Gold Nanobeads with Enhanced Absorbance for Improved Sensitivity in Competitive Lateral Flow Immunoassays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characterization
2.3. Synthesis of Hydrophobic AuNPs
2.4. Synthesis of Carboxylated GNBs
2.5. Synthesis of Anti-FB1 Monoclonal Antibodies Labeled GNB129 (GNB129–mAbs)
2.6. Preparation of BSA-FB1 Conjugate
2.7. Construction of GNB129-LFIA Test Strips
2.8. Quantitative Procedure of GNB-LFIA for FB1 Detection
2.9. Sample Preparation
3. Results and Discussion
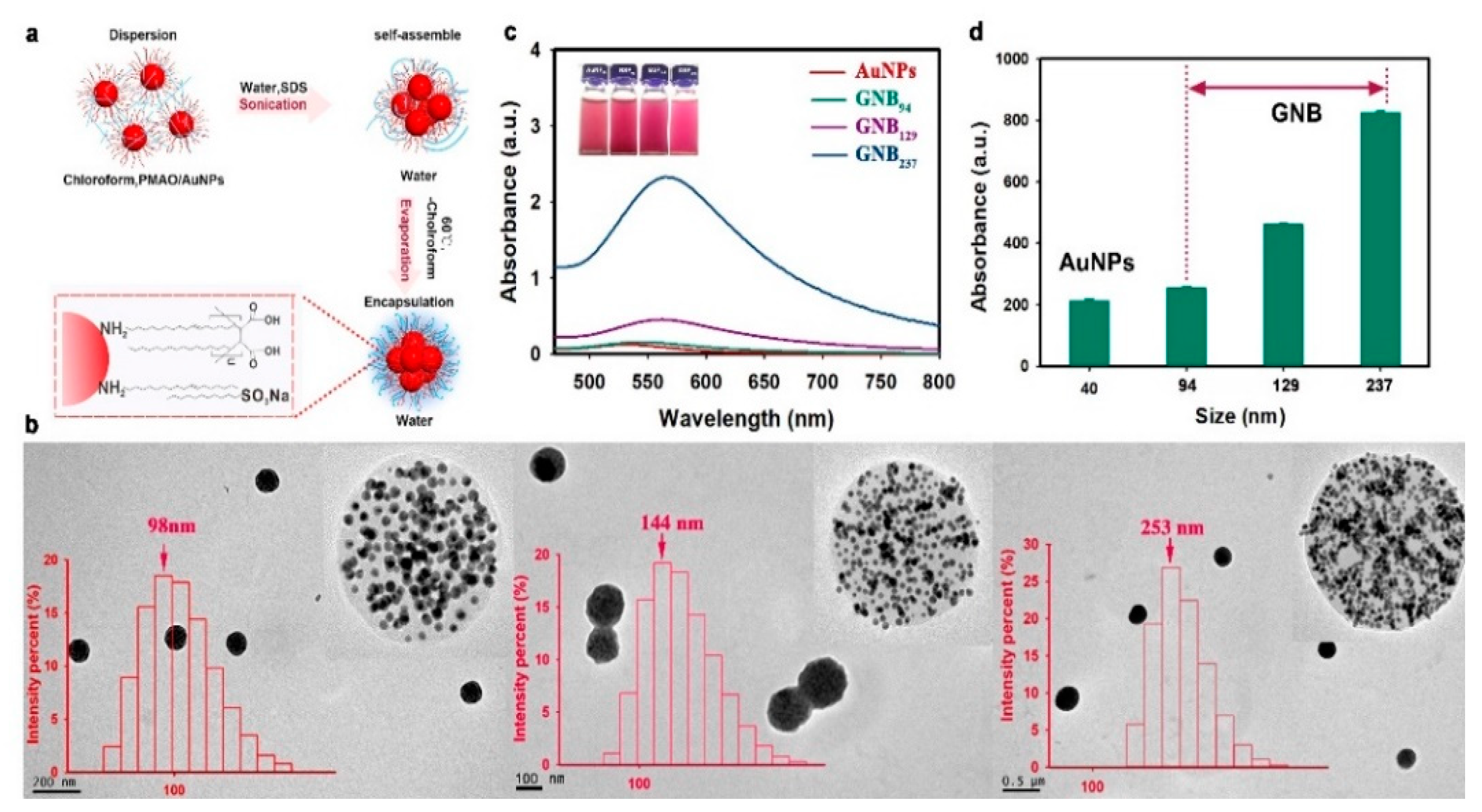
3.1. Synthesis and Characterization of GNBs
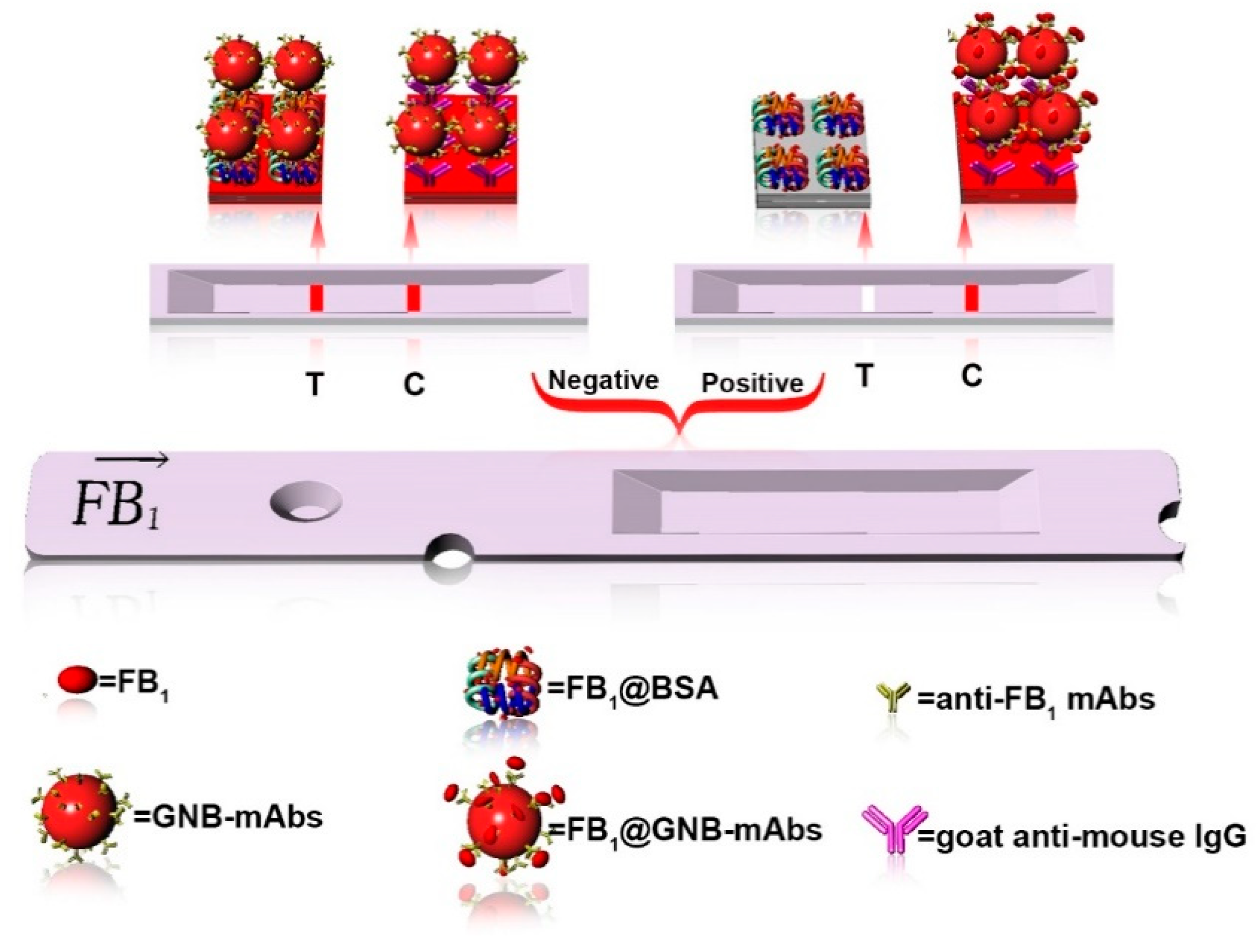
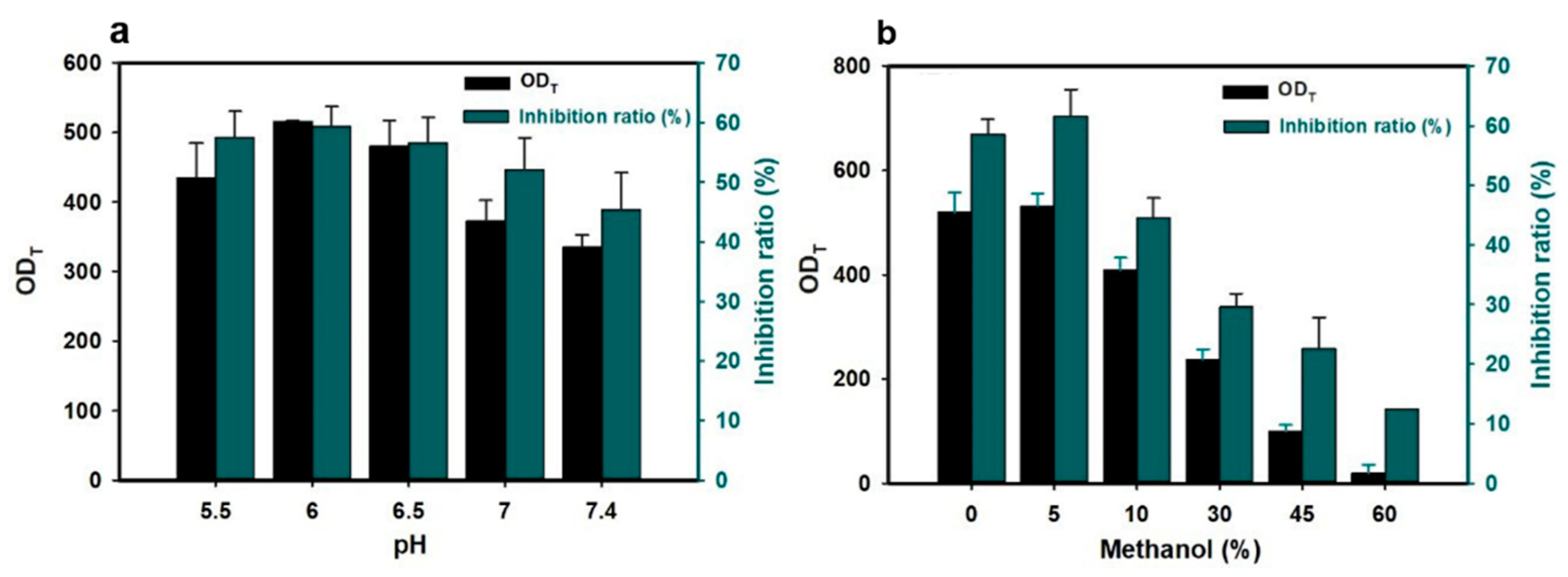
3.2. Detection Principle and Optimization of GNB-LFIA
3.3. Performance Evaluation of GNB129-LFIA for FB1 in Corn Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Loynachan, C.N.; Thomas, M.R.; Gray, E.R.; Richards, D.A.; Kim, J.; Miller, B.S. Platinum Nanocatalyst Amplification: Redefining the Gold Standard for Lateral Flow Immunoassays with Ultrabroad Dynamic Range. ACS Nano 2018, 12, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Ma, T.; Yang, J.; Li, S.; Liu, S.; Wang, S. Development of Lateral Flow Immunochromatographic Assays Using Colloidal Au Sphere and Nanorods as Signal Marker for the Determination of Zearalenone in Cereals. Foods 2020, 3, 281. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Ding, L.; Wu, Y.; Huang, X.; Lai, W.; Xiong, Y. Emerging Strategies to Develop Sensitive AuNP-Based ICTS Nanosensors. TrAC Trends Anal. Chem. 2019, 112, 147–160. [Google Scholar] [CrossRef]
- Luo, K.; Hu, L.; Guo, Q.; Wu, C.; Wu, S.; Liu, D. Comparison of 4 Label-Based Immunochromatographic Assays for the Detection of Escherichia Coli O157:H7 in Milk. J. Dairy Sci. 2017, 100, 5176–5187. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.N.; Tumskiy, R.S.; Burov, A.M.; Pylaev, T.E.; Khlebtsov, N.G. Quantifying the Numbers of Gold Nanoparticles in the Test Zone of Lateral Flow Immunoassay Strips. ACS Appl. Nano Mater. 2019, 2, 5020–5028. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.; Huang, X.; Shao, Y.; Zheng, L.; Guo, L.; Xiong, Y. Size-Dependent Immunochromatographic Assay with Quantum Dot Nanobeads for Sensitive and Quantitative Detection of Ochratoxin A in Corn. Anal. Chem. 2017, 89, 7062–7068. [Google Scholar] [CrossRef]
- Zhao, P.; Wu, Y.; Zhu, Y.; Yang, X.; Jiang, X.; Xiao, J. Upconversion Fluorescent Strip Sensor for Rapid Determination of Vibrio Anguillarum. Nanoscale 2014, 6, 3804–3809. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhao, G.; Dou, W. An Optical and Rapid Sandwich Immunoassay Method for Detection of Salmonella Pullorum and Salmonella Gallinarum Based on Immune Blue Silica Nanoparticles and Magnetic Nanoparticles. Sens. Actuators B 2016, 226, 69–75. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, S.; Xiong, Y.; Wei, H.; Xu, H.; Duan, H.; Lai, W. Application and Development of Superparamagnetic Nanoparticles in Sample Pretreatment and Immunochromatographic Assay. TrAC Trends Anal. Chem. 2019, 114, 151–170. [Google Scholar] [CrossRef]
- Dement’eva, O.; Naumova, K.; Zhigletsova, S.; Klykova, M.; Somov, A.; Dunaytsev, I.; Senchikhin, I.; Volkov, V.; Rudoy, V. Drug-Templated Mesoporous Silica Nanocontainers with Extra High Payload and Controlled Release Rate. Colloids Surf. B. 2020, 185, 110577. [Google Scholar] [CrossRef]
- Gómez-Hens, A.; Manuel Fernández-Romero, J. The Role of Liposomes in Analytical Processes. TrAC Trends Anal. Chem. 2005, 24, 9–19. [Google Scholar] [CrossRef]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.S.Y. Mesoporous Silica Nanoparticles for Drug Delivery and Biosensing Applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Hofmann, C.; Duerkop, A.; Baeumner, A.J. Nanocontainers for Analytical Applications. Angew. Chem. Int. Ed. 2019, 58, 12840–12860. [Google Scholar] [CrossRef]
- Bhushan, B.; Kumar, S.U.; Matai, I.; Sachdev, A.; Dubey, P.; Gopinath, P. Ferritin Nanocages: A Novel Platform for Biomedical Applications. J. Biomed. Nanotechnol. 2014, 10, 2950–2976. [Google Scholar] [CrossRef]
- Feng, A.; Yuan, J. Smart Nanocontainers: Progress on Novel Stimuli-Responsive Polymer Vesicles. Macromol. Rapid Commun. 2014, 35, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, X.; Han, N.; Chen, J.; Qian, X.; Deng, Y.; Tang, W.; Chen, Y. MOF-Derived Hierarchical Hollow ZnO Nanocages with Enhanced Low-Concentration VOCs Gas-Sensing Performance. Sens. Actuators B 2016, 225, 158–166. [Google Scholar] [CrossRef]
- Bi, J.; Wang, H.; Kamal, T.; Zhu, B.-W.; Tan, M. A Fluorescence Turn-Off-On Chemosensor Based on Carbon Nanocages for Detection of Ascorbic Acid. RSC Adv. 2017, 7, 30481–30487. [Google Scholar] [CrossRef] [Green Version]
- Misson, M.; Zhang, H.; Jin, B. Nanobiocatalyst Advancements and Bioprocessing Applications. J. R. Soc. Interface 2015, 12, 20140891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broz, P.; Driamov, S.; Ziegler, J.; Ben-Haim, N.; Marsch, S.; Meier, W.; Hunziker, P. Toward Intelligent Nanosize Bioreactors: A pH-Switchable, Channel-Equipped, Functional Polymer Nanocontainer. Nano Lett. 2006, 6, 2349–2353. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, S.; Huang, H.; Liu, D.; Zhuang, Z.; Zhong, C. ZIF-67 as Continuous Self-Sacrifice Template Derived NiCo2O4/Co,N-CNTs Nanocages as Efficient Bifunctional Electrocatalysts for Rechargeable Zn–Air Batteries. ACS Sustain. Chem. Eng. 2018, 6, 10021–10029. [Google Scholar] [CrossRef]
- Trantidou, T.; Friddin, M.; Elani, Y.; Brooks, N.J.; Law, R.V.; Seddon, J.M.; Ces, O. Engineering Compartmentalized Biomimetic Micro- and Nanocontainers. ACS Nano 2017, 11, 6549–6565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, P.; Xu, H.; Wang, Z.; Zhang, X.; Kabanov, A.V. Photocontrolled Self-Assembly and Disassembly of Block Ionomer Complex Vesicles: A Facile Approach toward Supramolecular Polymer Nanocontainers. Langmuir 2010, 26, 709–715. [Google Scholar] [CrossRef]
- Haase, M.F.; Grigoriev, D.O.; Möhwald, H.; Shchukin, D.G. Development of Nanoparticle Stabilized Polymer Nanocontainers with High Content of the Encapsulated Active Agent and Their Application in Water-Borne Anticorrosive Coatings. Adv. Mater. 2012, 24, 2429–2435. [Google Scholar] [CrossRef]
- Yagai, S.; Karatsu, T.; Kitamura, A. Photocontrollable Self-Assembly. Chem. Eur. J. 2005, 11, 4054–4063. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, C.P.; Suzuki, T.; Demurtas, D.; Finka, A.; Hubbell, J.A. A Novel Method for the Encapsulation of Biomolecules into Polymersomes via Direct Hydration. Langmuir 2009, 25, 9025–9029. [Google Scholar] [CrossRef]
- Lo, H.; Ponticiello, M.S.; Leong, K.W. Fabrication of Controlled Release Biodegradable Foams by Phase Separation. Tissue Eng. 1995, 1, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Capadona, J.R.; Van Den Berg, O.; Capadona, L.A.; Schroeter, M.; Rowan, S.J. A Versatile Approach for the Processing of Polymer Nanocomposites with Self-Assembled Nanofibre Templates. Nat. Nanotechnol. 2007, 2, 765–769. [Google Scholar] [CrossRef]
- Messager, L.; Burns, J.R.; Kim, J.; Cecchin, D.; Hindley, J.; Pyne, A.L.; Gaitzsch, J.; Battaglia, G.; Howorka, S. Biomimetic Hybrid Nanocontainers with Selective Permeability. Angew. Chem. 2016, 128, 11272–11275. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Xu, H.; Huang, X.; Kuang, M.; Xiong, Y.; Xu, H.; Xu, Y.; Chen, H.; Wang, A. Immunochromatographic Assay for Ultrasensitive Detection of Aflatoxin B1 in Maize by Highly Luminescent Quantum Dot Beads. ACS Appl. Mater. Interfaces 2014, 6, 14215–14222. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Leng, Y.; Hao, L.; Duan, H.; Yuan, J. Self-Assembled Colloidal Gold Superparticles to Enhance the Sensitivity of Lateral Flow Immunoassays with Sandwich Format. Theranostics 2020, 10, 3737–3748. [Google Scholar] [CrossRef]
- Zhan, L.; Guo, S.-Z.; Song, F.; Gong, Y.; Xu, F.; Boulware, D.R.; McAlpine, M.C.; Chan, W.C.W.; Bischof, J.C. The Role of Nanoparticle Design in Determining Analytical Performance of Lateral Flow Immunoassays. Nano Lett. 2017, 17, 7207–7212. [Google Scholar] [CrossRef] [Green Version]
- Petrarca, M.H.; Rodrigues, M.I.; Rossi, E.A.; de Sylos, C.M. Optimisation of A Sample Preparation Method for the Determination of Fumonisin B1 in Rice. Food Chem. 2014, 158, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Mexía-Salazar, A.L.; Hernández-López, J.; Burgos-Hernández, A.; Cortez-Rocha, M.O.; Castro-Longoria, R.; Ezquerra-Brauer, J.M. Role of Fumonisin B1 on the Immune System, Histopathology, and Muscle Proteins of White Shrimp (Litopenaeus Vannamei). Food Chem. 2008, 110, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Li, C.; Yu, Q.; Shen, J.; De Saeger, S. Development and Application of a Quantitative Fluorescence-Based Immunochromatographic Assay for Fumonisin B1 in Maize. J. Agric. Food Chem. 2014, 62, 6294–6298. [Google Scholar] [CrossRef] [PubMed]
- Anttila, A.; Bhat, R.V.; Bond, J.A.; Borghoff, S.J.; Bosch, F.X.; Carlson, G.P.; Castegnaro, M.; Cruzan, G.; Gelderblom, W.C.; Hass, U. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 2002; Volume 82. [Google Scholar]
- Hiramatsu, H.; Osterloh, F.E. A Simple Large-Scale Synthesis of Nearly Monodisperse Gold and Silver Nanoparticles with Adjustable Sizes and with Exchangeable Surfactants. Chem. Mater. 2004, 16, 2509–2511. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Y.; Zhang, W.; Leng, Y.; Xiong, Y. A Colorimetric Immunoassay Based on Glucose Oxidase-Induced AuNP Aggregation for Fumonisin B1. Talanta 2018, 186, 29–35. [Google Scholar] [CrossRef]
- Ji, Y.; Ren, M.; Li, Y.; Huang, Z.; Shu, M.; Yang, H.; Xiong, Y.; Xu, Y. Detection of Aflatoxin B1 with Immunochromatographic Test Strips: Enhanced Signal Sensitivity using Gold Nanoflowers. Talanta 2015, 142, 206–212. [Google Scholar] [CrossRef]
- Duan, H.; Chen, X.; Xu, W.; Fu, J.; Xiong, Y.; Wang, A. Quantum-Dot Submicrobead-Based Immunochromatographic Assay for Quantitative and Sensitive Detection of Zearalenone. Talanta 2015, 132, 126–131. [Google Scholar] [CrossRef] [PubMed]


| Spiked Concentration FB1 (mg kg−1) | Intra-Assay Precision | Inter-Assay Precision | ||||
|---|---|---|---|---|---|---|
| Detected Concentration a | CV | Recovery (%) | Detected Concentration b | CV | Recovery (%) | |
| 10 | 9.78 ± 1.52 | 15.6 | 97.82 | 9.24 ± 0.39 | 5.16 | 92.43 |
| 5 | 5.63 ± 0.39 | 6.96 | 112.64 | 5.23 ± 0.47 | 7.37 | 112.5 |
| 2 | 1.94 ± 0.24 | 12.51 | 97.01 | 2.18 ± 0.21 | 9.80 | 108.97 |
| 1 | 0.97 ± 0.06 | 7.12 | 96.93 | 0.91 ± 0.09 | 9.40 | 91.42 |
| 0.5 | 0.48 ± 0.02 | 7.62 | 95.97 | 0.447 ± 0.03 | 5.30 | 89.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Miao, X.; Ma, T.; Leng, Y.; Hao, L.; Duan, H.; Yuan, J.; Li, Y.; Huang, X.; Xiong, Y. Gold Nanobeads with Enhanced Absorbance for Improved Sensitivity in Competitive Lateral Flow Immunoassays. Foods 2021, 10, 1488. https://doi.org/10.3390/foods10071488
Chen X, Miao X, Ma T, Leng Y, Hao L, Duan H, Yuan J, Li Y, Huang X, Xiong Y. Gold Nanobeads with Enhanced Absorbance for Improved Sensitivity in Competitive Lateral Flow Immunoassays. Foods. 2021; 10(7):1488. https://doi.org/10.3390/foods10071488
Chicago/Turabian StyleChen, Xirui, Xintao Miao, Tongtong Ma, Yuankui Leng, Liangwen Hao, Hong Duan, Jing Yuan, Yu Li, Xiaolin Huang, and Yonghua Xiong. 2021. "Gold Nanobeads with Enhanced Absorbance for Improved Sensitivity in Competitive Lateral Flow Immunoassays" Foods 10, no. 7: 1488. https://doi.org/10.3390/foods10071488
APA StyleChen, X., Miao, X., Ma, T., Leng, Y., Hao, L., Duan, H., Yuan, J., Li, Y., Huang, X., & Xiong, Y. (2021). Gold Nanobeads with Enhanced Absorbance for Improved Sensitivity in Competitive Lateral Flow Immunoassays. Foods, 10(7), 1488. https://doi.org/10.3390/foods10071488







