1. Introduction
Nowadays, the world faces challenging crises related to health, demography, and nutrition, which require innovative and sustainable solutions. New sources of food and bioactive compounds are being investigated as adequate solutions for health and nutrition. Food bioactives were reported to reinforce the immune system against COVID-19 disease and promote human health [
1]. They can help prevent many diseases, including cancers, coughing, inflammatory, cardiovascular, and oxidative stress diseases [
2]. The phenolic compounds are one of the most popular bioactive compounds that have a wide range of biological functions and are used for food formulations and pharmaceutical industries [
3,
4]. The phenolic compounds are widely found in plants, fruits, and wastes. While seeking health and nutrition solutions, the valorization of food wastes for the recovery of phenolic compounds became imperative.
Although bioactives, mainly phenolic compounds, exhibited numerous beneficial effects, they are chemically unstable in neutral pH environments and when exposed to oxygen [
5,
6]. Furthermore, the chemical structure of phenolic compounds counts unsaturated bonds, which may change the structure and functions of the original compound when exposing to light, oxygen, heat, enzymatic activity, and water [
7,
8,
9]. Thus, encapsulation techniques were developed to protect the phenolic compounds and improve their bioavailability. Moreover, the encapsulation process can also prevent unwanted taste and odor and extend the shelf-life and usages of phenolic compounds [
10,
11]. The encapsulation process consists of rendering the bioactive compounds in a solution of wall material, followed by drying [
12]. The efficiency of encapsulation depends on the coating material and drying techniques [
13,
14] and the solution homogeneity. Additionally, the optimum concentration of the active compounds, the release mechanism, the final particle size, density, stability requirements, and cost constraints are other essential features to be taken into account [
3]. Many coating materials are used for the microencapsulation, including maltodextrin, gum arabic, pectin, proteins, lipids, cyclodextrins, chitosan, and xanthan their combination. These materials’ efficacy depends on the preparation parameters of emulsion and features of the targeted compounds [
9,
14].
Cornsilk is the byproduct of maize (
Zea maize L.), which is discarded after taking the corn. In ancient times, cornsilk was used in traditional medicine to treat kidney problems [
15]. It was also reported to reduce hyperglycemia by incrementing insulin and recovering the beta-cells and demonstrated diuretic and kaliuretic properties at specific doses [
15,
16]. It has shown antioxidant activity [
15,
17], antimicrobial, and antidepressant properties [
15,
16]. Cornsilk has been used to treat cystitis, edema, gout, rheumatoid, and arthritis [
16,
18]. Cornsilk has proven beneficial effects in reducing obesity [
19,
20] and protecting against radiation-induced oxidative stress [
21]. In addition, the cornsilk extract is anticancer since it induced apoptosis via the ROS-mediated mitochondrial pathway in MCF-7 cells [
22]. These biological activities rely on bioactive compounds such as alkaloids, steroids, flavonoids, phenolic acids, and terpenoids that cornsilk contained [
16,
23,
24,
25].
Corn is the third most-produced cereal crop globally after wheat and rice [
26] and consumed boiled and grilled or used to make flour, starch, oil, and syrup. The processing of corn results in a high amount of cornsilk, which is not adequately valorized. It would be beneficial to find a novel approach in the valorization of cornsilk. The phenolic compounds of cornsilk could be extracted to produce natural food additives using well-studied conventional and emerging technologies [
27,
28,
29].
The recovered phenolic compounds could find different applications in food, cosmetics, and pharmaceutical industries. However, the investigation of their encapsulation and product formation is also critical to ensure their functionality [
30]. To this end, the present study considers the optimization of phenolic compound extraction from cornsilk and its encapsulation with maltodextrin using freeze-drying, spray-drying, and microwave-drying.
2. Material and Methods
2.1. Plant Material
The maize was collected from a field in Samsun/Turkey during the October 2020 season. The cornsilk was separated, washed and stored at −20 °C (
Figure 1).
2.2. Chemical and Reagents
2,4,6-Tris(2-pyridyl)-1,3,5-triazine (TPTZ), 2,2-Diphenyl-1-Picrylhydrazyl (DPPH), 6-hydroxy 2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), hydrochloric acid (HCl, 37%), methanol (99,8%), acetone, sodium nitrite, sodium hydroxide Folin–Ciocalteau reagent, maltodextrin and standards were purchased from Sigma-Aldrich (St. Louis, MO, USA). Gallic acid and sodium carbonate from taken from Riedel-de Haen. Sodium acetate and glacial acetic acid were taken from Carlo Erba. Aluminum chloride and iron chloride were purchased from Merck.
2.3. Obtaining Cornsilk Extract
A predefined amount of cornsilk was mixed with 100 mL of distilled water. The mixtures were extracted in a water bath (Nuve, ST 30) at different times and temperatures. Afterward, the extracts were filtered and diluted adequately for further analyses.
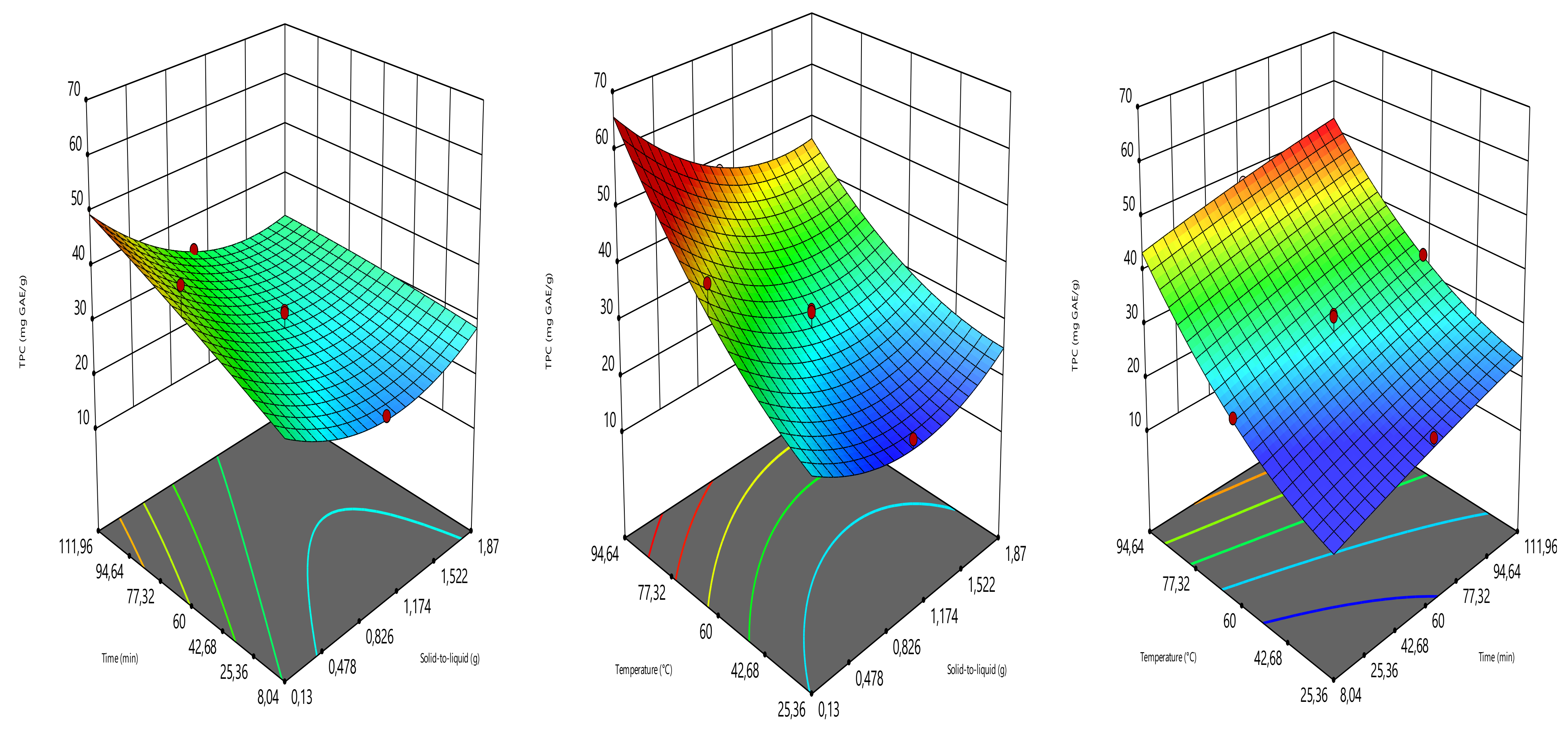
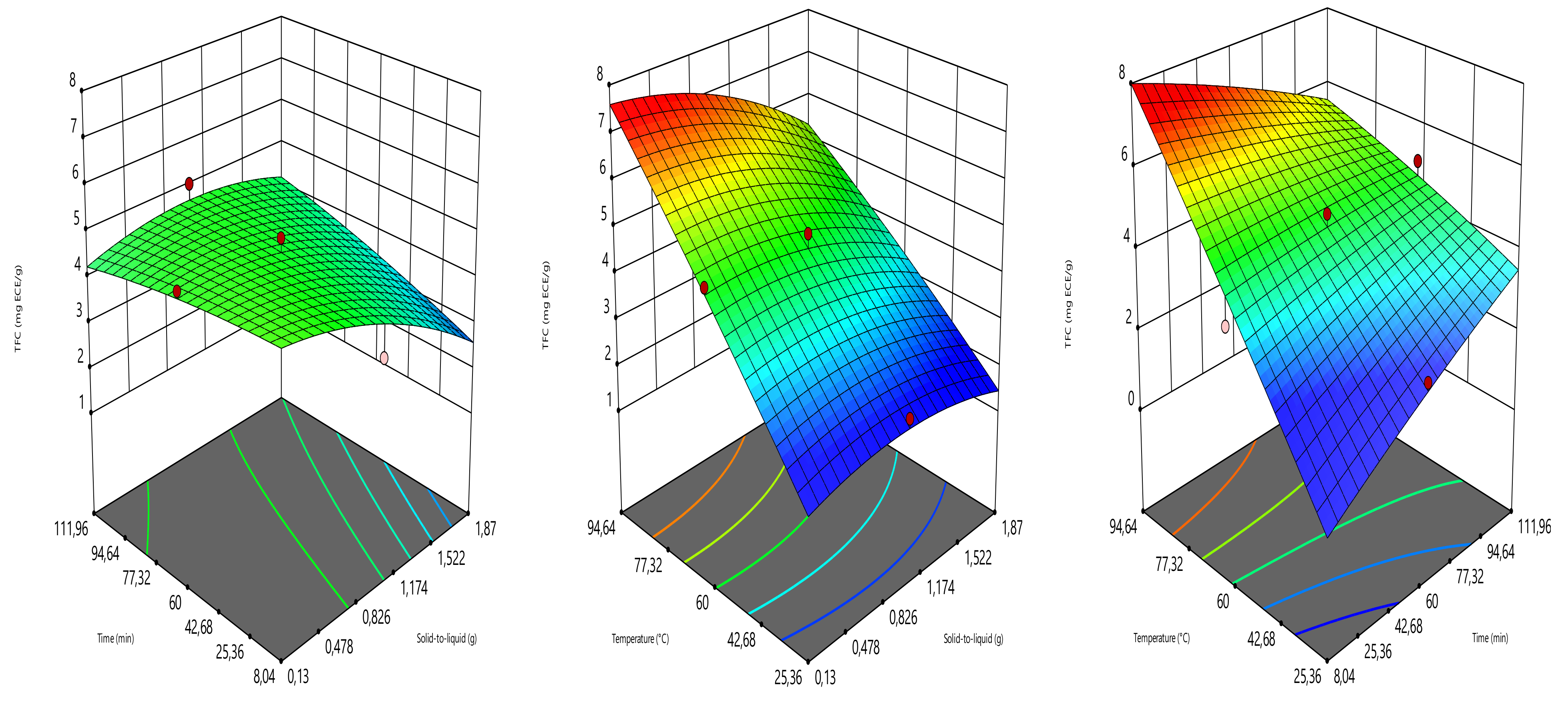
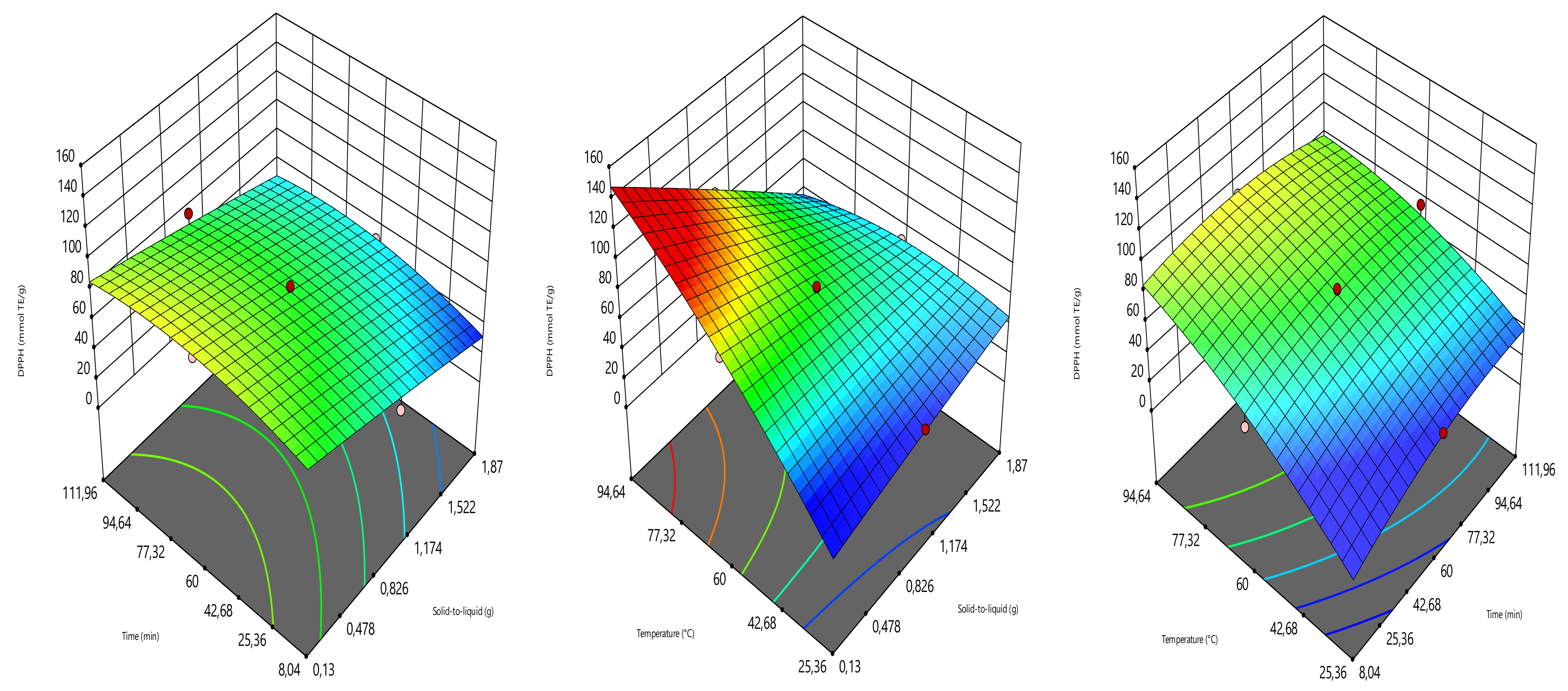
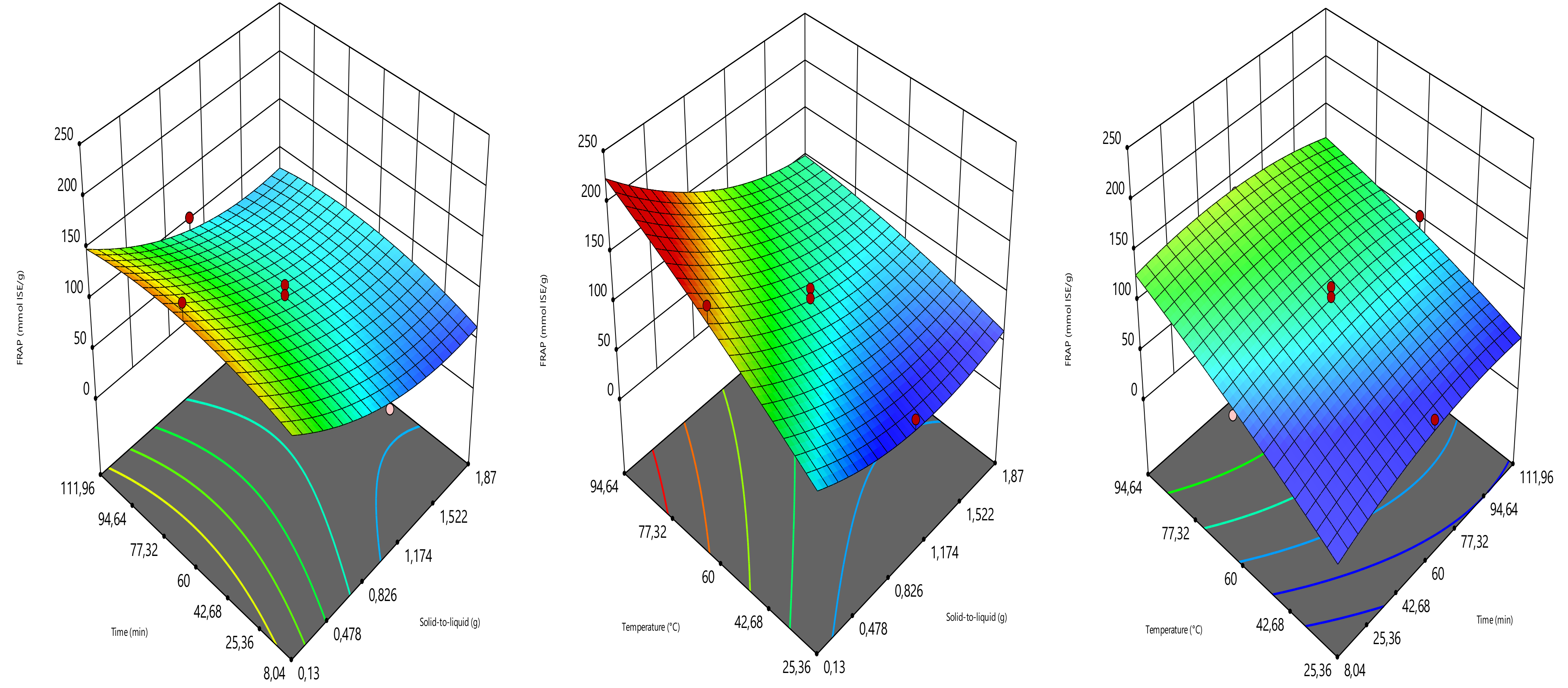
2.4. Optimization Design
The extraction parameters such as solid load (amount of sample in g), time (min), and temperature (°C) were optimized to provide the highest yield of total phenolic content (TPC), total flavonoid content (TFC), ferric reducing antioxidant power (FRAP), and DPPH radical scavenging activity (DPPH). The optimization was carried out using the three-level central composite design (Design expert software 11.0). The solid load, time, and temperature were assigned to the coded variables of X
1, X
2, and X
3, respectively (
Table 1). The combinations of the solid load (0.13, 0.5, 1, 1.5 and 1.87 g), time (8.04, 30, 60, 90 and 111.96 min), and temperature (25.36, 40, 60, 80, and 94.64 °C) generated 17 experimental points, counting three replicates at the central point. TPC, TFC, FRAP, and DPPH radical scavenging activity values were the responses (
Y) (
Table 2). The experimental data fitted to the polynomial model in Equation (1):
where
Xi and
Xj are the coded independent variables,
βij,
βii, and
βi interactive, quadratic, and linear coefficients, respectively,
β is the model intercept, and
Y is the predicted response.
2.5. Total Phenolic Content (TPC)
The TPC was determined using Folin–Ciocalteu method as described in Zannou and Koca [
31]. The absorbance was read at 760 nm using a UV- spectrometer (Thermo Spectronic) and compared to a standard curve as gallic acid equivalent (mg GAE/g).
2.6. Total Flavonoid Content (TFC)
The TFC was determined using the protocol [
31]. The absorbance was read at 510 nm, and TFC was calculated following the calibration curve of epigallocatechin. The results were given as mg epigallocatechin equivalents (ECE) per g.
2.7. DPPH Radical Scavenging Activity Assay
The DPPH assay was carried out following the method of Zannou et al. [
32]. The mixture was placed in the dark for 1 h at room temperature, followed by the absorbance recording at 517 nm. The DPPH solution was used as a control, and the scavenging ratio was calculated with Equation (2).
where
Ac is the absorbance of the control and
As is the absorbance of extract.
2.8. Ferric Reducing Antioxidant Power (FRAP) Assay
FRAP assay performed according to the procedure of Zannou et al. [
32] The FRAP values of the extracts were calculated from a calibration curve using FeSO4 as standard. The results were given as mmol FeSO4 equivalents (mmol ISE/g).
2.9. Encapsulation Process
For the encapsulation of the phenolic compounds of maize byproduct aqueous extracts, three techniques (freeze-drying, spray-drying, and microwave-drying) were used and compared. Maltodextrin (4.0–7.0 DE) was used as a coating agent. Maltodextrin and extract mixture was prepared by mixed 10 g of maltodextrin with 90 g of cornsilk aqueous extract. The mixture was heated at 60 °C for 1h and then subjected to ultraturax at 11,000 rpm for 5 min. The spray drying was performed using a Buchi mini spray dryer B-290 (Noble Park, VIC, Australia) as described in [
33]. The freeze-drying was carried out by initially frozen the samples using liquid nitrogen and then freeze-dried for 48 h (FD3 freeze dryer, Thomas Australia Pty. Ltd., Seven Hills, NSW, Australia) [
33]. The microwave drying was performed in a domestic microwave oven (Vestel, Turkey) at microwave power intensity of 750 W for 10 min, selected based on the preliminary experiments.
2.10. Production Yield
The production yield (
PY) is the percentage of the concentration ratio in powder to the total concentration in the extract.
PY of TPC, TFC, DPPH radical scavenging and FRAP was determined with Equation (3).
2.11. Surface Phenolic Content and Efficiency of Phenolic Compounds
The surface phenolic content was determined by the method of Cilek et al. [
14]. The encapsulation efficiency of microcapsules’ phenolic content was calculated according to Equation (4).
2.12. Physico-Chemical Characteristics
The moisture content was measured with an oven at 70 °C for 24 h. The solubility was determined according to the method used by Fazaeli et al. [
34] The bulk density and tapped density were determined according to [
35]. Carr index was calculated from the bulk and tapped densities of the powders as expressed in Equation (5).
2.13. Microstructure Analysis
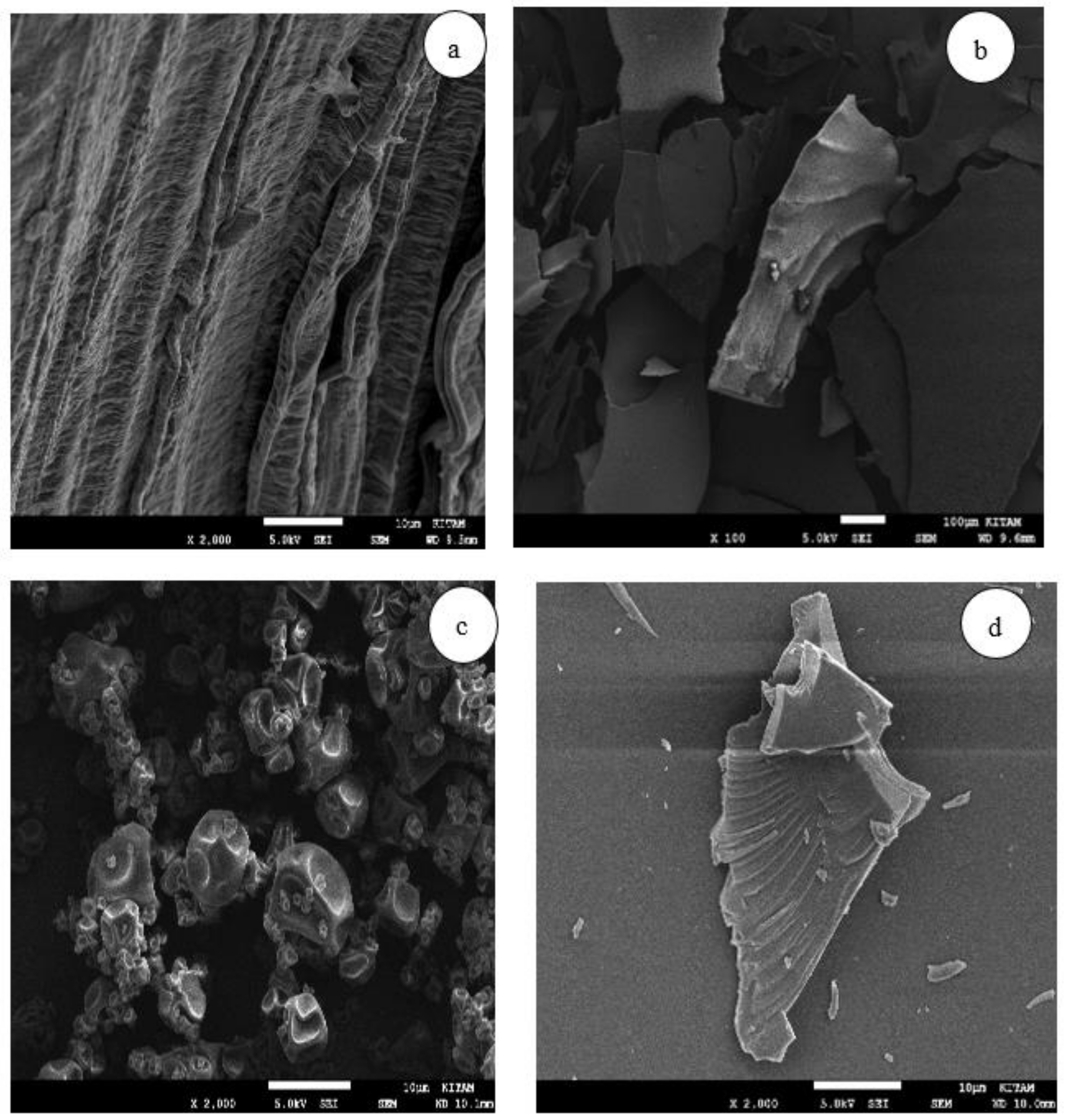
The microstructure analysis was performed using a scanning electron microscope (SEM, JEOL JSM-7001F). A small specimen was briefly taken from the samples and attached to a stainless stub with double sticky tape. The assembly was then immediately sputtered with a gold/palladium target (60/40) in approximately 10 nm using a sputter coater, functioning with argon and plasma current for 2 min. The images were recorded at an acceleration voltage of 10 kV and 15 kV.
2.14. Differential Scanning Calorimetry (DSC) Analysis
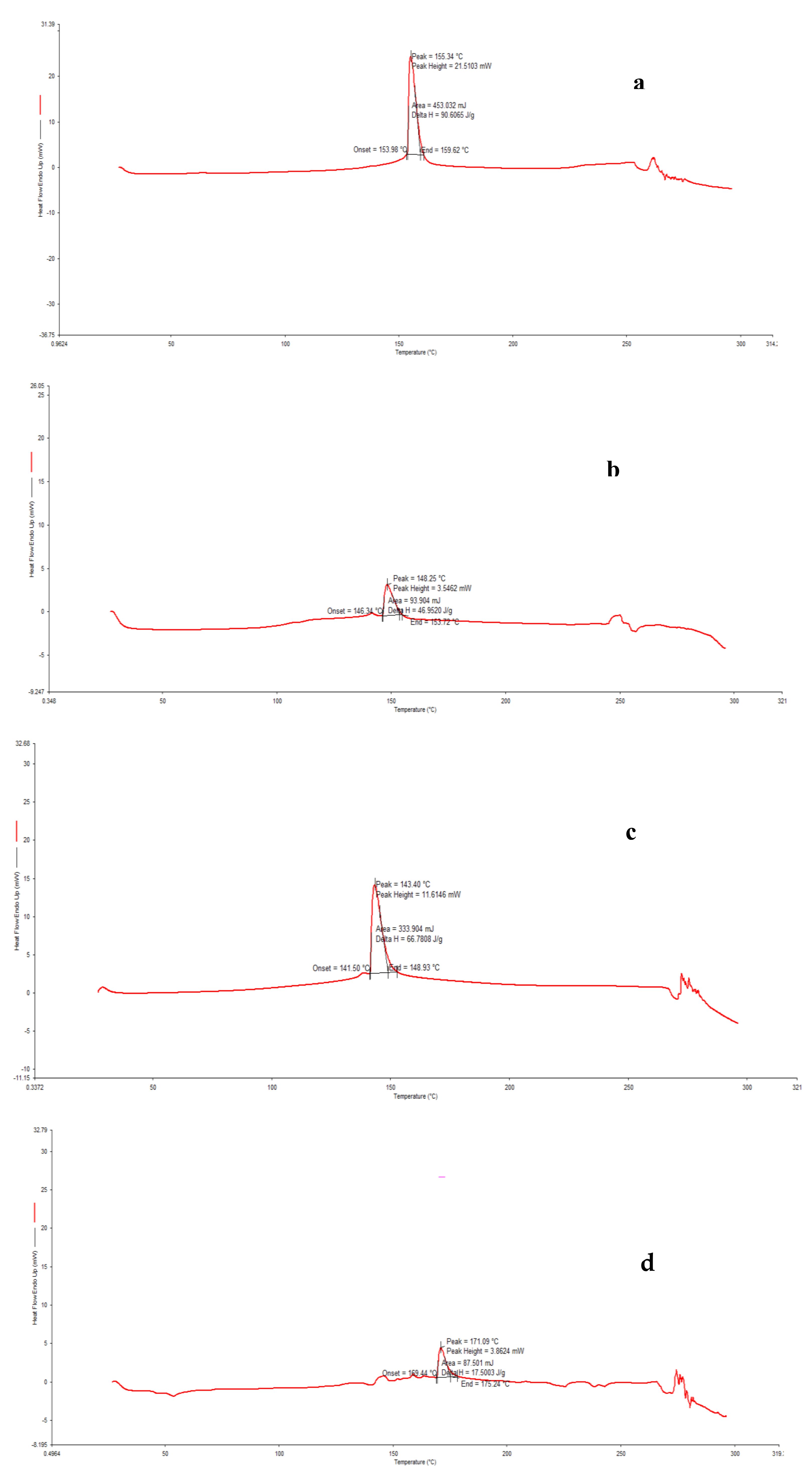
The differential scanning calorimeter (New Castle, DE; TA Instrument 2010) was used for the calorimetric measurements and calibrated with indium. The calorimetric scans’ temperature range varied from 10 to 60 °C, under nitrogen flow. The scan rate was set to 5 °C min−1. The Tm and ∆H were determined from the thermograms by the Universal 4.0 C. Software.
2.15. Fourier Transformed Infrared (FTIR) Analysis
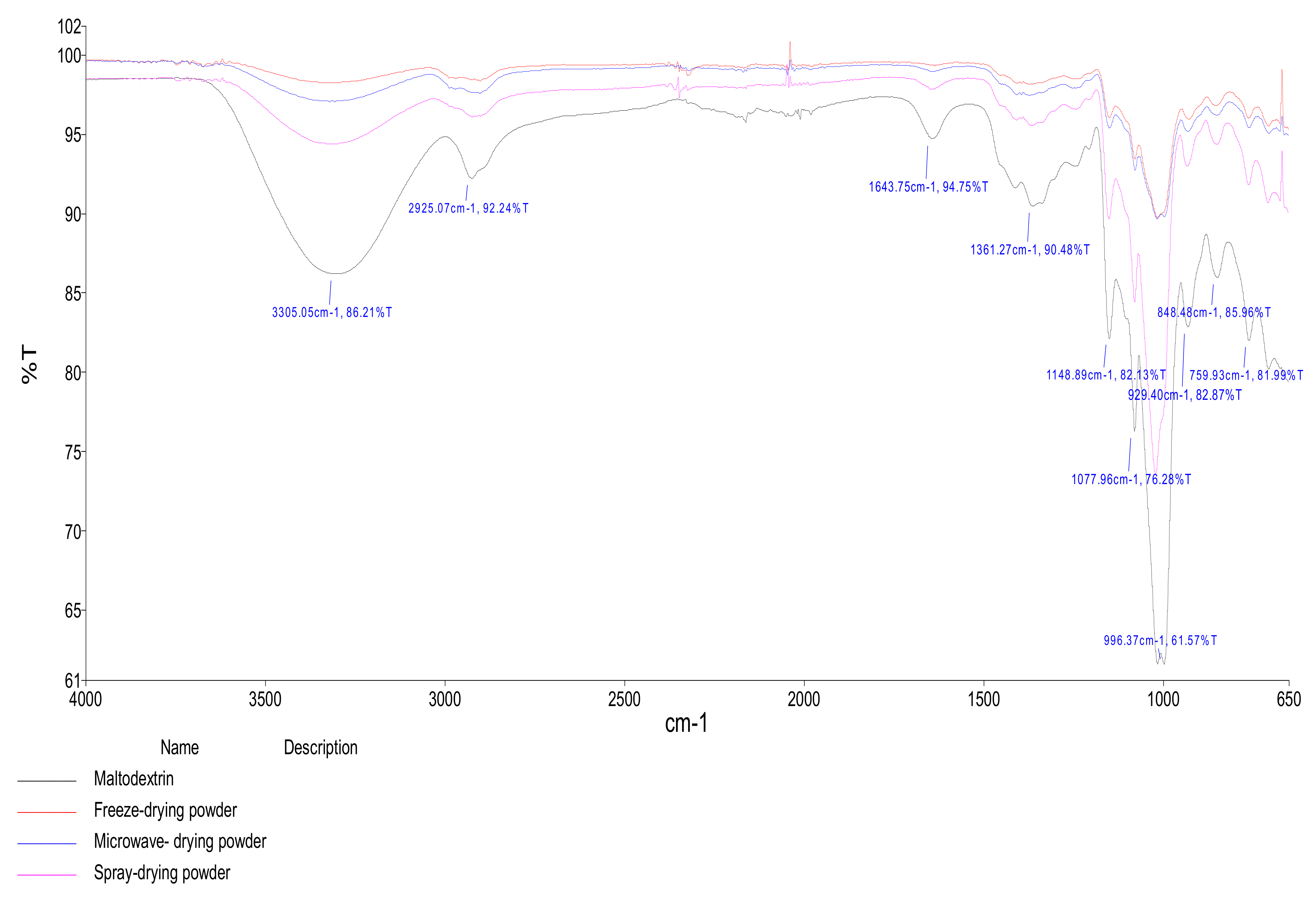
FTIR analysis was carried out according to [
32] using an FTIR spectrometer (Perkin Elmer, Spectrum-Two, USA, PEService 35).
2.16. LC/MS/MS Analysis
The phenolic compounds were determined using liquid chromatography coupled to a mass spectrometer detector (LC-MS/MS, Shimadzu LC-MS 8040) via electrospray ionization (ESI) and two pumps (LC-30 AD), a column oven (CTO-10AS VP), an autosampler (SIL-30AC) and a degassing unit (DGU-20A 3R), according to Zannou et al. [
36] The phenolic compounds were identified based on their elution time and quantified from their peak area. The identified compounds were quantified using a mixture of external standards (protocatechuic aldehyde, protocatechuic acid, gallic acid, catechin, vanilin, ρ-cumaric acid, caffeic acid, ferulic acid, 4-hydroxy benzoic acid, salicylic acid, ellagic acid and quercetin) at different concentrations.
2.17. Data Analysis
The Design-Expert software 9.0 (Trial version, Stat-Ease Inc., Minneapolis, MN, USA) was used to design the optimization process and to generate the models and 3D graphics. The ANOVA of response surface methodology was applied for the statistical significance. The coefficient of determination (R2), adjusted coefficient of determination (adj. R2), coefficient of variation (CV), and Fisher’s test value (F-value) were used for the validation of the models. The models and terms were considered significant at p < 0.05. The optimum conditions were determined based on the desirability function. Other analyses were carried out in three replicate, the one-way ANOVA with posthoc Duncan’s test was applied (SPSS, version 21) and the results were considered at p ≤ 0.05.
4. Conclusions
The response surface methodology was applied to determine the conditions that provide the highest values of TPC, TFC, FRAP and DPPH of cornsilk. The optimum conditions were 0.5 g for solid load, 62.10 min for decoction time and 80 °C for temperature. At the optimum conditions, the predicted results were 46.39 mg GAE/g, 6.29 mg ECE/g, 109.28 mmol TE/g, and 160.72 mmol ISE/g for TPC, TFC, DPPH radical scavenging, and FRAP, respectively, whereas the experimental results were found close to the predicted values as 58.87 ± 1.38 mg GAE/g, 6.64 ± 0.12 mg ECE/g, 132.63 ± 1.66 mmol TE/g, and 172.30 ± 3.40 for TPC, TFC, DPPH radical scavenging, and FRAP, respectively. The efficiency of 98.85 ± 0.90%, 97.95 ± 4.21%, 70.39 ± 6.20%, 82.45 ± 0.89% was obtained for TPC, TFC, DPPH radical scavenging, and FRAP, respectively. The results of antioxidant properties showed that the freeze-drying provided the highest efficiency. The highest recovery of phenolic compounds, microstructure, thermograms, and FTIR of the encapsulated samples showed that the encapsulation process was successful, and the freeze-drying was the best drying technique. The glass transition temperatures were 148.25 °C, 143.40 °C, and 171.09 °C for freeze-dried, spray-dried, and microwave-dried samples. The microwave-dried samples displayed the highest bulk and tapped bulk densities, which are desirable. However, the spray-dried samples had increased water activity, while the lowest values were found in freeze-dried and microwave-dried samples. This work confirmed the feasibility of the encapsulation of phenolic compounds of a cornsilk extract with maltodextrin using freeze-drying, spray-drying, and microwave-drying. Further studies should be carried out on the bioavailability and anti-microbial properties of the encapsulated products.