UV-C LED Irradiation Reduces Salmonella on Chicken and Food Contact Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures
2.2. UV-C LED Light and Surfaces Subjected to Irradiation
2.3. Chicken Inoculation and Treatment
2.4. Stainless Steel Inoculation and Treatments
2.5. High Density Polyethylene Inoculation and Treatments
2.6. Analysis of Chicken Rinse Fluid
2.7. Microbial Analysis
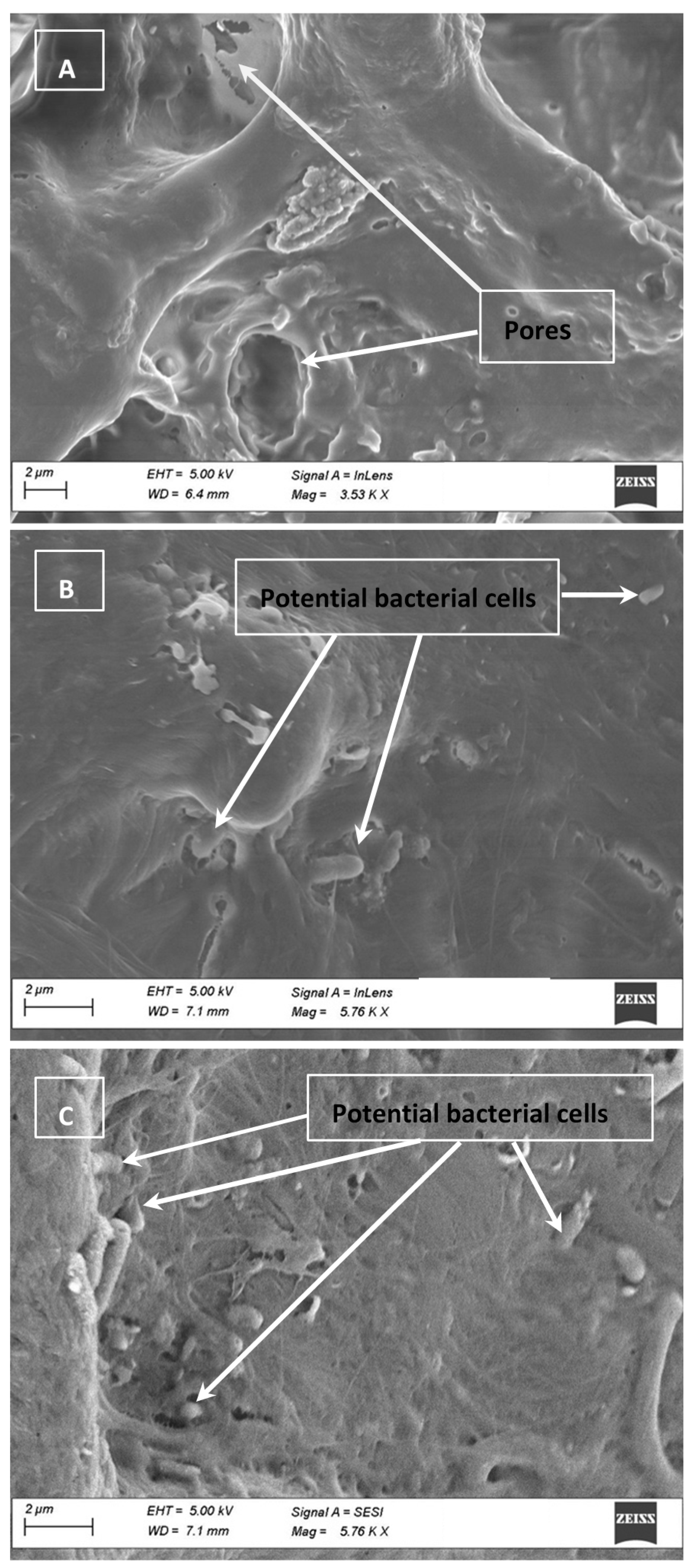
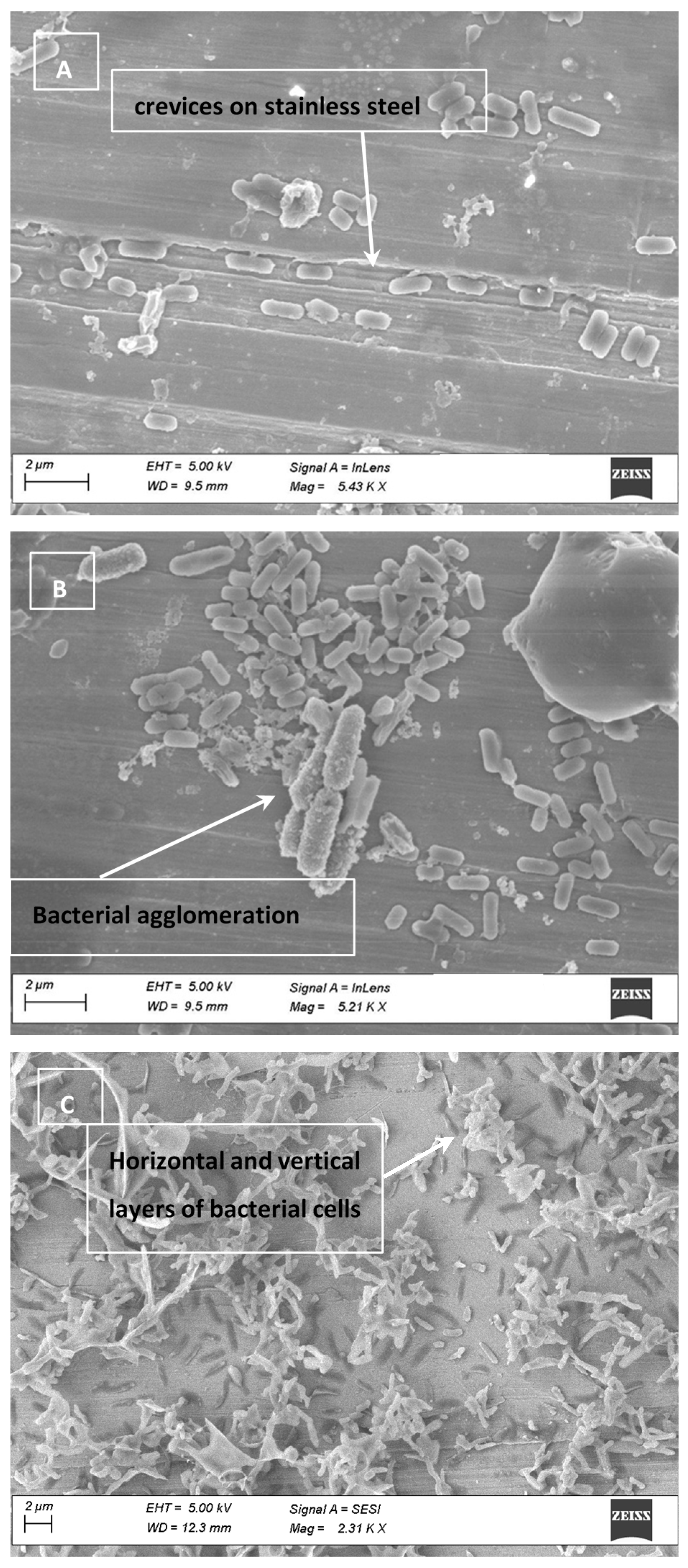
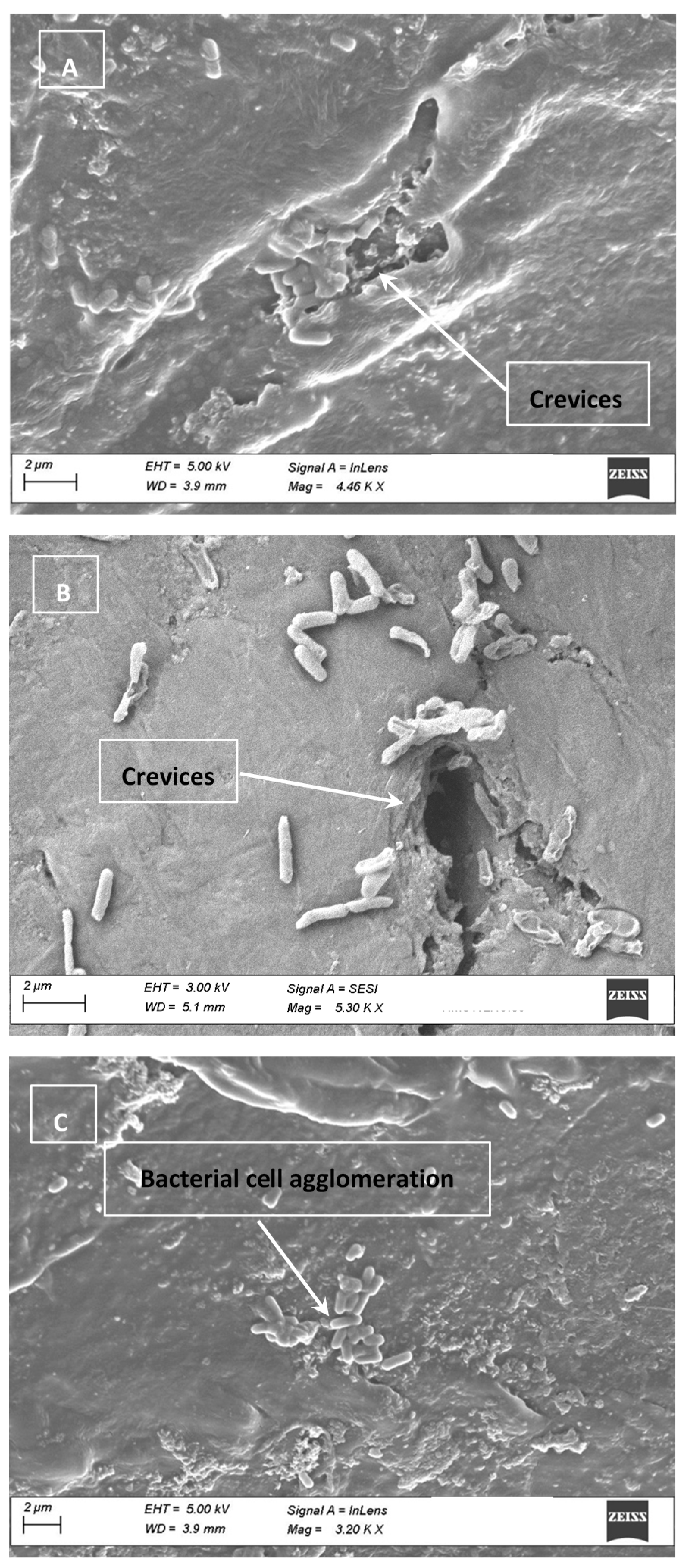
2.8. Electron Micrographs
2.9. Statistical Analysis
3. Results and Discussion
3.1. Boneless Skinless Chicken Breast (CB)
3.2. Stainless Steel (SS)
3.3. High Density Polyethylene (HD)
3.4. Cell Clumping and SEM Images
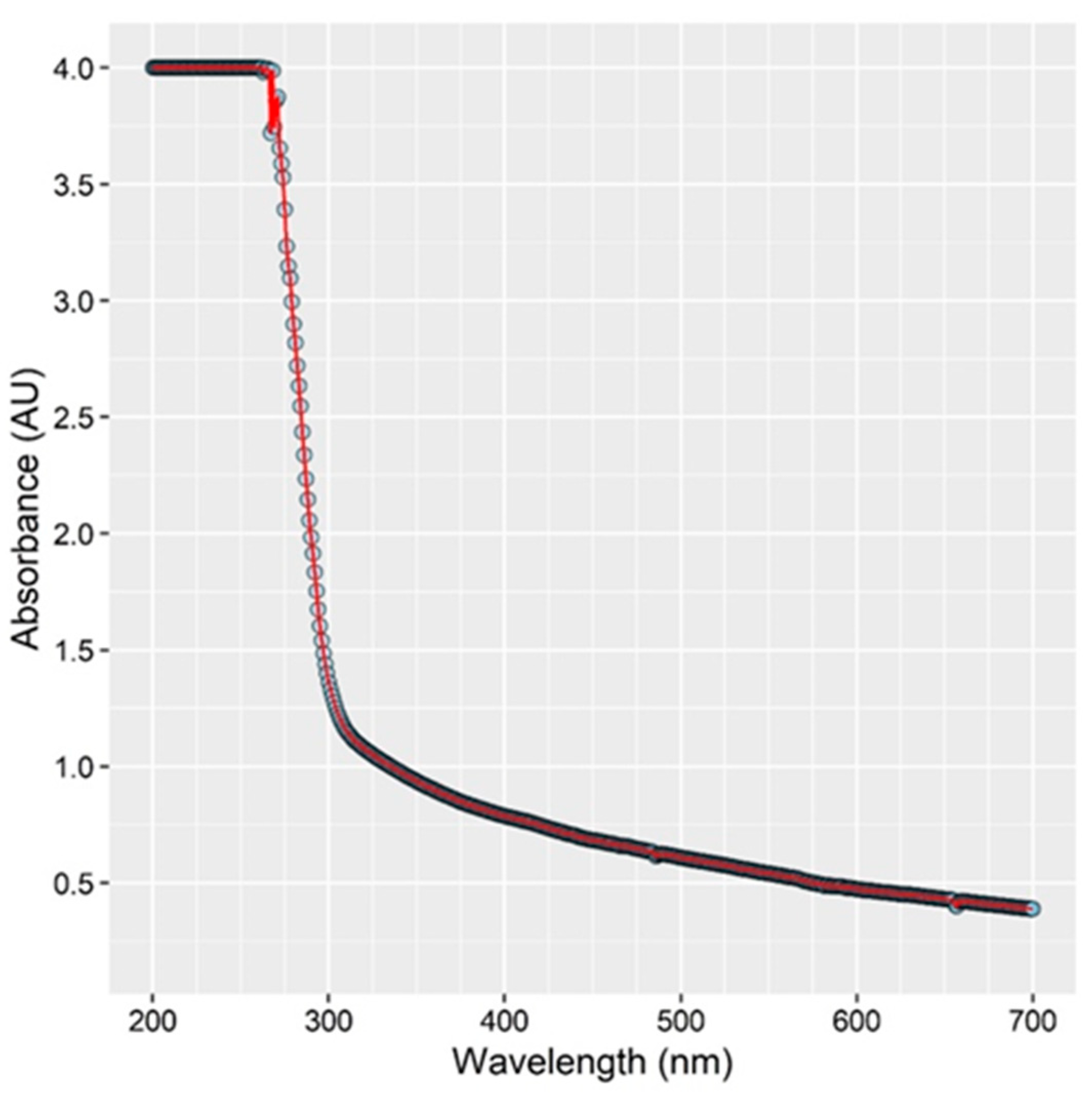
3.5. Absorption of UV Light by Chicken Rinse Fluid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA; FSIS. Serotypes Profile of Salmonella Isolates from Meat and Poultry Products January 1998 through December 2014. 2016. Available online: https://www.fsis.usda.gov/wps/wcm/connect/3866026a-582d-4f0e-a8ce-851b39c7390f/Salmonella-Serotype-Annual-2014.pdf?MOD=AJPERES (accessed on 17 April 2021).
- Tack, D.M.; Ray, L.; Griffin, P.M.; Cieslak, P.; Dunn, J.; Rissman, T.; Jervis, R.; Lathrop, S.; Muse, A.; Duwell, M.; et al. Preliminary Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2016–2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 509–514. [Google Scholar] [CrossRef]
- Grant, A.; Parveen, S.; Schwarz, J.; Hashem, F.; Vimini, B. Reduction of Salmonella in ground chicken using a bacteriophage. Poult. Sci. J. 2017, 96, 2845–2852. [Google Scholar] [CrossRef]
- Cox, N.A.; Richardson, L.J.; Cason, J.A.; Buhr, R.J.; Vizzier-Thaxton, Y.; Smith, D.P.; Fedorka-Cray, P.J.; Romanenghi, C.P.; Pereira, L.V.; Doyle, M.P. Comparison of neck skin excision and whole carcass rinse sampling methods for microbiological evaluation of broiler carcasses before and after immersion chilling. J. Food Prot. 2010, 73, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). National Center for Emerging and Zoonotic Infectious Diseases (NCEZID). December 2018. Available online: https://wwwn.cdc.gov/norsdashboard/ (accessed on 17 April 2019).
- Whyte, P.; Collins, J.D.; McGill, K.; Monahan, C.; O’Mahony, H. Quantitative investigation of the effects of chemical decontamination procedures on the microbiological status of broiler carcasses during processing. J. Food Prot. 2001, 64, 179–183. [Google Scholar] [CrossRef]
- Capita, R.; Prieto, M.; Alonsi-Calleja, C. Sampling methods for microbiological analysis of red meat and poultry carcasses. J. Food Prot. 2004, 67, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, C.; Macklin, K.; Kumar, S.; Bailey, M.; Ebner, P.; Oliver, H.; Martin-Gonzalez, F.; Singh, M. Prevalence and antimicrobial resistance patterns of Salmonella isolated from poultry farms in southeastern United States. Poult. Sci. J. 2018, 97, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture (USDA); Food Safety Inspection Service (FSIS). Roadmap to Reducing Salmonella. Driving Change through Science-Based Policy. 2020. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2020-12/FSISRoadmaptoReducingSalmonella.pdf (accessed on 20 April 2021).
- Loretz, M.; Stephan, R.; Zweifel, C. Antimicrobial activity of decontamination treatments for poultry carcasses: A literature survey. Food Control 2010, 21, 791–804. [Google Scholar] [CrossRef]
- Koutchma, T. Advances in Ultraviolet Light Technology for Non-thermal Processing of Liquid Foods. Food Bioproc. Technol. 2009, 2, 138–155. [Google Scholar] [CrossRef]
- Hessling, M.; Gross, A.; Hoenes, K.; Rath, M.; Stangl, F.; Tritschler, H.; Sift, M. Efficient Disinfection of Tap and Surface Water with Single High Power 285 nm LED and Square Quartz Tube. IEEE Photonics J. 2016, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Hijnen, W.; Beerendonk, E.; Medema, G. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Harrison, M.A. Effectiveness of UV light as a means to reduce Salmonella contamination on tomatoes and food contact surfaces. Food Control 2016, 66, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; MacGregor, S.; Anderson, J.; Woolsey, G. Pulsed ultra-violet inactivation spectrum of Escherichia coli. Water Res. 2005, 39, 2921–2925. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Kinetics of Microbial Inactivation for Alternative Food Processing Technologies–Ultraviolet Light. 2013. Available online: http://www.fda.gov/Food/FoodScienceResearch/SafePracticesforFoodProcesses/ucm103137.htm (accessed on 2 May 2021).
- Morehouse, K.; Komolprasert, V. Overview of Irradiation of Food and Packaging. ACS Symposium Series 875, Irradiation of Food and Packaging. 2004; Chapter 1; pp. 1–11. Available online: https://www.fda.gov/food/irradiation-food-packaging/overview-irradiation-food-and-packaging (accessed on 31 May 2021).
- Koutchma, T. UV Light for Processing Foods. Ozone Sci. Eng. 2008, 30, 93–98. [Google Scholar] [CrossRef]
- Koivunen, J.; Heinonen-Tanski, H. Inactivation of enteric microorganisms with chemical disinfectants, UV irradiation and combined chemical/UV treatments. Water Res. 2005, 39, 1519–1526. [Google Scholar] [CrossRef]
- Matak, K.; Churey, J.; Worobo, W.; Sumner, S.; Hovingh, E.; Hackney, C.; Pierson, M. Efficacy of UV Light for the Reduction of Listeria monocytogenes in Goat’s Milk. J. Food Prot. 2005, 68, 2212–2216. [Google Scholar] [CrossRef] [Green Version]
- Ozer, N.; Demirci, A. Inactivation of Escherichia coli O157:H7 and Listeria monocytogenes inoculated on raw salmon fillets by pulsed UV-light treatment. Int. J. Food Sci. Technol. 2006, 41, 354–360. [Google Scholar] [CrossRef]
- Keklik, N.; Krishnamurthy, K.; Demirci, A. Microbial Decontamination of Food by Ultraviolet (UV) and Pulsed UV Light. In Microbial Decontamination in the Food Industry: Novel Methods and Applications; Elsevier: Amsterdam, The Netherlands, 2012; pp. 344–369. [Google Scholar]
- Bialka, K.; Demirci, A.; Puri, V. Modeling the inactivation of Escherichia coli O157:H7 and Salmonella enterica on raspberries and strawberries resulting from exposure to ozone or pulsed UV-light. J. Food Eng. 2008, 85, 444–449. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Code of Federal Regulations Title 21, Part 179: Irradiation in the Production, Processing and Handling of Food. 2010. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=179&showFR=1 (accessed on 1 May 2021).
- McLeod, A.; Hovde Liland, K.; Haugen, J.E.; Sørheim, O.; Myhrer, K.S.; Holck, A.L. Chicken fillets subjected to UV-C and pulsed UV light: Reduction of pathogenic and spoilage bacteria, and changes in sensory quality. J. Food Saf. 2018, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Andino, A.; Hanning, I. Salmonella enterica: Survival, colonization, and virulence differences among serovars. Sci. World J. 2015, 2015, 520179. [Google Scholar] [CrossRef] [Green Version]
- Lagunas-Solar, M.C.; Piña, C.; MacDonald, J.D.; Bolkan, L. Development of pulsed UV light processes for surface fungal disinfection of fresh fruits. J. Food Prot. 2006, 69, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Oms-Oliu, G.; Martín-Belloso, O.; Soliva-Fortuny, R. Pulsed Light Treatments for Food Preservation. A Review. Food Bioproc. Tech. 2010, 3, 13–23. [Google Scholar] [CrossRef]
- Gómez-López, V.; Ragaert, P.; Debevere, J.; Devlieghere, F. Pulsed light for food decontamination: A review. Trends Food Sci. Technol. 2007, 9, 464–473. [Google Scholar] [CrossRef]
- Kim, D.; Kang, D. Effect of surface characteristics on the bactericidal efficacy of UVC LEDs. Food Control 2020, 108, 106869. [Google Scholar] [CrossRef]
- Bae, Y.-M.; Lee, S.-Y. Inhibitory Effects of UV Treatment and a Combination of UV and Dry Heat against Pathogens on Stainless Steel and Polypropylene Surfaces. J. Food Sci. 2012, 77, M61–M64. [Google Scholar] [CrossRef] [PubMed]
- Haughton, P.N.; Lyng, J.G.; Cronin, D.A.; Morgan, D.J.; Fanning, S.; Whyte, P. Efficacy of UV Light Treatment for the Microbiological Decontamination of Chicken, Associated Packaging, and Contact Surfaces. J. Food Prot. 2011, 74, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Sommers, C.H.; Sites, J.E.; Musgrove, M. Ultraviolet light (254 nm) inactivation of pathogens on foods and stainless steel surfaces. J. Food Saf. 2010, 30, 470–479. [Google Scholar] [CrossRef]
- Shama, G. Ultraviolet Light. In Encyclopedia of Food Microbiology-2; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: London, UK, 2014; pp. 665–671. [Google Scholar]
- Guerrero-Beltran, J.A.; Barbosa-Canovas, G.V. Advantages and Limitations on Processing Foods by UV Light. Int. J. Food Sci. Technol. 2004, 10, 137–147. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kim, S.J.; Kim, D.K.; Kang, D.H. Fundamental characteristics of deep-UV light-emitting diodes and their application to control foodborne pathogens. Appl. Environ. Microbiol. 2016, 82, 2–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza, C.; Yuk, H.; Hoon Khoo, G.; Zhou, W. Application of Light-Emitting Diodes in Food Production, Postharvest Preservation, and Microbiological Food Safety. Compr. Rev. Food Sci. Food Saf. 2015, 14, 719–740. [Google Scholar] [CrossRef]
- Jarvis, P.; Autin, O.; Goslan, E.H.; Hassard, F. Application of ultraviolet light-emitting diodes (UV-LED) to full-scale drinking-water disinfection. Water 2019, 11, 1894. [Google Scholar] [CrossRef] [Green Version]
| Illumination Time (s) | Irradiance 1 (mW/cm2) | UV 4 Dose (J/cm2) | Bacterial Count (Log CFU/cm2) | St. Dev. 5 | Reduction 2 (Log CFU/cm2) | Bacterial Reduction (%) 3 |
|---|---|---|---|---|---|---|
| 0 | 2 | 0 | 6.21 | 0.16 | - | - |
| 60 | 2 | 0.12 | 5.20 | 0.48 | 1.01 | 90.2 |
| 180 | 2 | 0.36 | 4.89 | 0.81 | 1.32 | 95.2 |
| 300 | 2 | 0.60 | 4.64 | 0.67 | 1.57 | 97.3 |
| 600 | 2 | 1.20 | 4.36 | 0.70 | 1.85 | 98.6 |
| 900 | 2 | 1.80 | 4.43 | 0.70 | 1.78 | 98.3 |
| 0 | 4 | 0 | 6.26 | 0.11 | - | - |
| 60 | 4 | 0.24 | 4.21 | 0.77 | 2.05 | 99.1 |
| 180 | 4 | 0.72 | 3.99 | 0.86 | 2.27 | 99.5 |
| 300 | 4 | 1.2 | 3.67 | 0.63 | 2.59 | 99.7 |
| 600 | 4 | 2.4 | 3.89 | 0.44 | 2.37 | 99.6 |
| 900 | 4 | 3.6 | 3.25 | 0.53 | 3.01 | 99.9 |
| Illumination Time (s) | Irradiance 1 (mW/cm2) | UV Dose (J/cm2) | Log CFU/cm2 | St. Dev. | Reduction 2 (Log CFU/cm2) | Bacterial Reduction (%) 3 |
|---|---|---|---|---|---|---|
| 0 | 2 | 0 | 3.4 | 0.61 | - | - |
| 15 | 2 | 0.03 | 2.1 | 0.72 | 1.3 | 93.7 |
| 30 | 2 | 0.06 | 1.94 | 0.83 | 1.46 | 95.6 |
| 45 | 2 | 0.09 | 1.87 | 0.72 | 1.53 | 96.3 |
| 60 | 2 | 0.12 | 1.43 | 0.41 | 1.97 | 98.7 |
| 0 | 4 | 0 | 6.27 | 0.49 | - | - |
| 15 | 4 | 0.06 | 4.91 | 0.56 | 1.36 | 95.6 |
| 30 | 4 | 0.12 | 3.78 | 1.5 | 2.49 | 99.7 |
| 45 | 4 | 0.18 | 3.47 | 0.65 | 2.8 | 99.8 |
| 60 | 4 | 0.24 | 2.79 | 1.76 | 3.48 | 99.9 |
| Illumination Time (s) | Irradiance 1 (mW/cm2) | UV Dose (J/cm2) | Log CFU/cm2 | St. Dev. | Reduction 2 (Log CFU/cm2) | Bacterial Reduction (%) 3 |
|---|---|---|---|---|---|---|
| 0 | 2 | 0 | 6.58 | 0.16 | - | - |
| 30 | 2 | 0.06 | 5.67 | 0.28 | 0.91 | 87.7 |
| 60 | 2 | 0.12 | 5.33 | 0.12 | 1.25 | 94.4 |
| 90 | 2 | 0.18 | 5.28 | 0.17 | 1.3 | 95.0 |
| 120 | 2 | 0.24 | 5.13 | 0.16 | 1.45 | 96.5 |
| 150 | 2 | 0.30 | 5.05 | 0.25 | 1.53 | 97.0 |
| 180 | 2 | 0.36 | 4.57 | 0.47 | 2.01 | 99.0 |
| 300 | 2 | 0.6 | 2.75 | 1.68 | 3.83 | 99.99 |
| 600 | 2 | 1.2 | 2.04 | 1.68 | 4.54 | 99.997 |
| 900 | 2 | 1.8 | 1.84 | 1.46 | 4.74 | 99.998 |
| 0 | 4 | 0 | 5.20 | 0.15 | - | - |
| 30 | 4 | 0.12 | 3.97 | 0.24 | 1.23 | 94.1 |
| 60 | 4 | 0.24 | 3.43 | 0.24 | 1.77 | 98.3 |
| 90 | 4 | 0.36 | 2.82 | 0.29 | 2.38 | 99.6 |
| 120 | 4 | 0.48 | 2.42 | 0.07 | 2.78 | 99.8 |
| 150 | 4 | 0.60 | 2.40 | 0.00 | 2.8 | 99.8 |
| 180 | 4 | 0.72 | 0 * | 0 * | 5.2 | 99.999 |
| 300 | 4 | 1.20 | 0 * | 0 * | 5.2 | 99.999 |
| 600 | 4 | 2.40 | 0 * | 0 * | 5.2 | 99.999 |
| 900 | 4 | 3.60 | 0 * | 0 * | 5.2 | 99.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calle, A.; Fernandez, M.; Montoya, B.; Schmidt, M.; Thompson, J. UV-C LED Irradiation Reduces Salmonella on Chicken and Food Contact Surfaces. Foods 2021, 10, 1459. https://doi.org/10.3390/foods10071459
Calle A, Fernandez M, Montoya B, Schmidt M, Thompson J. UV-C LED Irradiation Reduces Salmonella on Chicken and Food Contact Surfaces. Foods. 2021; 10(7):1459. https://doi.org/10.3390/foods10071459
Chicago/Turabian StyleCalle, Alexandra, Mariana Fernandez, Brayan Montoya, Marcelo Schmidt, and Jonathan Thompson. 2021. "UV-C LED Irradiation Reduces Salmonella on Chicken and Food Contact Surfaces" Foods 10, no. 7: 1459. https://doi.org/10.3390/foods10071459
APA StyleCalle, A., Fernandez, M., Montoya, B., Schmidt, M., & Thompson, J. (2021). UV-C LED Irradiation Reduces Salmonella on Chicken and Food Contact Surfaces. Foods, 10(7), 1459. https://doi.org/10.3390/foods10071459







