Antilisterial Effect of a Natural Formulation Based on Citrus Extract in Ready-To-Eat Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Used Growth Media
2.2. Citrus Extract
2.3. In Vitro Tests for the Evaluation of the Antimicrobial Activity
2.4. Challenge Tests
- The carne mechada samples were prepared from fresh pig head supplied by a local butcher shop (Alhendín, Spain). The piece of meat was cleaned, weighted, and cut. For the preparation of the brine, we solved 105 g in water of commercial preparation composed of salt, maltodextrin, corn starch, and sodium ascorbate (ref. 110249, from the DOMCA trademark, Granada, Spain). Then, the brine was injected inside the meat piece using a roving machine with an individual injector (Suministros Lizondo, Barcelona, Spain). The meat was then transferred to the mixing drum (Mixer RM-20, MAINCA SL, Barcelona, Spain). A potato starch solution (5%) was added and subsequently homogenized for 15 min. Then, the piece was manually stuffered (EC-12, MAINCA SL) in polyamide casing (FIBRACO, Barcelona, Spain) and finally cooked at 70–75 °C for 2 h. After 24 h, the meat was cut into 6–8 mm thickness slices, which were then divided into expanded polystyrene trays (135 × 80 mm, Bandesur SA, Jaén, Spain). Afterwards, these were vacuumed (Tecnotrip EVT-10–2-CV-SC, Barcelona, Spain) for further storage in refrigeration.
- Salami samples were prepared from pork supplied by a local butcher (Alhendín, Spain). Previously, 600 g of lean were frozen at −6 °C and chopped in a meat grinder (CUTTER (MAINCA SL) at 1500 rpm. A total of 130 g of ice and 70 g/kg of a commercial formulation based on salt, lactose, dextrin, pepper flavor, meat flavor, sodium ascorbate, and red dye were then added (ref. 70170162, DOMCA, Granada, Spain) and minced at 300 rpm. A total of 200 g/kg of frozen bacon was added and minced at 1500 rpm until a uniform dough was obtained. After that, this mixture was stuffed using a manual casing stuffer (FIBRAN) and refrigerated at 4–6 °C for 8–10 days, with a humidity of 90–95%. The sausage was then stored in the drying room for 10 days at 12–15 °C and humidity of 75%. Before use, salami was cut into 6–8 mm thickness slices.
- The piece of fresh salmon (Salmo salar) was supplied by a local fishmonger (Alhendín, Spain). It was cleaned by removing excess fat and cut into symmetrical heavy pieces of 5 × 5 cm (25 cm2).
- Lettuce of the iceberg variety was supplied by a local fruit store (Alhendín, Spain). The leaves were washed with sterile water and cut into symmetrical pieces of 5 × 5 cm (25 cm2).
- Home-made mozzarella cheese pearls made of buffalo milk were supplied by a local store (Alhendín, Spain). The original brine was discarded and a brine with sterile water at 10% NaCl was added. After applying treatments to the brines and their subsequent homogenization, we added 20 mozzarella pearls per batch and stored them at 4 °C for 25 days.
2.5. Monitoring
2.6. Statistical Analysis
3. Results
3.1. In Vitro Tests to Evaluate Antimicrobial Activity
3.2. Efficacy Evaluation Trials in Food
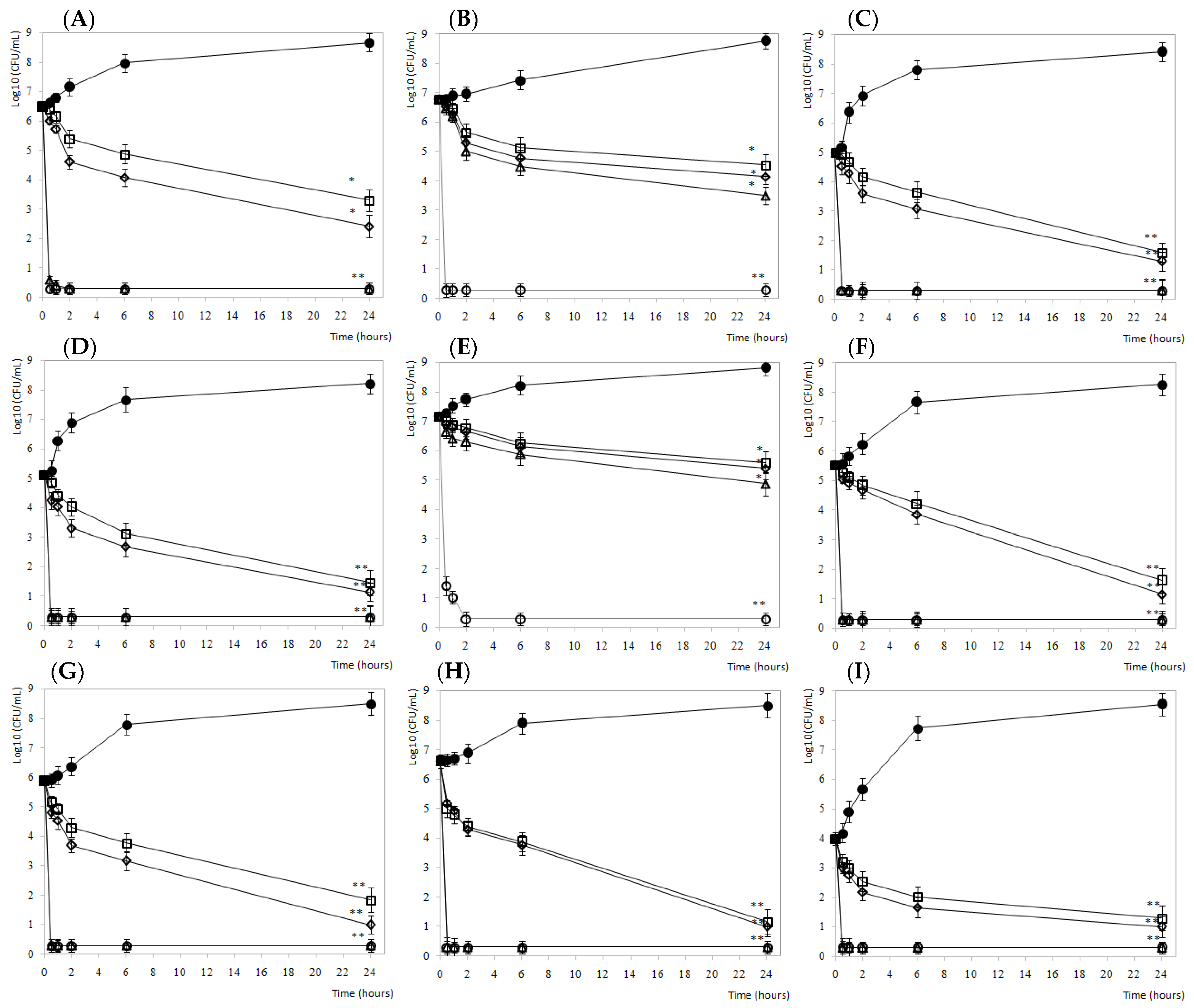
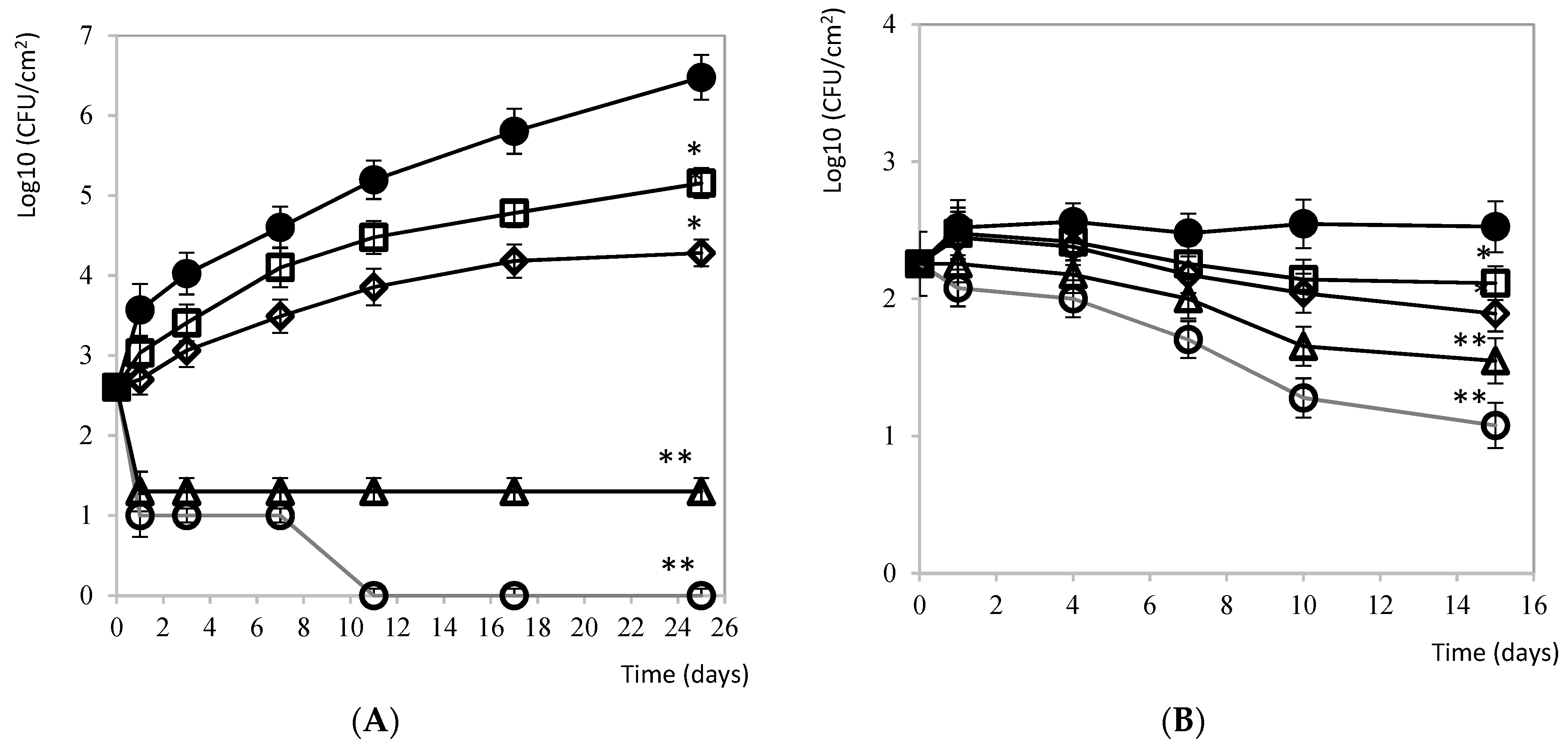
3.2.1. Carne Mechada
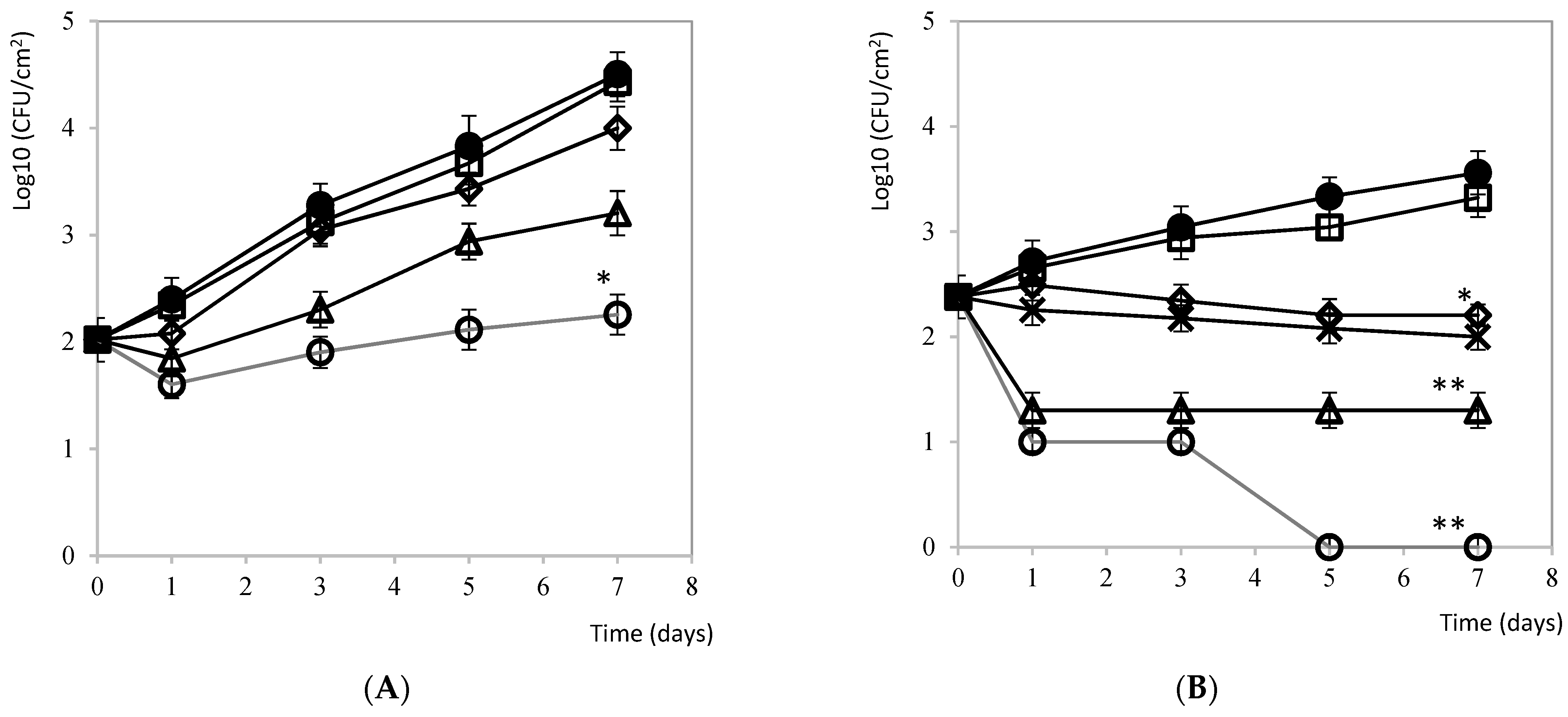
3.2.2. Salami
3.2.3. Fresh Salmon
3.2.4. Lettuce
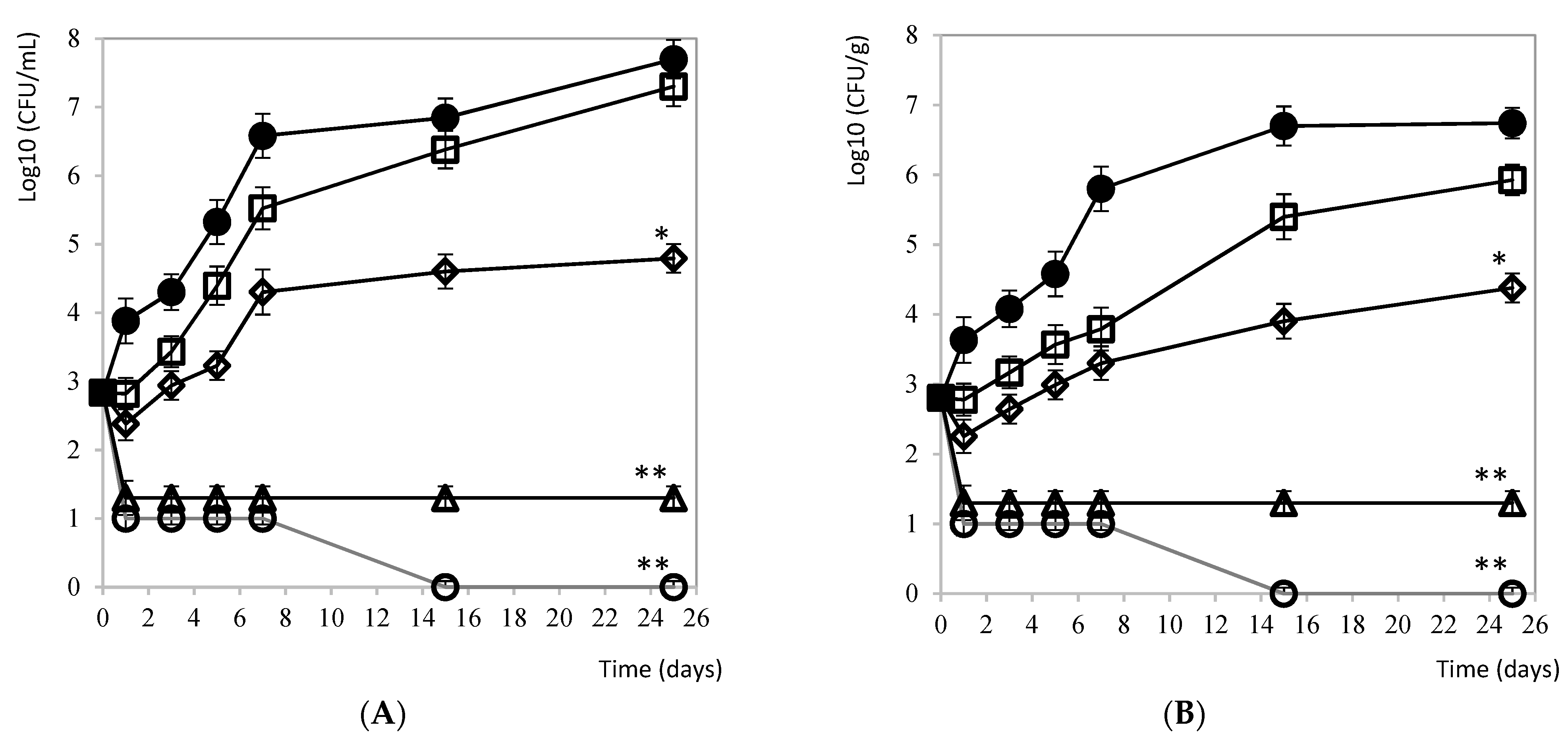
3.2.5. Mozzarella Cheese and Brine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 5926. [Google Scholar] [CrossRef]
- Luque-Sastre, L.; Arroyo, C.; Fox, E.M.; McMahon, B.J.; Bai, L.; Li, F.; Fanning, S. Antimicrobial resistance in Listeria species. In Antimicrobial Resistance in Bacteria from Livestock and Companion Animals; John Wiley & Sons: Hoboken, NJ, USA, 2018; Volume 6, pp. 237–259. [Google Scholar]
- Jami, M.; Ghanbari, M.; Zunabovic, M.; Domig, K.J.; Kneifel, W. Listeria Monocytogenes in Aquatic food products-A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 798–813. [Google Scholar] [CrossRef]
- Kurpas, M.; Wieczorek, K.; Osek, J. Ready-to-eat meat products as a source of Listeria monocytogenes. J. Vet. Res. 2018, 62, 49–55. [Google Scholar] [CrossRef] [PubMed]
- ELIKA Seguridad Alimentaria|Informe anual RASFF 2019 Sobre Alertas Alimentarias—ELIKA Seguridad Alimentaria. Available online: https://seguridadalimentaria.elika.eus/informe-anual-rasff-2019-sobre-alertas-alimentarias/ (accessed on 16 June 2021).
- Tucci, P.; Centorotola, G.; Salini, R.; Iannetti, L.; Sperandii, A.F.; D’Alterio, N.; Migliorati, G.; Pomilio, F. Challenge test studies on Listeria monocytogenes in ready-to-eat iceberg lettuce. Food Sci. Nutr. 2019, 7, 3845–3852. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Maisanaba, S.; Puerto, M.; Prieto, A.I.; Gutiérrez-Praena, D.; Jos, A.; Cameán, A.M. In vitro toxicological evaluation of essential oils and their main compounds used in active food packaging: A review. Food Chem. Toxicol. 2015, 81, 9–27. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.L.; Marceddu, S.; Venditti, T.; Pintore, G.; Zara, G.; Mannazzu, I.; Budroni, M.; Zara, S. Antimicrobial activity of gaseous Citrus limon var pompia leaf essential oil against Listeria monocytogenes on ricotta salata cheese. Food Microbiol. 2020, 87, 103386. [Google Scholar] [CrossRef]
- Wang, F.; You, H.; Guo, Y.; Wei, Y.; Xia, P.; Yang, Z.; Ren, M.; Guo, H.; Han, R.; Yang, D. Essential oils from three kinds of fingered citrons and their antibacterial activities. Ind. Crops Prod. 2020, 147, 112172. [Google Scholar] [CrossRef]
- Manso, S.; Pezo, D.; Gómez-Lus, R.; Nerín, C. Diminution of aflatoxin B1 production caused by an active packaging containing cinnamon essential oil. Food Control 2014, 45, 101–108. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef]
- Medina-Rodríguez, A.C.; Ávila-Sierra, A.; Ariza, J.J.; Guillamón, E.; Baños-Arjona, A.; Vicaria, J.M.; Jurado, E. Clean-in-place disinfection of dual-species biofilm (Listeria and Pseudomonas) by a green antibacterial product made from citrus extract. Food Control 2020, 118, 107422. [Google Scholar] [CrossRef]
- Harich, M.; Maherani, B.; Salmieri, S.; Lacroix, M. Antibacterial activity of cranberry juice concentrate on freshness and sensory quality of ready to eat (RTE) foods. Food Control 2017, 75, 134–144. [Google Scholar] [CrossRef]
- Tagg, J.R.; Mcgiven, A.R. Assay System for Bacteriocins. Appl. Microbiol. 1971, 21, 943. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Patel, J.B.; Bobenchik, A.M.; Campeau, S.; Cullen, S.K.; Galas, M.F.; Gold, H.; Humphries, R.M.; Kirn, T.J.; Lewis Ii, J.S.; et al. M100 Performance Standards for Antimicrobial Susceptibility Testing a CLSI Supplement for Global Application. Performance Standards for Antimicrobial Susceptibility Testing Performance Standards for Antimicrobial Susceptibility Testing; Standards Institute: Wayne, PA, USA, 2020; ISBN 9781684400324. [Google Scholar]
- Chen, H.; Zhong, Q. Lactobionic acid enhances the synergistic effect of nisin and thymol against Listeria monocytogenes Scott A in tryptic soy broth and milk. Int. J. Food Microbiol. 2017, 260, 36–41. [Google Scholar] [CrossRef]
- Guerillot, F.; Carret, G.; Flandrois, J.P. Mathematical model for comparison of time-killing curves. Antimicrob. Agents Chemother. 1993, 37, 1685–1689. [Google Scholar] [CrossRef][Green Version]
- Baños, A.; García-López, J.D.; Núñez, C.; Martínez-Bueno, M.; Maqueda, M.; Valdivia, E. Biocontrol of Listeria monocytogenes in fish by enterocin AS-48 and Listeria lytic bacteriophage P100. LWT Food Sci. Technol. 2016, 66, 672–677. [Google Scholar] [CrossRef]
- Hua, Z.; Korany, A.M.; El-Shinawy, S.H.; Zhu, M.J. Comparative evaluation of different sanitizers against listeria monocytogenes biofilms on major food-contact surfaces. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Bachelli, M.L.B.; Amaral, R.D.Á.; Benedetti, B.C. Alternative sanitization methods for minimally processed lettuce in comparison to sodium hypochlorite. Braz. J. Microbiol. 2013, 44, 673–678. [Google Scholar] [CrossRef]
- Madigan, M.T.; Jung, D.O. An overview of purple bacteria: Systematics, physiology, and habitats. In The Purple Phototrophic Bacteria; Springer: Dordrecht, The Netherlands, 2009; pp. 1–15. [Google Scholar]
- Gutiérrez-del-Río, I.; Fernández, J.; Lombó, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. BioFactors 2017, 43, 495–506. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Park, I.W.; Lee, J.I.; Shukla, S.; Nile, S.H.; Chun, H.S.; Khan, I.; Oh, S.Y.; Lee, H.; Huh, Y.S.; et al. Antioxidant and antimicrobial efficacy of a biflavonoid, amentoflavone from Nandina domestica in vitro and in minced chicken meat and apple juice food models. Food Chem. 2019, 271, 239–247. [Google Scholar] [CrossRef]
- Oficial, D. REGLAMENTO (CE) no 2073/2005 DE LA COMISIÓN de 15 de noviembre de 2005 relativo a los criterios microbiológicos aplicables a los productos alimenticios. D. Of. Unión Eur. 2005, L 338, 1–26. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017, 16. [Google Scholar] [CrossRef]
- Mhalla, D.; Bouaziz, A.; Ennouri, K.; Chawech, R.; Smaoui, S.; Jarraya, R.; Tounsi, S.; Trigui, M. Antimicrobial activity and bioguided fractionation of Rumex tingitanus extracts for meat preservation. Meat Sci. 2017, 125, 22–29. [Google Scholar] [CrossRef]
- Shukla, S.; Ahirwal, L.; Bajpai, V.K.; Huh, Y.S.; Han, Y.-K. Growth inhibitory effects of adhatoda vasica and its potential at reducing listeria monocytogenes in chicken meat. Front. Microbiol. 2017, 8, 1260. [Google Scholar] [CrossRef]
- Dussault, D.; Vu, K.D.; Lacroix, M. In vitro evaluation of antimicrobial activities of various commercial essential oils, oleoresin and pure compounds against food pathogens and application in ham. Meat Sci. 2014, 96, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.W. Detection and Isolation of Listeria monocytogenes from Food Samples: Implications of Sublethal Injury. J. AOAC Int. 2002, 85, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Mejlholm, O.; Bøknæs, N.; Dalgaard, P. Development and validation of a stochastic model for potential growth of Listeria monocytogenes in naturally contaminated lightly preserved seafood. Food Microbiol. 2015, 45, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Lee, H.W.; Yuk, H.G. Antimicrobial efficacy of Cinnamomum javanicum plant extract against Listeria monocytogenes and its application potential with smoked salmon. Int. J. Food Microbiol. 2017, 260, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Hadjivasileiou, L.; Primikyri, A.; Michael, C.; Botsaris, G.; Tzakos, A.G.; Gerothanassis, I.P. Valorization of carob fruit residues for the preparation of novel bi-functional polyphenolic coating for food packaging applications. Molecules 2019, 24, 3162. [Google Scholar] [CrossRef]
- Yu, H.H.; Song, M.W.; Song, Y.J.; Lee, N.K.; Paik, H.D. Antibacterial Effect of a Mixed Natural Preservative against Listeria monocytogenes on Lettuce and Raw Pork Loin. J. Food Prot. 2019, 82, 2001–2006. [Google Scholar] [CrossRef]
- Vázquez-Armenta, F.J.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Nazzaro, F.; Fratianni, F.; Ayala-Zavala, J.F. Antibacterial and antioxidant properties of grape stem extract applied as disinfectant in fresh leafy vegetables. J. Food Sci. Technol. 2017, 54, 3192–3200. [Google Scholar] [CrossRef]
- Larson, A.E.; Johnson, E.A.; Nelson, J.H. Survival of Listeria monocytogenes in commercial cheese brines. J. Dairy Sci. 1999, 82, 1860–1868. [Google Scholar] [CrossRef]
- Valderrama, W.B.; Mills, E.W.; Cutter, C.N. Efficacy of chlorine dioxide against listeria monocytogenes in brine chilling solutions. J. Food Prot. 2009, 72, 2272–2277. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. Potential application of spice and herb extracts as natural preservatives in cheese. J. Med. Food 2011, 14, 284–290. [Google Scholar] [CrossRef]
- Tayel, A.A.; Hussein, H.; Sorour, N.M.; El-Tras, W.F. Foodborne pathogens prevention and sensory attributes enhancement in processed cheese via flavoring with plant extracts. J. Food Sci. 2015, 80, M2886–M2891. [Google Scholar] [CrossRef]
- Pedonese, F.; Verani, G.; Torracca, B.; Turchi, B.; Felicioli, A.; Nuvoloni, R. Effect of an Italian propolis on the growth of Listeria monocytogenes, staphylococcus aureus and bacillus cereus in milk and whey cheese. Ital. J. Food Saf. 2019, 8, 218–222. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Chen, Y.; Ma, X.; Xia, M. Chitosan and procyanidin composite films with high antioxidant activity and pH responsivity for cheese packaging. Food Chem. 2021, 338, 128013. [Google Scholar] [CrossRef]
| Strain Used for Vitro Test and Challenge Test | Isolated |
| Listeria monocytogenes CECT 4032 | Clinical isolate of meningitis |
| Listeria monocytogenes CECT 5366 | Clinical isolate |
| Listeria monocytogenes DMC 1-23 | Minced meat isolated |
| Listeria monocytogenes DMC 3-17 | Fresh cheese isolated |
| Strain Used for In Vitro Test | Isolated |
| Listeria innocua CECT 4030 | Fresh cheese isolated |
| Listeria innocua DMC 4 | Minced meat isolated |
| Listeria innocua DMC 5-1 | Fresh cheese isolated |
| Listeria innocua DMC 6-2 | Food industry isolated |
| Listeria innocua DMC 7-3 | Food industry isolated |
| Strain | MBC (mg/L) | Inhibition Zone (mm) | Activity on Liquid Medium (mm) |
|---|---|---|---|
| Listeria monocytogenes CECT 4032 | 5000 | 13 ± 1 | 15 ± 1 |
| Listeria monocytogenes CECT 5366 | 5000 | 13 ± 1 | 13 ± 1 |
| Listeria monocytogenes DMC 1-23 | 5000 | 12 ± 0 | 14 ± 1 |
| Listeria monocytogenes DMC 3-17 | 5000 | 12 ± 0 | 14 ± 1 |
| Listeria innocua CECT 4030 | 5000 | 13 ± 1 | 17 ± 1 |
| Listeria innocua DMC 4 | 7812.5 | 12 ± 1 | 15 ± 0 |
| Listeria innocua DMC 5-1 | 15,625 | 12 ± 1 | 13 ± 1 |
| Listeria innocua DMC 6-2 | 7812.5 | 11 ± 2 | 13 ± 1 |
| Listeria innocua DMC 7-3 | 5000 | 14 ± 1 | 15 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariza, J.J.; García-López, D.; Sánchez-Nieto, E.; Guillamón, E.; Baños, A.; Martínez-Bueno, M. Antilisterial Effect of a Natural Formulation Based on Citrus Extract in Ready-To-Eat Foods. Foods 2021, 10, 1475. https://doi.org/10.3390/foods10071475
Ariza JJ, García-López D, Sánchez-Nieto E, Guillamón E, Baños A, Martínez-Bueno M. Antilisterial Effect of a Natural Formulation Based on Citrus Extract in Ready-To-Eat Foods. Foods. 2021; 10(7):1475. https://doi.org/10.3390/foods10071475
Chicago/Turabian StyleAriza, Juan José, David García-López, Esperanza Sánchez-Nieto, Enrique Guillamón, Alberto Baños, and Manuel Martínez-Bueno. 2021. "Antilisterial Effect of a Natural Formulation Based on Citrus Extract in Ready-To-Eat Foods" Foods 10, no. 7: 1475. https://doi.org/10.3390/foods10071475
APA StyleAriza, J. J., García-López, D., Sánchez-Nieto, E., Guillamón, E., Baños, A., & Martínez-Bueno, M. (2021). Antilisterial Effect of a Natural Formulation Based on Citrus Extract in Ready-To-Eat Foods. Foods, 10(7), 1475. https://doi.org/10.3390/foods10071475






