plant-based milk based on chickpea and coconut. LWT-Food Technol. 2020, 128, 109479. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- García, M.C.; Puchalska, P.; Esteve, C.; Marina, M.L. Vegetable foods: A cheap source of proteins and peptides with antihypertensive, antioxidant, and other less occurrence bioactivities. Talanta 2013, 106, 328–349. [Google Scholar] [CrossRef] [PubMed]
- Choct, M.; Dersjant-Li, Y.; McLeish, J.; Peisker, M. Soy oligosaccharides and soluble non-starch polysaccharides: A review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Asian-Australas. J. Anim. Sci. 2010, 23, 1386–1398. [Google Scholar] [CrossRef]
- Mishra, S.; Rai, T. Morphology and functional properties of corn, potato and tapioca starches. Food Hydrocoll. 2006, 20, 557–566. [Google Scholar] [CrossRef]
- Pérez, S.; Baldwin, P.M.; Gallant, D.J. Structural features of starch granules I. In Starch; Academic Press: Cambridge, MA, USA, 2009; pp. 149–192. [Google Scholar]
- Cai, J.; Cai, C.; Man, J.; Zhou, W.; Wei, C. Structural and functional properties of C-type starches. Carbohydr. Polym. 2014, 101, 289–300. [Google Scholar] [CrossRef]
- Hoover, R.; Sosulski, F.W. Composition, structure, functionality, and chemical modification of legume starches: A review. Can. J. Physiol. Pharmacol. 1991, 69, 79–92. [Google Scholar] [CrossRef]
- Hoover, R.; Sosulski, F. Studies on the functional characteristics and digestibility of starches from Phaseolus vulgaris biotypes. Starch Stärke 1985, 37, 181–191. [Google Scholar] [CrossRef]
- Wang, S.; Copeland, L. Effect of acid hydrolysis on starch structure and functionality: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1081–1097. [Google Scholar] [CrossRef]
- Hoover, R.; Zhou, Y. In vitro and in vivo hydrolysis of legume starches by α-amylase and resistant starch formation in legumes—A review. Carbohydr. Polym. 2003, 54, 401–417. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Prakash, J. Effect of germination and dehulling on functional properties of legume flours. J. Sci. Food Agric. 2006, 86, 1189–1195. [Google Scholar] [CrossRef]
- Brajdeș, C.; Vizireanu, C. Sprouted buckwheat an important vegetable source of antioxidants. Ann. Univ. Dunarea Jos Galati. Fascicle VI Food Technol. 2012, 36, 53–60. [Google Scholar]
- Patraşcu, L.; Vasilean, I.; Turtoi, M.; Garnai, M.; Aprodu, I. Pulse germination as tool for modulating their functionality in wheat flour sourdoughs. Qual. Assur. Saf. Crop. Foods 2019, 11, 269–282. [Google Scholar] [CrossRef]
- Segal, R. Biochimia Produselor Alimentare. (Biochemistry of Food Science); Academica Press: Galati, Romania, 2006. [Google Scholar]
- Robyt, J.F. Enzymes and their action on starch. In Starch; Academic Press: Cambridge, MA, USA, 2009; pp. 237–292. [Google Scholar]
- Jiménez-Martínez, C.; Hernández-Sánchez, H.; Dávila-Ortiz, G. Production of a yogurt-like product from Lupinus campestris seeds. J. Sci. Food Agric. 2003, 83, 515–522. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists International (AACC). Approved Methods of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Association of Official Agricultural Chemists (AOAC). Official Methods of AOAC International, 17th ed.; Official Method 962.09; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Asociatia de Standardizare din România (ASRO). Romanian Standards Catalog for Cereal and Milling Products Analysis; SR ISO 2171:2002, 91:2007; ASRO: Bucharest, Romania, 2008. [Google Scholar]
- Vidal, B.C., Jr.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Kinetics of granular starch hydrolysis in corn dry-grind process. Starch Stärke 2009, 61, 448–456. [Google Scholar] [CrossRef]
- Adthalungrong, C.; Temviriyanukul, S. Optimization of lactic acid production from tapioca starch hydrolysate by Lactobacillus casei TISTR 453. Asia-Pac. J. Sci. Technol. 2010, 15, 436–445. [Google Scholar]
- Vasilean, I.; Segal, R. The influence of biosynthesized exopolysaccharides on some characteristics of fermented dairy products. Ann. Univ. Dunarea Jos Galati. Fascicle VI Food Technol. 2011, 35, 71–76. [Google Scholar]
- López-Amorós, M.L.; Hernández, T.; Estrella, I. Effect of germination on legume phenolic compounds and their antioxidant activity. J. Food Compos. Anal. 2006, 19, 277–283. [Google Scholar] [CrossRef]
- Toker, O.S.; Karasu, S.; Yilmaz, M.T.; Karaman, S. Three interval thixotropy test (3ITT) in food applications: A novel technique to determine structural regeneration of mayonnaise under different shear conditions. Food Res. Int. 2015, 70, 125–133. [Google Scholar] [CrossRef]
- Vuorela, S.; Meyer, A.S.; Heinonen, M. Quantitative analysis of the main phenolics in rapeseed meal and oils processed differently using enzymatic hydrolysis and HPLC. Eur. Food Res. Technol. 2003, 217, 517–523. [Google Scholar] [CrossRef]
- Apostolidis, E.; Kwon, Y.I.; Ghaedian, R.; Shetty, K. Fermentation of milk and soymilk by Lactobacillus bulgaricus and Lactobacillus acidophilus enhances functionality for potential dietary management of hyperglycemia and hypertension. Food Biotechnol. 2007, 21, 217–236. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Kim, Y.; Goodner, K.L.; Park, J.-D.; Choi, J.; Talcott, S.T. Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chem. 2011, 129, 1331–1342. [Google Scholar] [CrossRef]
- USDA National Nutrient Database for Standard Reference. SR Legacy, 175205, NDB Number: 16052. 2018. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/175205/nutrients (accessed on 10 February 2021).
- USDA National Nutrient Database for Standard Reference. SR Legacy, 173756, NDB Number: 16056. 2018. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/173756/nutrients (accessed on 10 February 2021).
- Baysal, T.; Demirdöven, A. Lipoxygenase in fruits and vegetables: A review. Enzym. Microb. Technol. 2007, 40, 491–496. [Google Scholar] [CrossRef]
- Junqueira, R.M.; Cocato, M.L.; Colli, C.; Castro, I.A. Synergism between lipoxygenase-active soybean flour and ascorbic acid on rheological and sensory properties of wheat bread. J. Sci. Food Agric. 2008, 88, 194–198. [Google Scholar] [CrossRef]
- Chang, P.R.; McCurdy, A.R. Lipoxygenase activity in fourteen legumes. Can. Inst. Food Sci. Technol. J. 1985, 18, 94–96. [Google Scholar] [CrossRef]
- Clemente, A.; Olías, R.; Olías, J.M. Purification and characterization of broad bean lipoxygenase isoenzymes. J. Agric. Food Chem. 2000, 48, 1070–1075. [Google Scholar] [CrossRef]
- Nozzolillo, C.; Ricciardi, L.; Lattanzio, V. Flavonoid constituents of seed coats of Vicia faba (Fabaceae) in relation to genetic control of their color. Can. J. Bot. 1989, 67, 1600–1604. [Google Scholar] [CrossRef]
- Mathew, A.G.; Parpia, H.A.B. Food browning as a polyphenol reaction. In Advances in Food Research; Academic Press: Cambridge, MA, USA, 1971; Volume 9, pp. 75–145. [Google Scholar]
- Saigusa, N.; Terahara, N.; Ohba, R. Evaluation of DPPH-radical-scavenging activity and antimutagenicity and analysis of anthocyanins in an alcoholic fermented beverage produced from cooked or raw purple-fleshed sweet potato (Ipomoea batatas cv. Ayamurasaki) roots. Food Sci. Technol. Res. 2005, 11, 390–394. [Google Scholar] [CrossRef][Green Version]
- Saigusa, N.; Teramoto, Y.; Ueda, S.; Yoshizawa, K. Effects of β-glucosidase activity on the characteristics of aromatic red rice wine. J. Inst. Brew. 1994, 100, 159–162. [Google Scholar] [CrossRef]
- Sakamura, S.; Watanase, S.; Obata, Y. Oxidative decolorization of the anthocyanin by polyphenol oxidase. Agric. Biol. Chem. 1965, 29, 181–190. [Google Scholar]
- Su, M.S.; Silva, J.L. Antioxidant activity, anthocyanins, and phenolics of rabbiteye blueberry (Vaccinium ashei) by-products as affected by fermentation. Food Chem. 2006, 97, 447–451. [Google Scholar] [CrossRef]
- El-Sayed, S.T.; Al-Azzouny, R.A.; Ali, O.S. Purification of a novel monophenolase inhibitory peptides prepared from Vicia faba pods protein via enzymatic hydrolysis. Biocatal. Agric. Biotechnol. 2019, 19, 101123. [Google Scholar] [CrossRef]
- Karkouch, I.; Tabbene, O.; Gharbi, D.; Mlouka, M.A.B.; Elkahoui, S.; Rihouey, C.; Coquet, L.; Cosette, P.; Jouenne TLimam, F. Antioxidant, antityrosinase and antibiofilm activities of synthesized peptides derived from Vicia faba protein hydrolysate: A powerful agents in cosmetic application. Ind. Crop. Prod. 2017, 109, 310–319. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. CMLS 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Mezger, T.G. Applied Rheology; Anton Paar GmbH: Graz, Austria, 2015. [Google Scholar]
- Matheis, G.; Whitaker, J.R. A review: Enzymatic cross-linking of proteins applicable to foods. J. Food Biochem. 1987, 11, 309–327. [Google Scholar] [CrossRef]
| Water, g/100 g | Ash, g/100 g | Proteins, g/100 g | Fat, g/100 g | Fibers, g/100 g | Carbohydrates *, g/100 g | |
|---|---|---|---|---|---|---|
| Broad bean | 9.11 ± 0.08 | 3.68 ± 0.00 | 24.51 ± 0.80 | 0.92 ± 0.07 | 6.61 ± 0.49 | 55.17 |
| Chickpea | 9.53 ± 0.03 | 2.74 ± 0.00 | 17.36 ± 0.06 | 5.6 ± 0.18 | 2.29 ± 0.31 | 62.42 |
| Sample Treatment | IC50 of DPPH, mg D.W. | Total Phenols, mg Ferulic Acid/g D.W. | ||
|---|---|---|---|---|
| Broad Bean | Chickpea | Broad Bean | Chickpea | |
| Control | 4.50 ± 0.06 | 9.92 ± 0.86 | 4.59 ± 0.14 | 2.98 ± 0.13 |
| Hydrolyzed vegetable milk | 2.84 ± 0.03 | 5.99 ± 0.03 | 3.88 ± 0.04 | 2.42 ± 0.07 |
| Sample | IC50 of DPPH, mg D.W. | Total Phenols, mg Ferulic Acid/g D.W. | ||
|---|---|---|---|---|
| Initial | 2 Weeks of Storage | Initial | 2 Weeks of Storage | |
| B1 | 2.15 ± 0.26 a | 3.34 ± 0.02 a | 5.68 ± 0.05 a | 3.90 ± 0.13 b |
| B2 | 2.21 ± 0.08 a | 3.82 ± 0.07 a | 5.73 ± 0.04 a | 3.70 ± 0.08 b |
| Ch1 | 5.78 ± 0.10 b | 8.27 ± 0.12 b | 3.85 ± 0.02 b | 2.65 ± 0.10 a |
| Ch2 | 5.93 ± 0.02 b | 8.86 ± 0.00 b | 3.88 ± 0.01 b | 2.45 ± 0.02 a |
| Physicochemical Parameter | Sample | |||
|---|---|---|---|---|
| B1 | B2 | Ch1 | Ch2 | |
| Titratable acidity (ml NaOH 0.1n/100 g) | 132.09 ± 1.89 a | 124.23 ± 6.71 a | 110.23 ± 6.02 b | 118.59 ± 1.80 b |
| pH value | 4.24 ± 0.01 a | 4.36 ± 0.00 a | 4.13 ± 0.02 a | 4.00 ± 0.01 a |
| Syneresis, g liquid/100 g product | 35.81 ± 1.15 a | 32.14 ± 2.93 a | 41.58 ± 1.52 b | 41.84 ± 2.34 b |
| Color Parameters | |||
|---|---|---|---|
| L* | a* | b* | |
| Vegetable milk | |||
| Broad bean | 57.32 ± 3.57 a | 2.01 ± 0.02 bc | 4.70 ± 0.36 a |
| Chickpea | 75.87 ± 1.25 c | 1.58 ± 0.13 a | 17.31 ± 3.43 c |
| Fermented products—Immediately after fermentation | |||
| B1 | 71.45 ± 0.14 b | 2.32 ± 0.03 c | 10.51 ± 0.03 b |
| B2 | 71.00 ± 0.49 b | 2.12 ± 0.04 bc | 10.18 ± 0.03 b |
| Ch1 | 76.96 ± 0.51 c | 1.44 ± 0.11 a | 19.44 ± 0.13 c |
| Ch2 | 76.53 ± 0.54 c | 1.51 ± 0.35 a | 19.19 ± 0.33 c |
| Fermented products—After 2 weeks of storage | |||
| B1 | 71.64 ± 1.24 b | 2.27 ± 0.40 c | 10.6 ± 0.30 b |
| B2 | 71.18 ± 0.16 b | 2.12 ± 0.65 bc | 10.49 ± 0.45 b |
| Ch1 | 76.81 ± 0.17 c | 1.73 ± 0.79 ab | 18.74 ± 1.07 c |
| Ch2 | 75.93 ± 1.60 c | 1.44 ± 0.28 a | 18.71 ± 0.89 c |
| Rheological Parameter | Sample | |||
|---|---|---|---|---|
| B1 | B2 | Ch1 | Ch2 | |
| Complex viscosity in LVR (Pa.s) | 112.10 ± 28.82 a | 75.11 ± 2.74 a | 16.98 ± 3.46 b | 31.24 ± 1.89 b |
| Flow point %γ at G′-G′′ crossover | 13.31 ± 0.25 a | 16.27 ± 0.09 b | 37.55 ± 0.18 c | 42.10 ± 0.19 d |
| % of structure recovery in 3ITT | 82.57 ± 2.82 a | 62.65 ± 1.19 b | 57.85 ± 1.61 b | 54.12 ± 2.45 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilean, I.; Aprodu, I.; Garnai, M.; Munteanu, V.; Patrașcu, L. Preliminary Investigations into the Use of Amylases and Lactic Acid Bacteria to Obtain Fermented Vegetable Products. Foods 2021, 10, 1530. https://doi.org/10.3390/foods10071530
Vasilean I, Aprodu I, Garnai M, Munteanu V, Patrașcu L. Preliminary Investigations into the Use of Amylases and Lactic Acid Bacteria to Obtain Fermented Vegetable Products. Foods. 2021; 10(7):1530. https://doi.org/10.3390/foods10071530
Chicago/Turabian StyleVasilean, Ina, Iuliana Aprodu, Maria Garnai, Valeriu Munteanu, and Livia Patrașcu. 2021. "Preliminary Investigations into the Use of Amylases and Lactic Acid Bacteria to Obtain Fermented Vegetable Products" Foods 10, no. 7: 1530. https://doi.org/10.3390/foods10071530
APA StyleVasilean, I., Aprodu, I., Garnai, M., Munteanu, V., & Patrașcu, L. (2021). Preliminary Investigations into the Use of Amylases and Lactic Acid Bacteria to Obtain Fermented Vegetable Products. Foods, 10(7), 1530. https://doi.org/10.3390/foods10071530







