Sensory-Directed Identification of Creaminess-Enhancing Semi-Volatile Lactones in Crumb Chocolate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fat and Chocolate Samples
2.2. Cocoa Liquor Samples
2.3. Chemicals
2.4. Syntheses of δ-Hexa- and δ-Octadecalactones
2.4.1. δ-(7-Methyl)-Hexadecalactone
2.4.2. δ-Hexadecalactone
2.4.3. δ-Octadecalactone
2.4.4. δ-(7-Methyl)-Hexadecalactone
2.5. Solvent Extraction of Chocolate and Cocoa Liquor
2.6. Isolation of Semi-Volatiles from Fat
2.7. Sub-Fractionation of Fraction I
2.8. Sensory Analyses
2.8.1. Precaution Taken for Sensory Analysis of Food Fractions and Synthesized δ-Lactones
2.8.2. Sensory Profiling of Chocolate and Isolated Chocolate Fractions in TFLE
2.8.3. Two-Alternative Forced-Choice Test (2-AFC Test)
2.8.4. Recognition Thresholds of δ-Lactones in Crumb Chocolate
2.9. Identification and Quantification of δ-Lactones
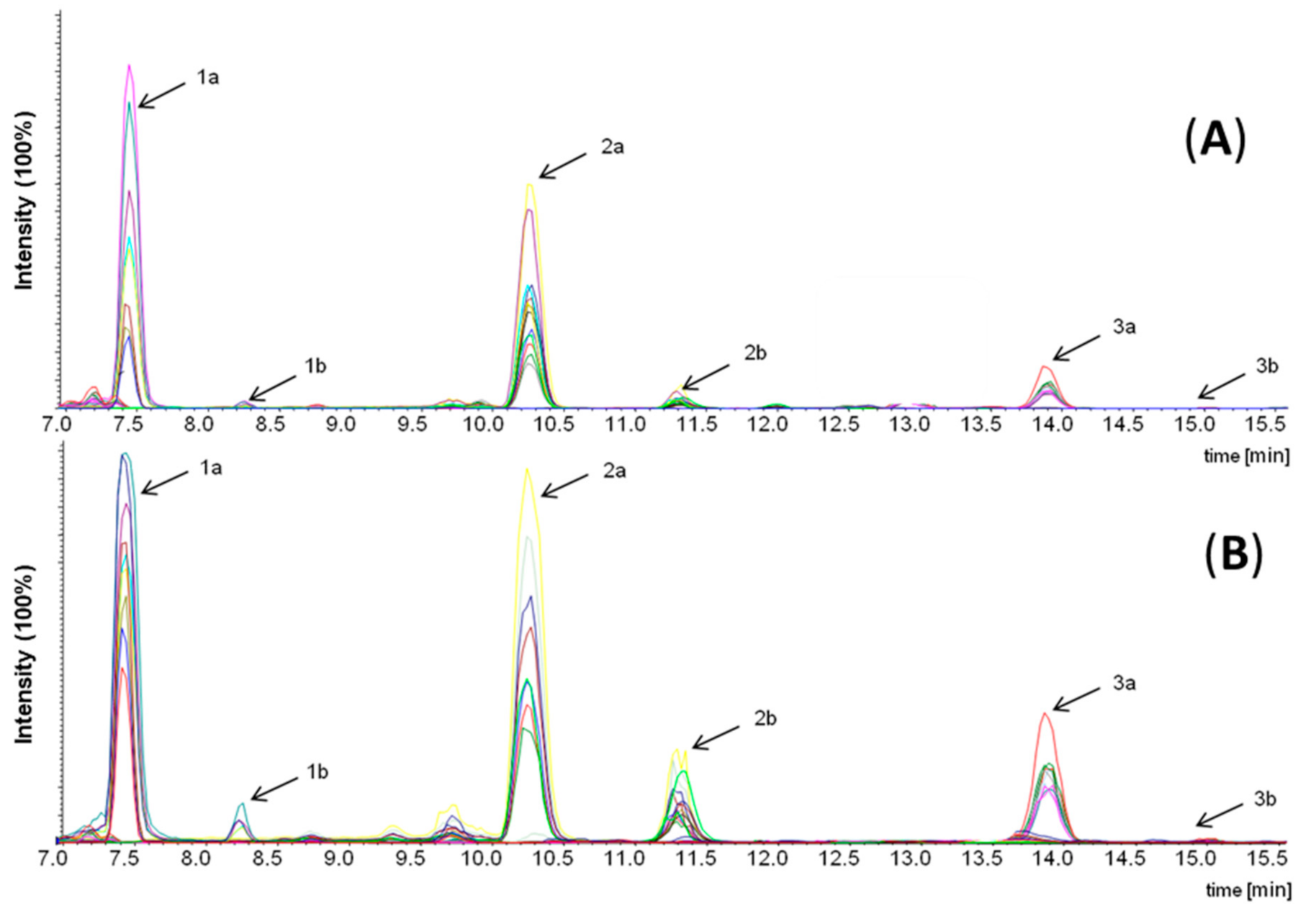
2.9.1. Identification of δ-Lactones in Fraction Ia
2.9.2. Quantification of δ-Lactones in Fraction Ia
2.10. Liquid Chromatography–Mass Spectrometry (LC–MS/MS)
2.11. UPLC/Time-of-Flight Mass Spectrometry (UPLC/TOF-MS)
2.12. Nuclear Magnetic Resonance Spectroscopy (NMR)
3. Results and Discussion
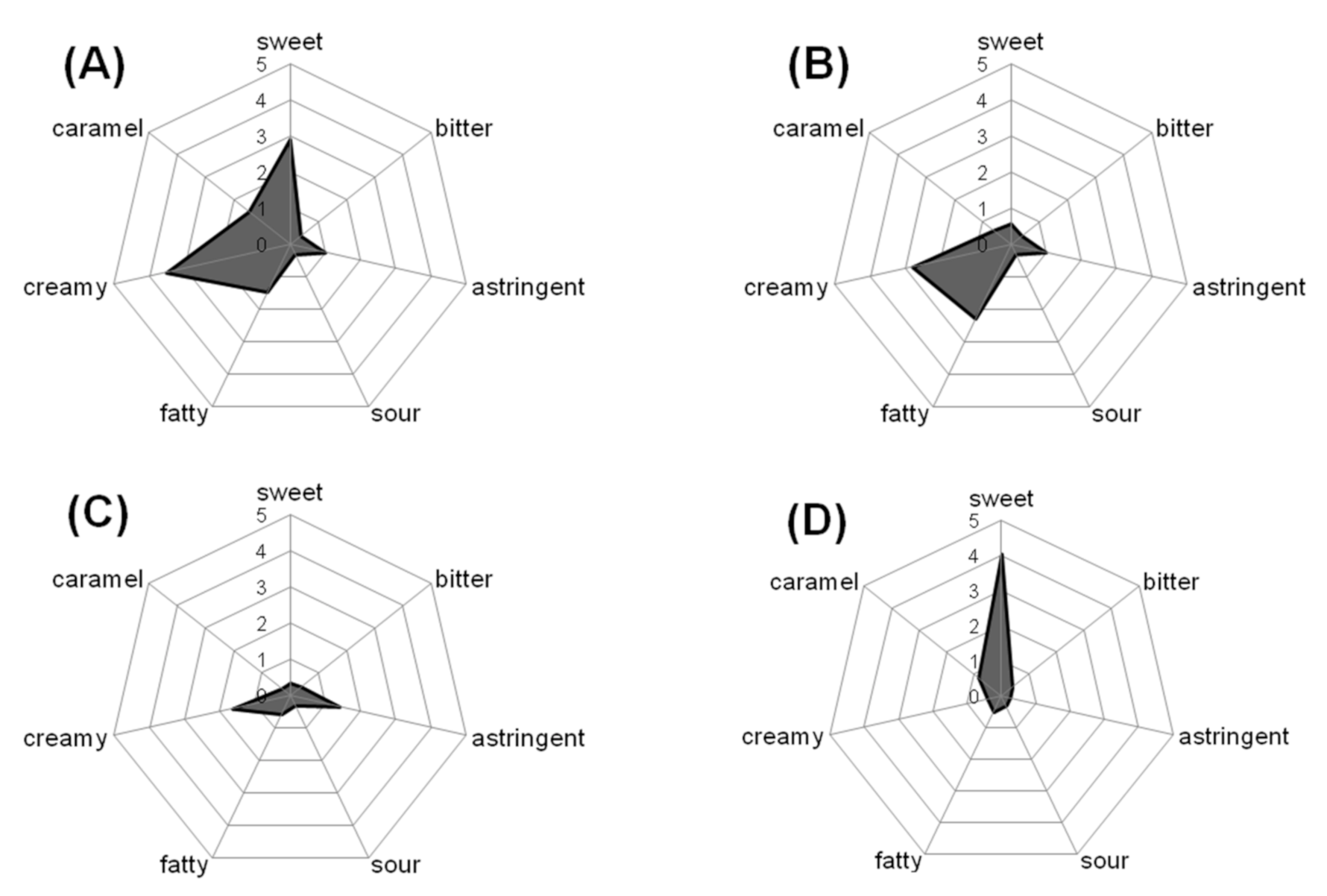
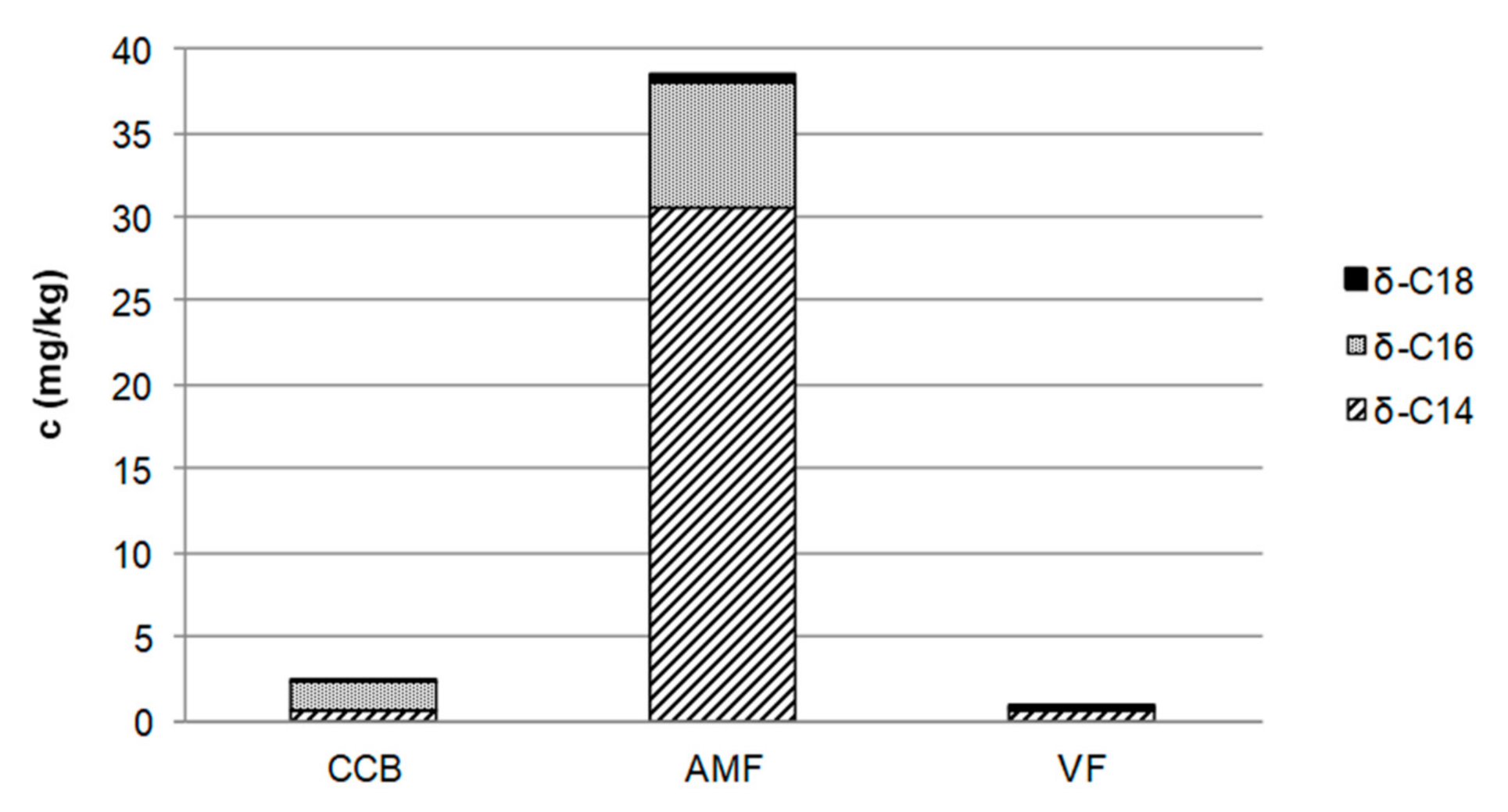
3.1. Identification of Sensory Active Semi-Volatiles
3.2. Sensory Properties of δ-C14/C16/C18-Lactones
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dillinger, T.L.; Barriga, P.; Escárcega, S.; Jimenez, M.; Lowe, D.S.; Grivetti, L.E. Food of the Gods: Cure for Humanity? A Cultural History of the Medicinal and Ritual Use of Chocolate. J. Nutr. 2000, 130, 2057S–2072S. [Google Scholar] [CrossRef]
- Tannenbaum, G. Chocolate: A Marvelous Natural Product of Chemistry. J. Chem. Educ. 2004, 81, 1131. [Google Scholar] [CrossRef]
- Beckett, S.T. Industrial Chocolate Manufacture and Use, 4th ed.; Wiley-Blackwell: Chichester, UK; Ames, IA, USA, 2009. [Google Scholar]
- Beckett, S.T. Is the taste of British milk chocolate different? Int. J. Dairy Technol. 2003, 56, 139–142. [Google Scholar] [CrossRef]
- Tournier, C.; Martin, C.; Guichard, E.; Issanchou, S.; Sulmont-Rossé, C. Contribution to the understanding of consumers’ creaminess concept: A sensory and a verbal approach. Int. Dairy J. 2007, 17, 555–564. [Google Scholar] [CrossRef]
- Frøst, M.; Janhøj, T. Understanding creaminess. Int. Dairy J. 2007, 17, 1298–1311. [Google Scholar] [CrossRef] [Green Version]
- Antmann, G.; Ares, G.; Salvador, A.N.A.; Varela, P.; Fiszman, S.M. Exploring and explaning Creaminess Perception: Consumers‘ underlying concepts. J. Sens. Stud. 2011, 26, 40–47. [Google Scholar] [CrossRef]
- Chen, J.; Eaton, L. Multimodal mechanisms of food creaminess sensation. Food Funct. 2012, 3, 1265–1270. [Google Scholar] [CrossRef]
- De Wijk, R.A.; Van Gemert, L.J.; Terpstra, M.E.; Wilkinson, C.L. Texture of semi-solids; sensory and instrumental measurements on vanilla custard desserts. Food Qual. Prefer. 2003, 14, 305–317. [Google Scholar] [CrossRef]
- Elmore, J.R.; Heymann, H.; Johnson, J.; Hewett, J.E. Preference mapping: Relating acceptance of “creaminess” to a descriptive sensory map of a semi-solid. Food Qual. Prefer. 1999, 10, 465–475. [Google Scholar] [CrossRef]
- Kirkmeyer, S.V. Understanding Creaminess Perception of Dairy Products Using Free-Choice Profiling and Genetic Responsivity to 6-n-Propylthiouracil. Chem. Senses 2003, 28, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Schlutt, B.; Moran, N.; Schieberle, P.; Hofmann, T. Sensory-Directed Identification of Creaminess-Enhancing Volatiles and Semivolatiles in Full-Fat Cream. J. Agric. Food Chem. 2007, 55, 9634–9645. [Google Scholar] [CrossRef]
- Chen, Q.; Deng, H.; Zhao, J.; Lu, Y.; He, M.; Zhai, H. Two efficient four-step routes to marine toxin tanikolide. Tetrahedron 2005, 61, 8390–8393. [Google Scholar] [CrossRef]
- Mino, T.; Masuda, S.; Nishio, M.; Yamashita, M. Synthesis of Lactones by Baeyer-Villiger Oxidation with Magnesium Monoperphthalate Hexahydrate. J. Org. Chem. 1997, 62, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Chen, Q.; Zhao, J.; Luo, S.; Jia, X. A new synthesis of tanikolide. Tetrahedron Lett. 2003, 44, 2893–2894. [Google Scholar] [CrossRef]
- Frank, O.; Kreissl, J.; Daschner, A.; Hofmann, T. Accurate Determination of Reference Materials and Natural Isolates by Means of Quantitative 1H NMR Spectroscopy. J. Agric. Food Chem. 2014, 62, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Galindo, M.M.; Voigt, N.; Stein, J.; Van Lengerich, J.; Raguse, J.-D.; Hofmann, T.; Meyerhof, W.; Behrens, M. G Protein-Coupled Receptors in Human Fat Taste Perception. Chem. Senses 2011, 37, 123–139. [Google Scholar] [CrossRef] [Green Version]
- Kinsella, J.E.; Patton, S.; Dimick, P.S. The flavor potential of milk fat. A review of its chemical nature and biochemical origin. J. Am. Oil Chem. Soc. 1967, 44, 449–454. [Google Scholar] [CrossRef]
- Wyatt, C.J.; Pereira, R.; Day, E. Lactone Precursor in Fresh Milk Fat: Isolation and Characterization of the Precursor. J. Dairy Sci. 1967, 50, 1760–1763. [Google Scholar] [CrossRef]
- Alewijn, M.; Smit, B.; Sliwinski, E.; Wouters, J. The formation mechanism of lactones in Gouda cheese. Int. Dairy J. 2007, 17, 59–66. [Google Scholar] [CrossRef]
- Urbach, G. The Effect of Different Feeds on the Lactone and Methyl Ketone Precursors of Milk Fat. Lebensm. Wiss. Technol. 1982, 15, 62–67. [Google Scholar]
- Keeney, P.G.; Patton, S. The coconut-like flavor defect of milk fat. II. Demonstration of δ-decalactone in dried cream, dry whole milk, and evaporated milk. Int. Dairy J. 1956, 39, 1114–1119. [Google Scholar]
- Boldingh, J.; Taylor, R.J. Trace Constituents of Butterfat. Nat. Cell Biol. 1962, 194, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Dimick, P.S.; Walker, N.J.; Patton, S. Occurence and biochemical origin of aliphatic lactones in milk fat. A Review. J. Agric. Food Chem. 1969, 17, 649–655. [Google Scholar] [CrossRef]
- Dimick, P.S.; Patton, S.; Kinsella, J.E.; Walker, N.J. The prevalence of aliphatic delta-lactones or their precursors in animal fats. Lipids 1966, 1, 387–390. [Google Scholar] [CrossRef]
- Patton, S.; Keeney, P.G.; Herald, C.T. The Role of Lactones in Flavor Deterioration of Milk Fat. Science 1954, 119, 218–219. [Google Scholar] [CrossRef]
- Urbach, G.; Stark, W. The effect of diet on the γ- and δ-lactone and methyl ketone potentials of bovine butterfat. J. Dairy Res. 1978, 45, 223–229. [Google Scholar] [CrossRef]
- Marquez-Ruiz, G.; Rodríguez-Pino, V.; De La Fuente, M. Ángel Determination of 10-hydroxystearic, 10-ketostearic, 8-hydroxypalmitic, and 8-ketopalmitic acids in milk fat by solid-phase extraction plus gas chromatography-mass spectrometry. J. Dairy Sci. 2011, 94, 4810–4819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cozma, A.; Miere, D.; Filip, L.; Andrei, S.; Banc, R.; Loghin, F. A Review of the Metabolic Origins of Milk Fatty Acids. Not. Sci. Biol. 2013, 5, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Barber, M.C.; Clegg, R.A.; Travers, M.T.; Vernon, R.G. Lipid metabolism in the lactating mammary gland. Biochim. Biophys. Acta Lipids Lipid Metab. 1997, 1347, 101–126. [Google Scholar] [CrossRef]
- Harvatine, K.; Boisclair, Y.; Bauman, D. Recent advances in the regulation of milk fat synthesis. Animal 2009, 3, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Shingfield, K.J.; Bernard, L.; Leroux, C.; Chilliard, Y. Role of trans fatty acids in the nutritional regulation of mammary lipogenesis in ruminants. Animal 2010, 4, 1140–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
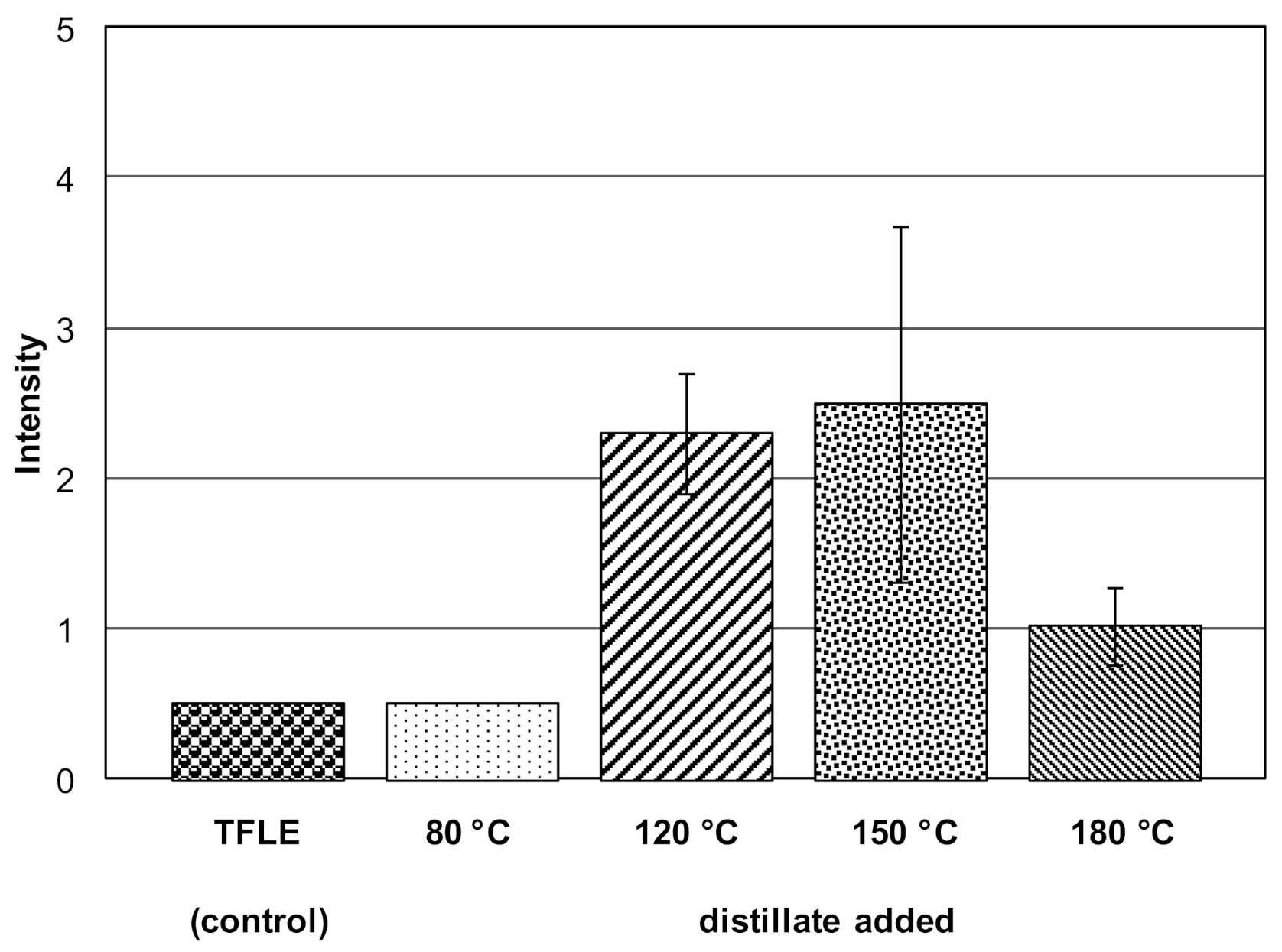
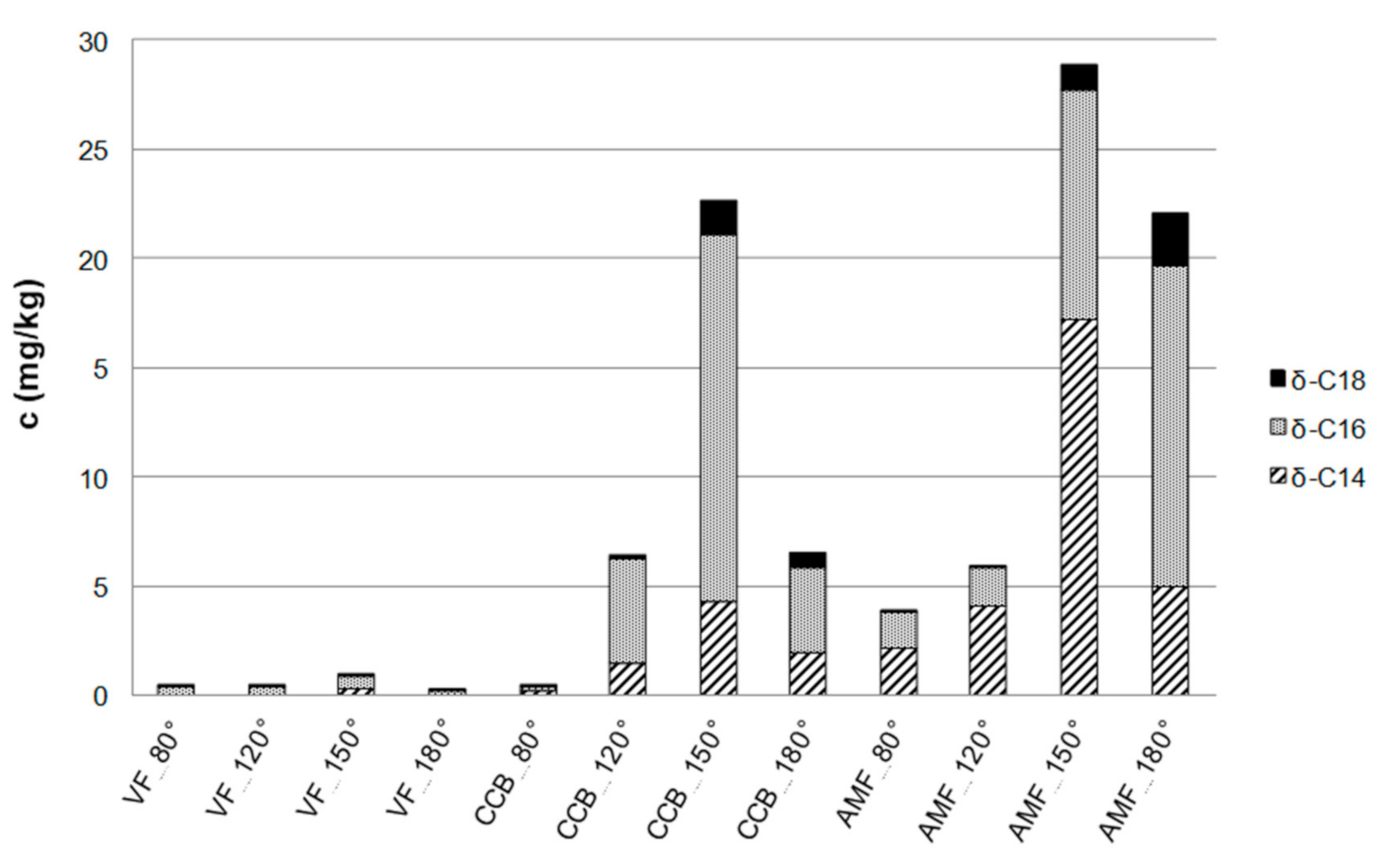
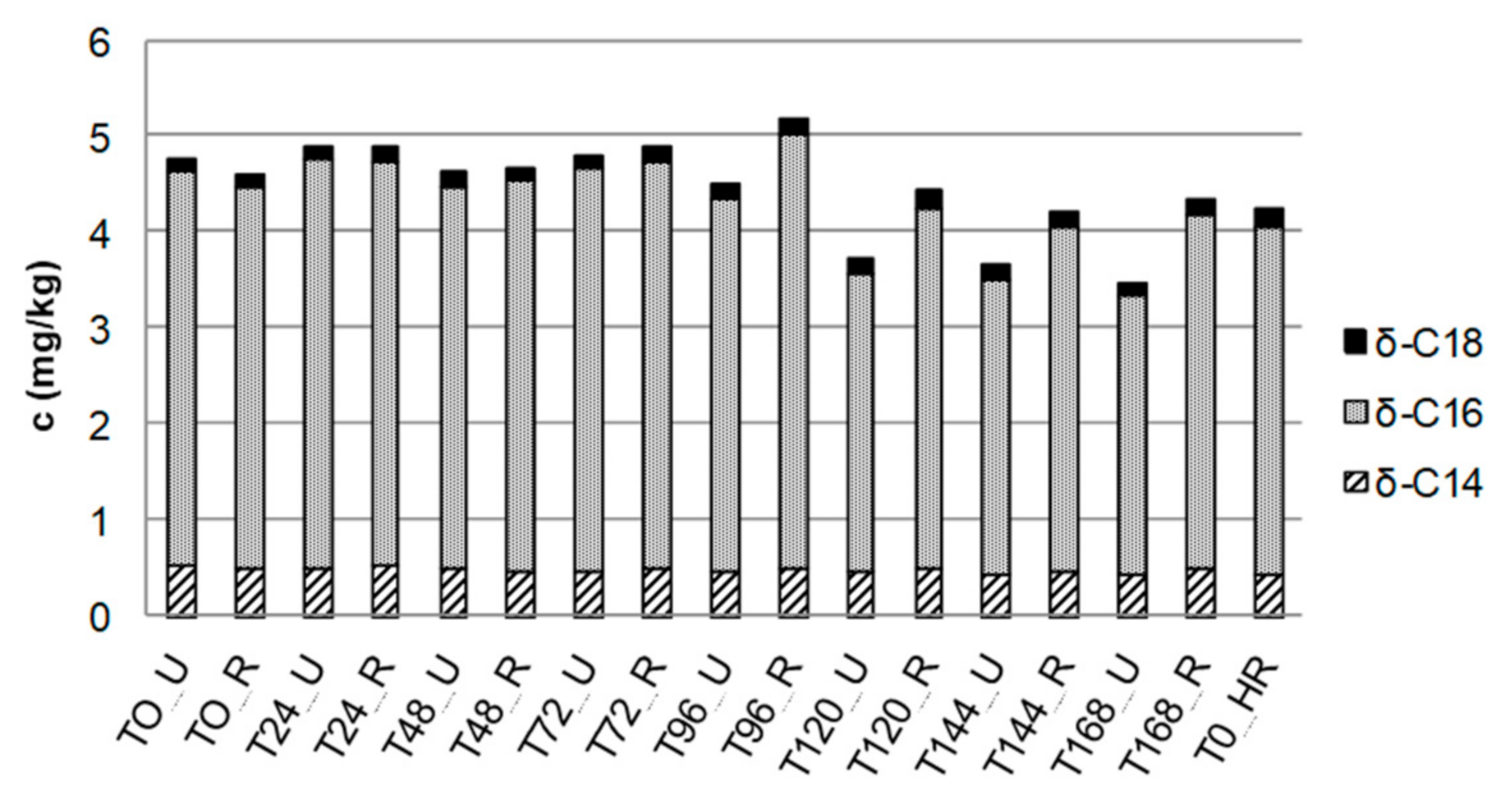
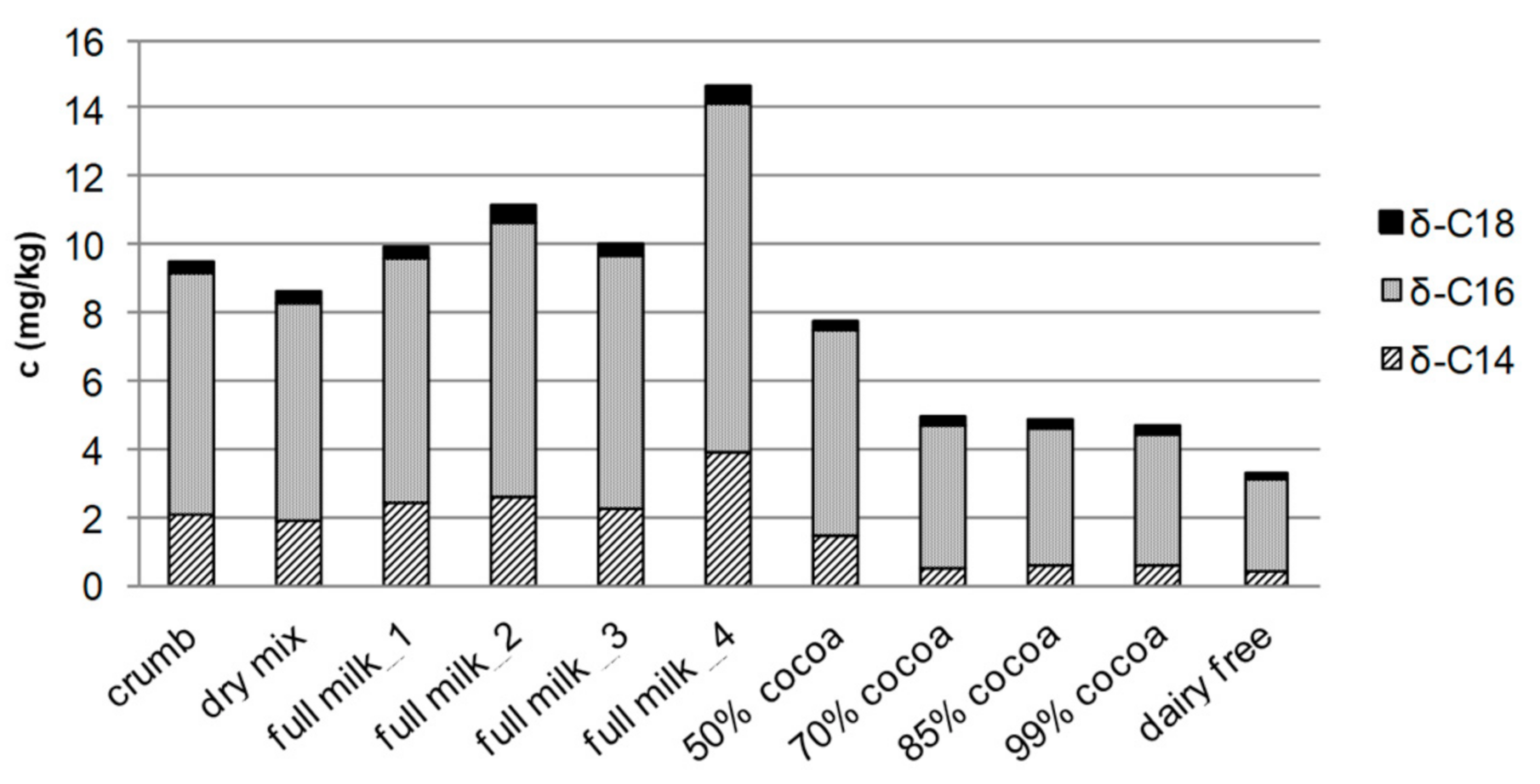
| High-Vacuum Distillation | Temperature (°C) | |||
|---|---|---|---|---|
| Fraction | 80 | 120 | 150 | 180 |
| cocoa butter | - | - a | - b | - |
| anhydrous milk fatb c | +/- | ++ | ++ | ++ |
| vegetable fat | - | - | - | - |
| Lactone | Recognition Threshold (µmol/kg) a | Flavor Quality (Retro-Nasal) | Mouth Coating Effect |
|---|---|---|---|
| δ-tetradecalactone | 40 | creamy | sticky |
| δ-hexadecalactone | 32 | melted butter | film-forming |
| δ-octadecalactone | 29 | fatty | long-lasting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samfaß, J.; Stark, T.D.; Hofmann, T.F. Sensory-Directed Identification of Creaminess-Enhancing Semi-Volatile Lactones in Crumb Chocolate. Foods 2021, 10, 1483. https://doi.org/10.3390/foods10071483
Samfaß J, Stark TD, Hofmann TF. Sensory-Directed Identification of Creaminess-Enhancing Semi-Volatile Lactones in Crumb Chocolate. Foods. 2021; 10(7):1483. https://doi.org/10.3390/foods10071483
Chicago/Turabian StyleSamfaß, Julia, Timo D. Stark, and Thomas F. Hofmann. 2021. "Sensory-Directed Identification of Creaminess-Enhancing Semi-Volatile Lactones in Crumb Chocolate" Foods 10, no. 7: 1483. https://doi.org/10.3390/foods10071483
APA StyleSamfaß, J., Stark, T. D., & Hofmann, T. F. (2021). Sensory-Directed Identification of Creaminess-Enhancing Semi-Volatile Lactones in Crumb Chocolate. Foods, 10(7), 1483. https://doi.org/10.3390/foods10071483






