Co-Encapsulated Synbiotics and Immobilized Probiotics in Human Health and Gut Microbiota Modulation
Abstract
1. Introduction
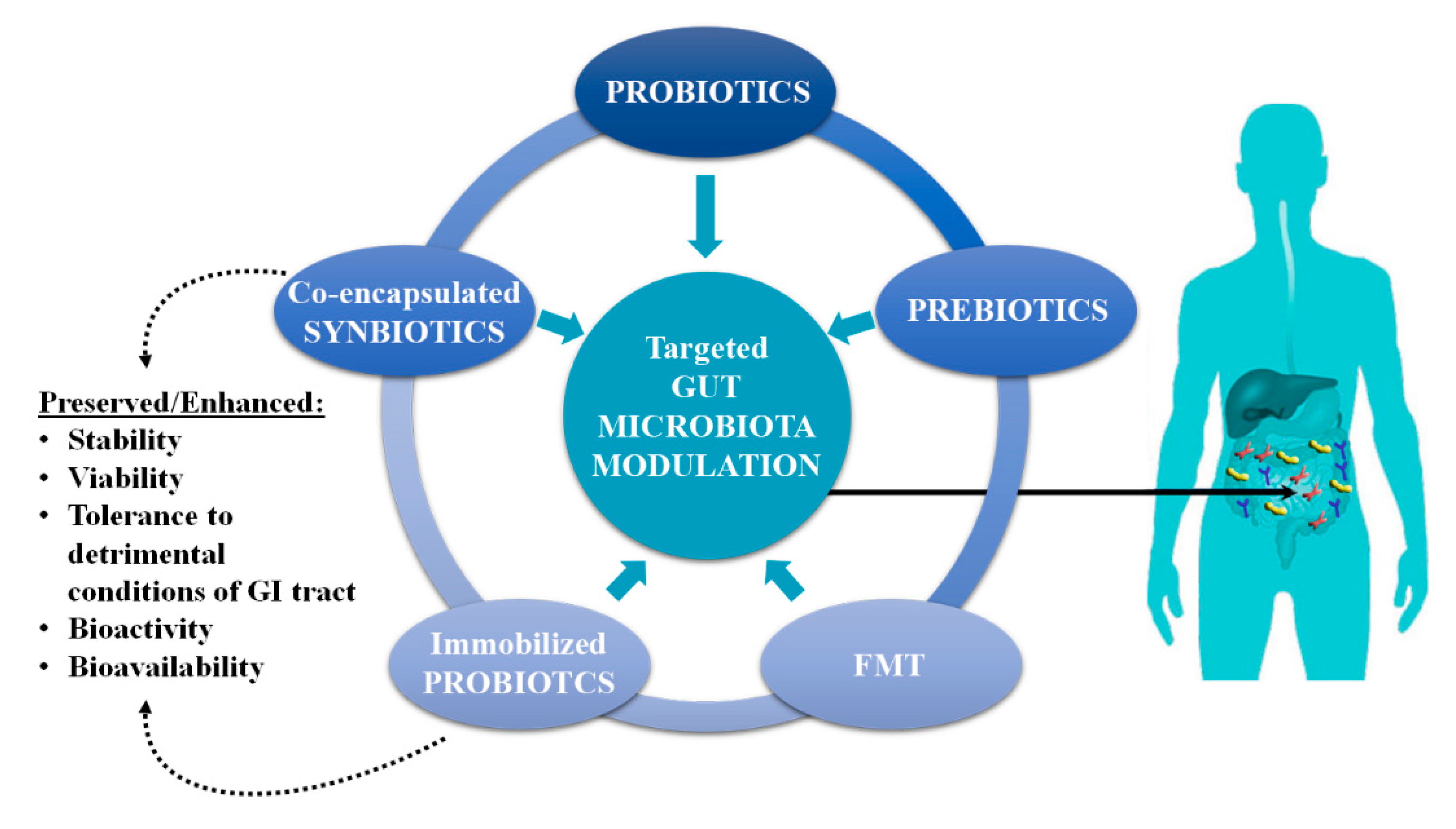
2. Gut Microbiota Modulation
2.1. Modulation by Probiotics
2.2. Modulation by Prebiotics
2.3. Modulation by FMT in Severe Dysbiotic States
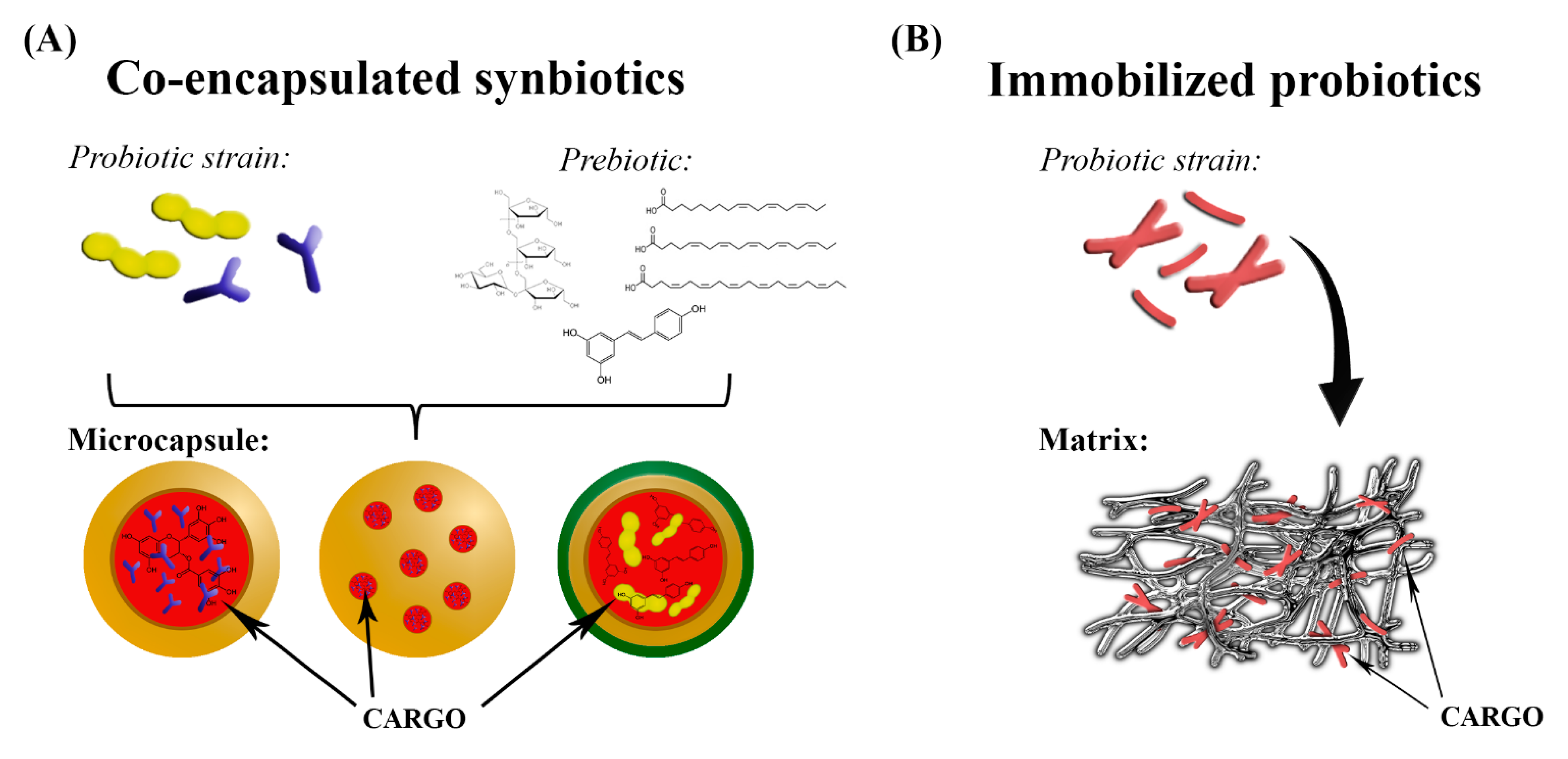
3. Co-Encapsulated Synbiotics
3.1. Synbiotics
3.2. Technologies and Carrier Materials Used in Fabrication of Co-Encapsulated Synbiotics
3.2.1. Co-Encapsulation with Omega-3 PUFAs and GABA
3.2.2. Co-Encapsulation with Phytochemicals
3.2.3. Co-Encapsulation with Dietary Fibers
- non-starch polysaccharides: cellulose, hemicelluloses, pectins, hydrocolloids;
- resistant oligosaccharides: FOS, GOS, inulin (which can selectively promote the growth of Bifidobacterium spp. and Lactobacillus spp.), and other resistant oligosaccharides;
- resistant starch: consisting of physically enclosed starch, chemically and/or physically modified starches, retrograded amylose, and some types of raw starch granules;
- lignin associated with the DF polysaccharides;
4. Gut Microbiota Modulation by Immobilized Probiotics
5. “Side Effects” of Gut Microbiota Functional Redundancy
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peluzio, M.D.C.G.; Martinez, J.A.; Milagro, F.I. Postbiotics: Metabolites and mechanisms involved in microbiota-host interactions. Trends Food Sci. Technol. 2021, 108, 11–26. [Google Scholar] [CrossRef]
- Wu, S.; Bekhit, A.E.-D.A.; Wu, Q.; Chen, M.; Liao, X.; Wang, J.; Ding, Y. Bioactive peptides and gut microbiota: Candidates for a novel strategy for reduction and control of neurodegenerative diseases. Trends Food Sci. Technol. 2021, 108, 164–176. [Google Scholar] [CrossRef]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Saha, S.; Das, S. Metagenomic Surveys of Gut Microbiota. Genom. Proteom. Bioinform. 2015, 13, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Nagao-Kitamoto, H.; Shreiner, A.B.; Gillilland, M.G.; Kitamoto, S.; Ishii, C.; Hirayama, A.; Kuffa, P.; El-Zaatari, M.; Grasberger, H.; Seekatz, A.M.; et al. Functional Characterization of Inflammatory Bowel Disease–Associated Gut Dysbiosis in Gnotobiotic Mice. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 468–481. [Google Scholar] [CrossRef]
- Eid, H.M.; Wright, M.L.; Kumar, N.V.A.; Qawasmeh, A.; Hassan, S.T.S.; Mocan, A.; Nabavi, S.M.; Rastrelli, L.; Atanasov, A.G.; Haddad, P.S. Significance of Microbiota in Obesity and Metabolic Diseases and the Modulatory Potential by Medicinal Plant and Food Ingredients. Front. Pharmacol. 2017, 8, 387. [Google Scholar] [CrossRef]
- Sharma, V.R.; Singh, M.; Kumar, V.; Yadav, M.; Sehrawat, N.; Sharma, D.K.; Sharma, A.K. Microbiome dysbiosis in cancer: Exploring therapeutic strategies to counter the disease. Semin. Cancer Biol. 2021, 70, 61–70. [Google Scholar] [CrossRef]
- Wu, W.; Kong, Q.; Tian, P.; Zhai, Q.; Wang, G.; Liu, X.; Zhao, J.; Zhang, H.; Lee, Y.K.; Chen, W. Targeting Gut Microbiota Dysbiosis: Potential Intervention Strategies for Neurological Disorders. Engineering 2020, 6, 415–423. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Berin, M.C. Food allergy and the microbiome: Current understandings and future directions. J. Allergy Clin. Immunol. 2019, 144, 1468–1477. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, T.C.; da Costa, W.K.A.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2021, 140, 110033. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, L.; Zeng, X.; Zhang, X.; Liu, Y.; Wu, Z.; Weng, P. The intervention of unique plant polysaccharides—Dietary fiber on depression from the gut-brain axis. Int. J. Biol. Macromol. 2021, 170, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, W.W.; Langille, M.G.; Rupasinghe, H.V. Polyphenol-based prebiotics and synbiotics: Potential for cancer chemoprevention. Curr. Opin. Food Sci. 2018, 20, 51–57. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Del Campo, S.S.F.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Lević, S.; Petrović, T.; Ivanović, S.; Nedović, V.; Kourkoutas, Y. Encapsulation of Lactobacillus casei ATCC 393 in alginate capsules for probiotic fermented milk production. LWT 2019, 116, 108501. [Google Scholar] [CrossRef]
- Terpou, A.; Bekatorou, A.; Bosnea, L.; Kanellaki, M.; Ganatsios, V.; Koutinas, A.A. Wheat bran as prebiotic cell immobilisation carrier for industrial functional Feta-type cheese making: Chemical, microbial and sensory evaluation. Biocatal. Agric. Biotechnol. 2018, 13, 75–83. [Google Scholar] [CrossRef]
- Champagne, C.P.; Raymond, Y.; Guertin, N.; Bélanger, G. Effects of storage conditions, microencapsulation and inclusion in chocolate particles on the stability of probiotic bacteria in ice cream. Int. Dairy J. 2015, 47, 109–117. [Google Scholar] [CrossRef]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Sidira, M.; Karapetsas, A.; Galanis, A.; Kanellaki, M.; Kourkoutas, Y. Effective survival of immobilized Lactobacillus casei during ripening and heat treatment of probiotic dry-fermented sausages and investigation of the microbial dynamics. Meat Sci. 2014, 96, 948–955. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.; Yeoh, Y.K.; Li, A.Y.; Zhan, H.; Wan, Y.; Chung, A.C.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Tursi, A.; Papa, A. Intestinal microbiome modulation during covid-19: Another chance to manage the disease? Gastroenterology 2020. [Google Scholar] [CrossRef]
- Janda, L.; Mihalčin, M.; Šťastná, M. Is a healthy microbiome responsible for lower mortality in COVID-19? Biologia 2021, 76, 819–829. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Lin, W.; Tang, W.; Chan, F.K.; Ng, S.C. Review article: Probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends Food Sci. Technol. 2021, 108, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zha, Y.; Chong, H.; Zhong, C.; Ning, K. Utilizing microbiome approaches to assist source tracking, treatment and prevention of COVID-19: Review and assessment. Comput. Struct. Biotechnol. J. 2020, 18, 3615–3622. [Google Scholar] [CrossRef]
- Khatiwada, S.; Subedi, A. Lung microbiome and coronavirus disease 2019 (COVID-19): Possible link and implications. Hum. Microbiome J. 2020, 17, 100073. [Google Scholar] [CrossRef]
- Villapol, S. Gastrointestinal symptoms associated with COVID-19: Impact on the gut microbiome. Transl. Res. 2020, 226, 57–69. [Google Scholar] [CrossRef]
- Din, A.U.; Mazhar, M.; Waseem, M.; Ahmad, W.; Bibi, A.; Hassan, A.; Ali, N.; Gang, W.; Qian, G.; Ullah, R.; et al. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed. Pharmacother. 2021, 133, 110947. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.R.; Li, B.; Al Sabbah, H.; Xu, W.; Mráz, J. Effects of prebiotic dietary fibers and probiotics on human health: With special focus on recent advancement in their encapsulated formulations. Trends Food Sci. Technol. 2020, 102, 178–192. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. 2006. Available online: www.fao.org (accessed on 18 February 2021).
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Silva, D.R.; Sardi, J.D.C.O.; Pitangui, N.D.S.; Roque, S.M.; da Silva, A.C.B.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Sidira, M.; Santarmaki, V.; Kiourtzidis, M.; Argyri, A.A.; Papadopoulou, O.; Chorianopoulos, N.; Tassou, C.; Kaloutsas, S.; Galanis, A.; Kourkoutas, Y. Evaluation of immobilized Lactobacillus plantarum 2035 on whey protein as adjunct probiotic culture in yoghurt production. LWT 2017, 75, 137–146. [Google Scholar] [CrossRef]
- Rodrigues, F.; Cedran, M.; Bicas, J.; Sato, H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications—A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef]
- Jayani, T.; Sanjeev, B.; Marimuthu, S.; Uthandi, S. Bacterial Cellulose Nano Fiber (BCNF) as carrier support for the immobilization of probiotic, Lactobacillus acidophilus 016. Carbohydr. Polym. 2020, 250, 116965. [Google Scholar] [CrossRef]
- Kosciow, K.; Deppenmeier, U. Characterization of three novel β-galactosidases from Akkermansia muciniphila involved in mucin degradation. Int. J. Biol. Macromol. 2020, 149, 331–340. [Google Scholar] [CrossRef]
- Poletto, G.; Raddatz, G.C.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; Wagner, R.; de Menezes, C.R. Study of viability and storage stability of Lactobacillus acidophillus when encapsulated with the prebiotics rice bran, inulin and Hi-maize. Food Hydrocoll. 2019, 95, 238–244. [Google Scholar] [CrossRef]
- Pereira, G.V.D.M.; Coelho, B.D.O.; Júnior, A.I.M.; Soccol, V.T.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Rudzki, L.; Stone, T.W.; Maes, M.; Misiak, B.; Samochowiec, J.; Szulc, A. Gut microbiota-derived vitamins—Underrated powers of a multipotent ally in psychiatric health and disease. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2021, 107, 110240. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, J.; Laiño, J.E.; Del Valle, M.J.; Vannini, V.; Van Sinderen, D.; Taranto, M.; De Valdez, G.F.; De Giori, G.S.; Sesma, F. B-Group vitamin production by lactic acid bacteria—Current knowledge and potential applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Xue, F.; Yu, S.-F.; Li, X.-S.; Liu, L.; Jia, Y.-Y.; Yan, W.-J.; Tan, Q.-R.; Wang, H.-N.; Peng, Z.-W. Gut microbiota dysbiosis in depressed women: The association of symptom severity and microbiota function. J. Affect. Disord. 2021, 282, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Jin, H.; Kwok, L.-Y.; Sun, Z.; Liong, M.-T.; Zhang, H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol. Stress 2021, 14, 100294. [Google Scholar] [CrossRef] [PubMed]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut feelings: A randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Wu, Y.-J.; Hu, C.-Y. Monosaccharide composition influence and immunomodulatory effects of probiotic exopolysaccharides. Int. J. Biol. Macromol. 2019, 133, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, S.; Neupane, S.; Bhurtel, S.; Katila, N.; Maharjan, S.; Choi, H.; Hong, J.T.; Choi, D.-Y. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J. Nutr. Biochem. 2019, 69, 73–86. [Google Scholar] [CrossRef]
- Valdovinos-García, L.; Abreu, A.; Valdovinos-Díaz, M. Probiotic use in clinical practice: Results of a national survey of gastroenterologists and nutritionists. Rev. Gastroenterol. Mex. 2019, 84, 303–309. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Li, Q.; Shi, Z.; Wu, H.; Zhang, H.; Tang, L.; Yi, R.; Su, H.; Sun, X. Oral administration of a mixture of probiotics protects against food allergy via induction of CD103+ dendritic cells and modulates the intestinal microbiota. J. Funct. Foods 2019, 55, 65–75. [Google Scholar] [CrossRef]
- Abdrabou, A.M.; Osman, E.Y.; Aboubakr, O.A. Comparative therapeutic efficacy study of Lactobacilli probiotics and citalopram in treatment of acute stress-induced depression in lab murine models. Hum. Microbiome J. 2018, 10, 33–36. [Google Scholar] [CrossRef]
- Sidira, M.; Galanis, A.; Ypsilantis, P.; Karapetsas, A.; Progaki, Z.; Simopoulos, C.; Kourkoutas, Y. Effect of Probiotic-Fermented Milk Administration onGastrointestinal Survival of Lactobacillus casei ATCC 393 and Modulation of Intestinal Microbial Flora. Microb. Physiol. 2010, 19, 224–230. [Google Scholar] [CrossRef]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R. Probiotics as treatment for food allergies among pediatric patients: A meta-analysis. World Allergy Organ. J. 2018, 11, 25. [Google Scholar] [CrossRef]
- Tripathi, M.; Giri, S. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef]
- Kailasapathy, K. Encapsulation technologies for functional foods and nutraceutical product development. CAB Rev. 2009, 4, 1–19. [Google Scholar] [CrossRef]
- Doleyres, Y.; Lacroix, C. Technologies with free and immobilised cells for probiotic bifidobacteria production and protection. Int. Dairy J. 2005, 15, 973–988. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Ballan, R.; Battistini, C.; Xavier-Santos, D.; Saad, S.M.I. Interactions of Probiotics and Prebiotics with the Gut Microbiota. In Progress in Molecular Biology and Translational Science; Academic Press: Amsterdam, The Netherlands, 2020; Volume 171, pp. 265–300. [Google Scholar]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.I.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef]
- Ramnani, P.; Gaudier, E.; Bingham, M.; Van Bruggen, P.; Tuohy, K.M.; Gibson, G.R. Prebiotic effect of fruit and vegetable shots containing Jerusalem artichoke inulin: A human intervention study. Br. J. Nutr. 2010, 104, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kleessen, B.; Schwarz, S.; Boehm, A.; Fuhrmann, H.; Richter, A.; Henle, T.; Krueger, M. Jerusalem artichoke and chicory inulin in bakery products affect faecal microbiota of healthy volunteers. Br. J. Nutr. 2007, 98, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Kjølbæk, L.; Benítez-Páez, A.; del Pulgar, E.M.G.; Brahe, L.K.; Liebisch, G.; Matysik, S.; Rampelli, S.; Vermeiren, J.; Brigidi, P.; Larsen, L.H.; et al. Arabinoxylan oligosaccharides and polyunsaturated fatty acid effects on gut microbiota and metabolic markers in overweight individuals with signs of metabolic syndrome: A randomized cross-over trial. Clin. Nutr. 2020, 39, 67–79. [Google Scholar] [CrossRef]
- Vigsnæs, L.K.; Holck, J.; Meyer, A.S.; Licht, T.R. In VitroFermentation of Sugar Beet Arabino-Oligosaccharides by Fecal Microbiota Obtained from Patients with Ulcerative Colitis To Selectively Stimulate the Growth of Bifidobacterium spp. and Lactobacillus spp. Appl. Environ. Microbiol. 2011, 77, 8336–8344. [Google Scholar] [CrossRef]
- Warren, F.J.; Fukuma, N.M.; Mikkelsen, D.; Flanagan, B.M.; Williams, B.A.; Lisle, A.T.; Cuiv, P.O.; Morrison, M.; Gidley, M.J. Food Starch Structure Impacts Gut Microbiome Composition. mSphere 2018, 3, e00086-18. [Google Scholar] [CrossRef]
- Singh, P.; Medronho, B.; Alves, L.; da Silva, G.; Miguel, M.; Lindman, B. Development of carboxymethyl cellulose-chitosan hybrid micro- and macroparticles for encapsulation of probiotic bacteria. Carbohydr. Polym. 2017, 175, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wan, Z.; Ou, A.; Liang, X.; Guo, X.; Zhang, Z.; Wu, L.; Xue, X. Monofloral honey from a medical plant, Prunella Vulgaris, protected against dextran sulfate sodium-induced ulcerative colitisviamodulating gut microbial populations in rats. Food Funct. 2019, 10, 3828–3838. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Haas, B.; Grenier, D. Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum. Sci. Rep. 2017, 7, 44815. [Google Scholar] [CrossRef]
- Yuan, L.; Li, X.; He, S.; Gao, C.; Wang, C.; Shao, Y. Effects of Natural Flavonoid Isoorientin on Growth Performance and Gut Microbiota of Mice. J. Agric. Food Chem. 2018, 66, 9777–9784. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, S.; Fan, S.; Ma, Y.; Li, D.; Tao, Y.; Han, Y. Encapsulation of bioactive polyphenols by starch and their impacts on gut microbiota. Curr. Opin. Food Sci. 2021, 38, 102–111. [Google Scholar] [CrossRef]
- Scheeler, A. Where Stool is a Drug: International Approaches to Regulating the use of Fecal Microbiota for Transplantation. J. Law Med. Ethics 2019, 47, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Haziri, D.; Brzozowski, T.; Hess, T.; Heyman, S.; Kwiecien, S.; Konturek, S.J.; Koziel, J. Emerging role of fe-cal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J. Physiol. Pharmacol. 2015, 66, 483–491. [Google Scholar]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.; Bogaerde, J.V.D.; Samuel, D.; Leong, R.W.L.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Kakiyama, G.; Savidge, T.; Takei, H.; Kassam, Z.A.; Fagan, A.; Gavis, E.A.; Pandak, W.M.; Nittono, H.; Hylemon, P.B.; et al. Antibiotic-Associated Disruption of Microbiota Composition and Function in Cirrhosis Is Restored by Fecal Transplant. Hepatology 2018, 68, 1549–1558. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Kassam, Z.; Fagan, A.; Gavis, E.A.; Liu, E.; Cox, I.J.; Kheradman, R.; Heuman, D.; Wang, J.; Gurry, T.; et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 2017, 66, 1727–1738. [Google Scholar] [CrossRef]
- Kim, K.O.; Gluck, M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin. Endosc. 2019, 52, 137–143. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Lam, K.; Lui, R.; Cheung, K.; Tang, W.; Ching, J.Y.L.; Chan, P.K.S.; Chan, M.C.W.; Wu, J.C.Y.; et al. Bacteriophage transfer during faecal microbiota transplantation inClostridium difficileinfection is associated with treatment outcome. Gut 2017, 67, 634–643. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, T.; El-Nezami, H.; Savidge, T.C. Food ingredients in human health: Ecological and metabolic perspectives implicating gut microbiota function. Trends Food Sci. Technol. 2020, 100, 103–117. [Google Scholar] [CrossRef]
- Venema, K.; Abbeele, P.V.D. Experimental models of the gut microbiome. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Abbeele, P.V.D.; Roos, S.; Eeckhaut, V.; MacKenzie, D.A.; Derde, M.; Verstraete, W.; Marzorati, M.; Possemiers, S.; Vanhoecke, B.; Van Immerseel, F.; et al. Incorporating a mucosal environment in a dynamic gut model results in a more representative colonization by lactobacilli. Microb. Biotechnol. 2011, 5, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Spinler, J.K.; Auchtung, J.; Brown, A.; Boonma, P.; Oezguen, N.; Ross, C.L.; Luna, R.A.; Runge, J.; Versalovic, J.; Peniche, A.; et al. Next-Generation Probiotics Targeting Clostridium difficile through Precursor-Directed Antimicrobial Biosynthesis. Infect. Immun. 2017, 85, e00303-17. [Google Scholar] [CrossRef]
- Li, C.; Niu, Z.; Zou, M.; Liu, S.; Wang, M.; Gu, X.; Lu, H.; Tian, H.; Jha, R. Probiotics, prebiotics, and synbiotics regulate the intestinal microbiota differentially and restore the relative abundance of specific gut microorganisms. J. Dairy Sci. 2020, 103, 5816–5829. [Google Scholar] [CrossRef]
- Newman, A.M.; Arshad, M. The Role of Probiotics, Prebiotics and Synbiotics in Combating Multidrug-Resistant Organisms. Clin. Ther. 2020, 42, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Santiago-López, L.; Hernández-Mendoza, A.; Garcia, H.S.; Mata-Haro, V.; Vallejo-Cordoba, B.; González-Córdova, A.F. The effects of consuming probiotic-fermented milk on the immune system: A review of scientific evidence. Int. J. Dairy Technol. 2015, 68, 153–165. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Rodriguez, J.; Taminiau, B.; Amadieu, C.; Herpin, F.; Allaert, F.-A.; Cani, P.D.; Daube, G.; Bindels, L.B.; Delzenne, N.M. Improvement of gastrointestinal discomfort and inflammatory status by a synbiotic in middle-aged adults: A double-blind randomized placebo-controlled trial. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Phavichitr, N.; COLOR Study Group; Wang, S.; Chomto, S.; Tantibhaedhyangkul, R.; Kakourou, A.; Intarakhao, S.; Jongpiputvanich, S.; Roeselers, G.; Knol, J. Impact of synbiotics on gut microbiota during early life: A randomized, double-blind study. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiary, M.; Morvaridzadeh, M.; Agah, S.; Rahimlou, M.; Christopher, E.; Zadro, J.R.; Heshmati, J. Effect of Probiotic, Prebiotic, and Synbiotic Supplementation on Cardiometabolic and Oxidative Stress Parameters in Patients With Chronic Kidney Disease: A Systematic Review and Meta-analysis. Clin. Ther. 2021, 43, e71–e96. [Google Scholar] [CrossRef]
- Scorletti, E.; Afolabi, P.R.; Miles, E.A.; Smith, D.E.; Almehmadi, A.; AlShathry, A.; Childs, C.E.; Del Fabbro, S.; Bilson, J.; Moyses, H.E.; et al. Synbiotics Alter Fecal Microbiomes, But Not Liver Fat or Fibrosis, in a Randomized Trial of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 158, 1597–1610.e7. [Google Scholar] [CrossRef]
- Askari, G.; Ghavami, A.; Shahdadian, F.; Moravejolahkami, A.R. Effect of synbiotics and probiotics supplementation on autoimmune diseases: A systematic review and meta-analysis of clinical trials. Clin. Nutr. 2021, 40, 3221–3234. [Google Scholar] [CrossRef]
- Kambale, R.M.; Nancy, F.I.; Ngaboyeka, G.A.; Kasengi, J.B.; Bindels, L.B.; Van der Linden, D. Effects of probiotics and synbiotics on diarrhea in undernourished children: Systematic review with meta-analysis. Clin. Nutr. 2021, 40, 3158–3169. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Sánchez, M.A.; Herisson, F.M.; Cluzel, G.L.; Caplice, N.M. Metabolic syndrome and synbiotic targeting of the gut microbiome. Curr. Opin. Food Sci. 2021, 41, 60–69. [Google Scholar] [CrossRef]
- Mohammadi-Sartang, M.; Bellissimo, N.; de Zepetnek, J.O.T.; Brett, N.R.; Mazloomi, S.M.; Fararouie, M.; Bedeltavana, A.; Famouri, M.; Mazloom, Z. The effect of daily fortified yogurt consumption on weight loss in adults with metabolic syndrome: A 10-week randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 565–574. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Bahrami, A.; Rehman, A.; Rezaei, A.; Babazadeh, A.; Singh, H.; Jafari, S.M. Co-encapsulation of probiotics with prebiotics and their application in functional/synbiotic dairy products. Crit. Rev. Food Sci. Nutr. 2020, 1–25. [Google Scholar] [CrossRef]
- Román, G.; Jackson, R.; Gadhia, R.; Román, A.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation Improved Probiotics Survival During Gastric Transit. HAYATI J. Biosci. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Cui, L.-H.; Yan, C.-G.; Li, H.-S.; Kim, W.-S.; Hong, L.; Kang, S.-K.; Choi, Y.-J.; Cho, C.-S. A New Method of Producing a Natural Antibacterial Peptide by Encapsulated Probiotics Internalized with Inulin Nanoparticles as Prebiotics. J. Microbiol. Biotechnol. 2018, 28, 510–519. [Google Scholar] [CrossRef]
- García-Díaz, M.; Birch, D.; Wan, F.; Nielsen, H.M. The role of mucus as an invisible cloak to transepithelial drug delivery by nanoparticles. Adv. Drug Deliv. Rev. 2018, 124, 107–124. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Ephrem, E.; Najjar, A.; Charcosset, C.; Greige-Gerges, H. Encapsulation of natural active compounds, enzymes, and probiotics for fruit juice fortification, preservation, and processing: An overview. J. Funct. Foods 2018, 48, 65–84. [Google Scholar] [CrossRef]
- Milea, Ș.A.; Vasile, M.A.; Crăciunescu, O.; Prelipcean, A.-M.; Bahrim, G.E.; Râpeanu, G.; Oancea, A.; Stănciuc, N. Co-Microencapsulation of Flavonoids from Yellow Onion Skins and Lactic Acid Bacteria Lead to Multifunctional Ingredient for Nutraceutical and Pharmaceutics Applications. Pharmaceutics 2020, 12, 1053. [Google Scholar] [CrossRef]
- Annunziata, G.; Jiménez-García, M.; Capó, X.; Moranta, D.; Arnone, A.; Tenore, G.; Sureda, A.; Tejada, S. Microencapsulation as a tool to counteract the typical low bioavailability of polyphenols in the management of diabetes. Food Chem. Toxicol. 2020, 139, 111248. [Google Scholar] [CrossRef]
- Yus, C.; Gracia, R.; Larrea, A.; Andreu, V.; Irusta, S.; Sebastian, V.; Mendoza, G.; Arruebo, M. Targeted Release of Probiotics from Enteric Microparticulated Formulations. Polymer 2019, 11, 1668. [Google Scholar] [CrossRef]
- Huq, T.; Fraschini, C.; Khan, A.; Riedl, B.; Bouchard, J.; Lacroix, M. Alginate based nanocomposite for microencapsulation of probiotic: Effect of cellulose nanocrystal (CNC) and lecithin. Carbohydr. Polym. 2017, 168, 61–69. [Google Scholar] [CrossRef]
- Sánchez, M.T.; Ruiz, M.A.; Lasserrot, A.; Hormigo, M.; Morales, M.E. An improved ionic gelation method to encapsulate Lactobacillus spp. bacteria: Protection, survival and stability study. Food Hydrocoll. 2017, 69, 67–75. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. J. Funct. Foods 2015, 19, 868–881. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Forghani, Z.; Jafari, S.M. A systematic review and meta-analysis of fish oil encapsulation within different micro/nanocarriers. Crit. Rev. Food Sci. Nutr. 2020, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Fan, G.-Q.; Zhang, Z.; Zhang, R.; Deng, Z.-Y.; McClements, D.J. Encapsulation of omega-3 fatty acids in nanoemulsions and microgels: Impact of delivery system type and protein addition on gastrointestinal fate. Food Res. Int. 2017, 100, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Peredo-Lovillo, A.; Beristain, C.; Pascual, L.; Azuara, E.; Jimenez, M. The effect of prebiotics on the viability of encapsulated probiotic bacteria. LWT 2016, 73, 191–196. [Google Scholar] [CrossRef]
- El-Abd, M.M.; Abdel-Hamid, M.; El-Sayed, S.H.; El Metwaly, A.H.; El-Demerdash, M.E.; Zeinab, F.A.M. Viability of Micro-encapsulated Probiotics Combined with Plant Extracts in Fermented Camel Milk under Simulated Gastrointestinal Condi-tions. Middle East J. Appl. Sci. 2018, 8, 837–850. [Google Scholar]
- Zhang, Y.; Lin, J.; Zhong, Q. S/O/W emulsions prepared with sugar beet pectin to enhance the viability of probiotic Lactobacillus salivarius NRRL B-30514. Food Hydrocoll. 2016, 52, 804–810. [Google Scholar] [CrossRef]
- Alehosseini, A.; del Pulgar, E.-M.G.; Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Fabra, M.J.; Sanz, Y.; Sarabi-Jamab, M.; Ghorani, B.; Lopez-Rubio, A. Unpurified Gelidium-extracted carbohydrate-rich fractions improve probiotic protection during storage. LWT 2018, 96, 694–703. [Google Scholar] [CrossRef]
- Alfaro-Galarza, O.; Villegas, E.O.L.; Rivero-Perez, N.; Maruri, D.T.-; Jiménez-Aparicio, A.; Palma-Rodríguez, H.; Vargas-Torres, A. Protective effects of the use of taro and rice starch as wall material on the viability of encapsulated Lactobacillus paracasei subsp. Paracasei. LWT 2020, 117, 108686. [Google Scholar] [CrossRef]
- Colín-Cruz, M.; Pimentel-González, D.; Carrillo-Navas, H.; Alvarez-Ramírez, J.; Guadarrama-Lezama, A. Co-encapsulation of bioactive compounds from blackberry juice and probiotic bacteria in biopolymeric matrices. LWT 2019, 110, 94–101. [Google Scholar] [CrossRef]
- Pinto, S.S.; Fritzen-Freire, C.B.; Benedetti, S.; Murakami, F.S.; Petrus, J.C.C.; Prudêncio, E.S.; Amboni, R.D. Potential use of whey concentrate and prebiotics as carrier agents to protect Bifidobacterium-BB-12 microencapsulated by spray drying. Food Res. Int. 2015, 67, 400–408. [Google Scholar] [CrossRef]
- Okuro, P.K.; Thomazini, M.; Balieiro, J.C.; Liberal, R.D.; Favarotrindade, C.S. Co- encapsulation of Lactobacillus acidophilus with inulin or polydextrose in solid lipid microparticles provides protection and improves stability. Food Res. Int. 2013, 53, 96–103. [Google Scholar] [CrossRef]
- Singh, P.; Medronho, B.; Valente, A.J.; Miguel, M.G.; Lindman, B. Exploring the prebiotic effect of cyclodextrins on probiotic bacteria entrapped in carboxymetyl cellulose-chitosan particles. Colloids Surf. B Biointerfaces 2018, 168, 156–162. [Google Scholar] [CrossRef]
- Damodharan, K.; Palaniyandi, S.A.; Yang, S.H.; Suh, J.W. Co-encapsulation of lactic acid bacteria and prebiotic with alginate-fenugreek gum-locust bean gum matrix: Viability of encapsulated bacteria under simulated gastrointestinal condition and during storage time. Biotechnol. Bioprocess Eng. 2017, 22, 265–271. [Google Scholar] [CrossRef]
- Arslan, S.; Erbas, M.; Tontul, I.; Topuz, A. Microencapsulation of probiotic Saccharomyces cerevisiae var. boulardii with different wall materials by spray drying. LWT 2015, 63, 685–690. [Google Scholar] [CrossRef]
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R. Double layer co-encapsulation of probiotics and prebiotics by electro-hydrodynamic atomization. LWT 2019, 110, 102–109. [Google Scholar] [CrossRef]
- Atia, A.; Gomaa, A.; Fernandez, B.; Subirade, M.; Fliss, I. Study and Understanding Behavior of Alginate-Inulin Synbiotics Beads for Protection and Delivery of Antimicrobial-Producing Probiotics in Colonic Simulated Conditions. Probiotics Antimicrob. Proteins 2018, 10, 157–167. [Google Scholar] [CrossRef]
- Serrano-Casas, V.; Pérez-Chabela, M.L.; Cortés-Barberena, E.; Totosaus, A. Improvement of lactic acid bacteria viability in acid conditions employing agroindustrial co-products as prebiotic on alginate ionotropic gel matrix co-encapsulation. J. Funct. Foods 2017, 38, 293–297. [Google Scholar] [CrossRef]
- Seifert, A.; Kashi, Y.; Livney, Y.D. Delivery to the gut microbiota: A rapidly proliferating research field. Adv. Colloid Interface Sci. 2019, 274, 102038. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.W.; Chen, M.; Li, Y.; Liang, R.; Xu, F.; Zhong, F. Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2863–2878. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.E.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Physiological protection of probiotic microcapsules by coatings. Crit. Rev. Food Sci. Nutr. 2018, 58, 1864–1877. [Google Scholar] [CrossRef]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T. Probiotic Encapsulation Technology: From Microencapsulation to Release into the Gut. Pharmaceutics 2012, 4, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Ding, Z.; Hou, D.; Prakash, S.; Zhao, Y.; Fan, Z.; Zhang, D.; Wang, Z.; Liu, M.; Han, J. Influence of polysaccharide as co-encapsulant on powder characteristics, survival and viability of microencapsulated Lactobacillus paracasei Lpc-37 by spray drying. J. Food Eng. 2019, 252, 10–17. [Google Scholar] [CrossRef]
- Enache, I.M.; Vasile, A.M.; Enachi, E.; Barbu, V.; Stănciuc, N.; Vizireanu, C. Co-Microencapsulation of Anthocyanins from Black Currant Extract and Lactic Acid Bacteria in Biopolymeric Matrices. Molecules 2020, 25, 1700. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, H.; Champagne, C.P.; Remondetto, G.E.; Gomaa, A.; Subirade, M. Co-encapsulation of Lactobacillus helveticus cells and green tea extract: Influence on cell survival in simulated gastrointestinal conditions. J. Funct. Foods 2016, 26, 451–459. [Google Scholar] [CrossRef]
- Phoem, A.N.; Mayiding, A.; Saedeh, F.; Permpoonpattana, P. Evaluation of Lactobacillus plantarum encapsulated with Eleutherine americana oligosaccharide extract as food additive in yoghurt. Braz. J. Microbiol. 2019, 50, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Eratte, D.; McKnight, S.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. Co-encapsulation and characterisation of omega-3 fatty acids and probiotic bacteria in whey protein isolate–gum Arabic complex coacervates. J. Funct. Foods 2015, 19, 882–892. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Khaza’Ai, H.; Abed, Y.; Rahmat, A.; Ismail, P.; Ranneh, Y. Role of fish oil in human health and possible mechanism to reduce the inflammation. Inflammopharmacology 2015, 23, 79–89. [Google Scholar] [CrossRef]
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622. [Google Scholar] [CrossRef]
- Kralovec, J.A.; Zhang, S.; Zhang, W.; Barrow, C.J. A review of the progress in enzymatic concentration and microencapsulation of omega-3 rich oil from fish and microbial sources. Food Chem. 2012, 131, 639–644. [Google Scholar] [CrossRef]
- Boran, G.; Karaçam, H.; Boran, M. Changes in the quality of fish oils due to storage temperature and time. Food Chem. 2006, 98, 693–698. [Google Scholar] [CrossRef]
- Andrejčáková, Z.; Sopková, D.; Vlčková, R.; Hertelyová, Z.; Gancarčíková, S.; Nemcová, R. The Application of Lactobacillus reuteri CCM 8617 and Flaxseed Positively Improved the Health of Mice Challenged with Enterotoxigenic E. coli O149:F4. Probiotics Antimicrob. Proteins 2019, 12, 937–951. [Google Scholar] [CrossRef]
- Jamshidi, A.; Cao, H.; Xiao, J.; Simal-Gandara, J. Advantages of techniques to fortify food products with the benefits of fish oil. Food Res. Int. 2020, 137, 109353. [Google Scholar] [CrossRef]
- Ruiz, J.C.R.; Ortiz-Vázquez, E.; Campos, M.R.S. Encapsulation of vegetable oils as source of omega-3 fatty acids for enriched functional foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liang, L.; Zhang, Z.; Deng, Z.; Decker, E.A.; McClements, D.J. Inhibition of lipid oxidation in nanoemulsions and filled microgels fortified with omega-3 fatty acids using casein as a natural antioxidant. Food Hydrocoll. 2017, 63, 240–248. [Google Scholar] [CrossRef]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: A review. Food Hydrocoll. 2016, 61, 442–457. [Google Scholar] [CrossRef]
- Barrow, C.J.; Nolan, C.; Holub, B.J. Bioequivalence of encapsulated and microencapsulated fish-oil supplementation. J. Funct. Foods 2009, 1, 38–43. [Google Scholar] [CrossRef]
- Eratte, D.; Dowling, K.; Barrow, C.J.; Adhikari, B. Recent advances in the microencapsulation of omega-3 oil and probiotic bacteria through complex coacervation: A review. Trends Food Sci. Technol. 2018, 71, 121–131. [Google Scholar] [CrossRef]
- Kankaanpää, P.E.; Salminen, S.J.; Isolauri, E.; Lee, Y.K. The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol. Lett. 2001, 194, 149–153. [Google Scholar] [CrossRef]
- Nemcova, R.; Borovská, D.; Koščová, J.; Gancarcikova, S.; Mudroňová, D.; Buleca, V.; Pistl, J. The effect of supplementation of flax-seed oil on interaction of Lactobacillus plantarum—Biocenol™ LP96 and Escherichia coli O8:K88ab:H9 in the gut of germ-free piglets. Res. Vet. Sci. 2012, 93, 39–41. [Google Scholar] [CrossRef]
- Bomba, A.; Nemcová, R.; Gancarcíková, S.; Herich, R.; Pistl, J.; Révajová, V.; Jonecová, Z.; Bugarský, A.; Levkut, M.; Kasteĺ, R.; et al. The influence of omega-3 polyunsaturated fatty acids (omega-3 pufa) on lactobacilli adhesion to the intestinal mucosa and on immunity in gnotobiotic piglets. Berl. Munch. Tierarztl. Wochenschr. 2003, 116, 312–316. [Google Scholar]
- Durkin, L.; Childs, C.; Calder, P. Omega-3 Polyunsaturated Fatty Acids and the Intestinal Epithelium—A Review. Foods 2021, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Falalyeyeva, T.; Boyko, N.; Tsyryuk, O.; Beregova, T.; Ostapchenko, L. Probiotics and nutraceuticals as a new frontier in obesity prevention and management. Diabetes Res. Clin. Pract. 2018, 141, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Falalyeyeva, T.; Mykhalchyshyn, G.; Molochek, N.; Savchuk, O.; Kyriienko, D.; Komisarenko, I. Probiotic and omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance, improves glycemia and obesity parameters in individuals with type 2 diabetes: A randomised controlled trial. Obes. Med. 2020, 19, 100248. [Google Scholar] [CrossRef]
- Eratte, D.; Dowling, K.; Barrow, C.; Adhikari, B.P. In-vitro digestion of probiotic bacteria and omega-3 oil co-microencapsulated in whey protein isolate-gum Arabic complex coacervates. Food Chem. 2017, 227, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Eratte, D.; Wang, B.; Dowling, K.; Barrow, C.J.; Adhikari, B. Survival and fermentation activity of probiotic bacteria and oxidative stability of omega-3 oil in co-microcapsules during storage. J. Funct. Foods 2016, 23, 485–496. [Google Scholar] [CrossRef]
- Vaziri, A.S.; Alemzadeh, I.; Vossoughi, M.; Khorasani, A.C. Co-microencapsulation of Lactobacillus plantarum and DHA fatty acid in alginate-pectin-gelatin biocomposites. Carbohydr. Polym. 2018, 199, 266–275. [Google Scholar] [CrossRef]
- Vega-Sagardía, M.; Rocha, J.; Sáez, K.; Smith, C.T.; Gutierrez-Zamorano, C.; García-Cancino, A. Encapsulation, with and without oil, of biofilm forming Lactobacillus fermentum UCO-979C strain in alginate-xanthan gum and its anti- Helicobacter pylori effect. J. Funct. Foods 2018, 46, 504–513. [Google Scholar] [CrossRef]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. Front. Neurosci. 2020, 14, 14. [Google Scholar] [CrossRef]
- Pandey, P.; Mishra, H.N. Co-microencapsulation of γ-Aminobutyric acid (GABA) and Probiotic Bacteria in Thermostable and Biocompatible Exopolysaccharides Matrix. LWT 2021, 136, 110293. [Google Scholar] [CrossRef]
- Shrishrimal, N.; Das, D. Retraction Notice: Phytochemicals from Plants to Combat Cardiovascular Disease. Curr. Med. Chem. 2012, 19, 2242–2251. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Sharma, R.; Padwad, Y. Plant-polyphenols based second-generation synbiotics: Emerging concepts, challenges, and opportunities. Nutrition 2020, 77, 110785. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chu, Q.; Yan, F.; Yang, Y.; Han, W.; Zheng, X. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J. Gastroenterol. Hepatol. 2016, 31, 1462–1469. [Google Scholar] [CrossRef]
- Van Der Lugt, B.; Van Beek, A.A.; Aalvink, S.; Meijer, B.; Sovran, B.; Vermeij, W.; Brandt, R.M.C.; De Vos, W.M.; Savelkoul, H.F.J.; Steegenga, W.T.; et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1−/Δ7 mice. Immun. Ageing 2019, 16, 1–17. [Google Scholar] [CrossRef]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; De Vos, W.M.; Satokari, R. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, J.; Sun, L.; Lyu, X.; Chang, X.-Y.; Mi, X.; Hu, M.-G.; Wu, C.; Chen, X. Akkermansia muciniphila: A potential novel mechanism of nuciferine to improve hyperlipidemia. Biomed. Pharmacother. 2021, 133, 111014. [Google Scholar] [CrossRef]
- Deng, L.; Ou, Z.; Huang, D.; Li, C.; Lu, Z.; Liu, W.; Wu, F.; Nong, C.; Gao, J.; Peng, Y. Diverse effects of different Akkermansia muciniphila genotypes on Brown adipose tissue inflammation and whitening in a high-fat-diet murine model. Microb. Pathog. 2020, 147, 104353. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Almeida, D.; Seabra, C.; Andrade, J.C.; Gomes, A.M.; Freitas, A.C. Uncovering Akkermansia muciniphila resilience or susceptibility to different temperatures, atmospheres and gastrointestinal conditions. Anaerobe 2020, 61, 102135. [Google Scholar] [CrossRef] [PubMed]
- Ansaldo, E.; Slayden, L.C.; Ching, K.L.; Koch, M.A.; Wolf, N.K.; Plichta, D.R.; Brown, E.M.; Graham, D.B.; Xavier, R.J.; Moon, J.J.; et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 2019, 364, 1179–1184. [Google Scholar] [CrossRef]
- Ottman, N.; Geerlings, S.Y.; Aalvink, S.; De Vos, W.M.; Belzer, C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 637–642. [Google Scholar] [CrossRef]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, Y.; Xu, N.; Mu, H.; Zhang, H.; Duan, J. Improved viability of Akkermansia muciniphila by encapsulation in spray dried succinate-grafted alginate doped with epigallocatechin-3-gallate. Int. J. Biol. Macromol. 2020, 159, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Kiskova, T.; Kubatka, P.; Büsselberg, D.; Kassayova, M. The Plant-Derived Compound Resveratrol in Brain Cancer: A Review. Biomolecules 2020, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.S.; Berrones, A.J.; Krishnakumar, I.; Charnigo, R.J.; Westgate, P.M.; Fleenor, B.S. Responsiveness to curcumin intervention is associated with reduced aortic stiffness in young, obese men with higher initial stiffness. J. Funct. Foods 2017, 29, 154–160. [Google Scholar] [CrossRef]
- Su, J.; Cai, Y.; Zhi, Z.; Guo, Q.; Mao, L.; Gao, Y.; Yuan, F.; Van der Meeren, P. Assembly of propylene glycol alginate/β-lactoglobulin composite hydrogels induced by ethanol for co-delivery of probiotics and curcumin. Carbohydr. Polym. 2021, 254, 117446. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Yu, B. The Bidirectional Interactions between Resveratrol and Gut Microbiota: An Insight into Oxidative Stress and Inflammatory Bowel Disease Therapy. BioMed Res. Int. 2019, 2019, 5403761–5403769. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, D.V.; Espinosa-Solis, V.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Martinez-Gutierrez, F.; Román-Aguirre, M.; Saavedra-Leos, M.Z. Preparation of Spray-Dried Functional Food: Effect of Adding Bacillus clausii Bacteria as a Co-Microencapsulating Agent on the Conservation of Resveratrol. Processes 2020, 8, 849. [Google Scholar] [CrossRef]
- Chavarri, M.; Maranon, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; Villarán, M.D.C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Hernández-Barrueta, T.; Martínez-Bustos, F.; Castaño-Tostado, E.; Lee, Y.; Miller, M.J.; Amaya-Llano, S.L. Encapsulation of probiotics in whey protein isolate and modified huauzontle’s starch: An approach to avoid fermentation and stabilize polyphenol compounds in a ready-to-drink probiotic green tea. LWT 2020, 124, 109131. [Google Scholar] [CrossRef]
- Shinde, T.; Sun-Waterhouse, D.; Brooks, J. Co-extrusion Encapsulation of Probiotic Lactobacillus acidophilus Alone or Together with Apple Skin Polyphenols: An Aqueous and Value-Added Delivery System Using Alginate. Food Bioproc. Technol. 2013, 7, 1581–1596. [Google Scholar] [CrossRef]
- Holkem, A.T.; Favaro-Trindade, C.S. Potential of solid lipid microparticles covered by the protein-polysaccharide complex for protection of probiotics and proanthocyanidin-rich cinnamon extract. Food Res. Int. 2020, 136, 109520. [Google Scholar] [CrossRef] [PubMed]
- Hijová, E.; Bertková, I.; Štofilová, J. Dietary fibre as prebiotics in nutrition. Central Eur. J. Public Health 2019, 27, 251–255. [Google Scholar] [CrossRef]
- Jones, J.M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr. J. 2014, 13, 34. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- Prado, S.B.R.D.; Minguzzi, B.T.; Hoffmann, C.; Fabi, J.P. Modulation of human gut microbiota by dietary fibers from unripe and ripe papayas: Distinct polysaccharide degradation using a colonic in vitro fermentation model. Food Chem. 2021, 348, 129071. [Google Scholar] [CrossRef]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R.; et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol. Motil. 2020, 32, e13914. [Google Scholar] [CrossRef]
- Ayua, E.O.; Kazem, A.E.; Hamaker, B.R. Whole grain cereal fibers and their support of the gut commensal Clostridia for health. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100245. [Google Scholar] [CrossRef]
- Sawicki, C.M.; Livingston, K.A.; Obin, M.; Roberts, S.B.; Chung, M.; McKeown, N.M. Dietary Fiber and the Human Gut Microbiota: Application of Evidence Mapping Methodology. Nutrients 2017, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.A.; Million, M.; Raoult, D. The Butyrogenic and Lactic Bacteria of the Gut Microbiota Determine the Outcome of Allogenic Hematopoietic Cell Transplant. Front. Microbiol. 2020, 11, 1642. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Albadran, H.A.; Chatzifragkou, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Stability of probiotic Lactobacillus plantarum in dry microcapsules under accelerated storage conditions. Food Res. Int. 2015, 74, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Ying, D.; Sanguansri, L.; Weerakkody, R.; Bull, M.; Singh, T.K.; Augustin, M.A. Effect of encapsulant matrix on stability of microencapsulated probiotics. J. Funct. Foods 2016, 25, 447–458. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- dos Santos, D.X.; Casazza, A.A.; Aliakbarian, B.; Bedani, R.; Saad, S.M.I.; Perego, P. Improved probiotic survival to in vitro gastrointestinal stress in a mousse containing Lactobacillus acidophilus La-5 microencapsulated with inulin by spray drying. LWT 2019, 99, 404–410. [Google Scholar] [CrossRef]
- Kumherová, M.; Veselá, K.; Jokešová, K.; Klojdová, I.; Horáčková, Š. Influence of co-encapsulation of Bifidobacterium animalis subsp. lactis Bb12 with inulin and ascorbic acid on its viability. Czech J. Food Sci. 2020, 38, 57–62. [Google Scholar] [CrossRef]
- Fayed, B.; Abood, A.; El-Sayed, H.S.; Hashem, A.M.; Mehanna, N.S. A synbiotic multiparticulate microcapsule for enhancing inulin intestinal release and Bifidobacterium gastro-intestinal survivability. Carbohydr. Polym. 2018, 193, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, X.; Xu, H.; Aguilar, Z.P.; Wei, H. Effect of skim milk coated inulin-alginate encapsulation beads on viability and gene expression of Lactobacillus plantarum during freeze-drying. LWT 2016, 68, 8–13. [Google Scholar] [CrossRef]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Khangwal, I.; Shukla, P. Potential prebiotics and their transmission mechanisms: Recent approaches. J. Food Drug Anal. 2019, 27, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Sathyabama, S.; Kumar, M.R.; Devi, P.B.; Vijayabharathi, R.; Priyadharisini, V.B. Co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT 2014, 57, 419–425. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of a synbiotic into PLGA/alginate multiparticulate gels. Int. J. Pharm. 2014, 466, 400–408. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Li, C.; Zheng, S.; Li, H.; Yu, J. Development of a microencapsulated synbiotic product and its application in yoghurt. LWT 2020, 122, 109033. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Watcharapoka, S. Effect of addition of inulin and galactooligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT 2014, 57, 761–766. [Google Scholar] [CrossRef]
- Pinto, S.S.; Verruck, S.; Vieira, C.R.; Prudêncio, E.S.; Amante, E.R.; Amboni, R.D. Influence of microencapsulation with sweet whey and prebiotics on the survival of Bifidobacterium-BB-12 under simulated gastrointestinal conditions and heat treatments. LWT 2015, 64, 1004–1009. [Google Scholar] [CrossRef]
- Darjani, P.; Hosseininezhad, M.; Kadkhodaee, R.; Milani, E. Influence of prebiotic and coating materials on morphology and survival of a probiotic strain of Lactobacillus casei exposed to simulated gastrointestinal conditions. LWT 2016, 73, 162–167. [Google Scholar] [CrossRef]
- Nunes, G.L.; Etchepare, M.D.A.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; Flores, É.M.D.M.; Silva, C.D.B.D.; De Menezes, C.R. Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Rosolen, M.D.; Bordini, F.W.; de Oliveira, P.D.; Conceição, F.R.; Pohndorf, R.S.; Fiorentini, Â.M.; da Silva, W.P.; Pieniz, S. Symbiotic microencapsulation of Lactococcus lactis subsp. lactis R7 using whey and inulin by spray drying. LWT 2019, 115, 108411. [Google Scholar] [CrossRef]
- Raddatz, G.C.; Fonseca, B.D.S.D.; Poletto, G.; Jacob-Lopes, E.; Cichoski, A.J.; Muller, E.I.; Flores, E.M.M.; Silva, C.D.B.D.; de Menezes, C.R. Influence of the prebiotics hi-maize, inulin and rice bran on the viability of pectin microparticles containing Lactobacillus acidophilus LA-5 obtained by internal gelation/emulsification. Powder Technol. 2020, 362, 409–415. [Google Scholar] [CrossRef]
- Raddatz, G.C.; Poletto, G.; de Deus, C.; Codevilla, C.F.; Cichoski, A.J.; Jacob-Lopes, E.; Muller, E.I.; Flores, E.M.M.; Esmerino, E.A.; de Menezes, C.R. Use of prebiotic sources to increase probiotic viability in pectin microparticles obtained by emulsification/internal gelation followed by freeze-drying. Food Res. Int. 2020, 130, 108902. [Google Scholar] [CrossRef] [PubMed]
- Maleki, O.; Khaledabad, M.A.; Amiri, S.; Asl, A.K.; Makouie, S. Microencapsulation of Lactobacillus rhamnosus ATCC 7469 in whey protein isolate-crystalline nanocellulose-inulin composite enhanced gastrointestinal survivability. LWT 2020, 126, 109224. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Nedovic, V.; Goyal, A.; Kourkoutas, Y. Immobilization Technologies in Probiotic Food Production. J. Nutr. Metab. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, V.; Ramaiyan, B.; Subhasree, R. Encapsulation “The Future of Probiotics”—A Review. Adv. Biol. Res. 2009, 3, 96–103. [Google Scholar]
- Kourkoutas, Y.; Xolias, V.; Kallis, M.; Bezirtzoglou, E.; Kanellaki, M. Lactobacillus casei cell immobilization on fruit pieces for probiotic additive, fermented milk and lactic acid production. Process. Biochem. 2005, 40, 411–416. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Bosnea, L.; Taboukos, S.; Baras, C.; Lambrou, D.; Kanellaki, M. Probiotic Cheese Production Using Lactobacillus casei Cells Immobilized on Fruit Pieces. J. Dairy Sci. 2006, 89, 1439–1451. [Google Scholar] [CrossRef]
- Sidira, M.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Effect of immobilized Lactobacillus casei on volatile compounds of heat treated probiotic dry-fermented sausages. Food Chem. 2015, 178, 201–207. [Google Scholar] [CrossRef]
- Sidira, M.; Kourkoutas, Y.; Kanellaki, M.; Charalampopoulos, D. In vitro study on the cell adhesion ability of immobilized lactobacilli on natural supports. Food Res. Int. 2015, 76, 532–539. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Kourkoutas, Y.; Albantaki, N.; Tzia, C.; Koutinas, A.A.; Kanellaki, M. Functionality of freeze-dried L. casei cells immobilized on wheat grains. LWT 2009, 42, 1696–1702. [Google Scholar] [CrossRef]
- Vitola, H.R.S.; Dannenberg, G.; Marques, J.D.L.; Lopes, G.V.; Da Silva, W.P.; Fiorentini, Â.M. Probiotic potential of Lactobacillus casei CSL3 isolated from bovine colostrum silage and its viability capacity immobilized in soybean. Process. Biochem. 2018, 75, 22–30. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Kourkoutas, Y.; Kanellaki, M. Novel probiotic whey cheese with immobilized lactobacilli on casein. LWT 2017, 86, 627–634. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Sidira, M.; Koutinas, A.A.; Kourkoutas, Y. Free and immobilized Lactobacillus casei ATCC 393 on whey protein as starter cultures for probiotic Feta-type cheese production. J. Dairy Sci. 2014, 97, 4675–4685. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou, D.; Kourkoutas, Y.; Koutinas, A.; Kanellaki, M.; Kourkoutas, I. Thermally-dried immobilized kefir on casein as starter culture in dried whey cheese production. Food Microbiol. 2009, 26, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou, D.; Tsaousi, K.; Kourkoutas, Y.; Panas, P.; Kanellaki, M.; Koutinas, A.A. Fermentation efficiency of thermally dried immobilized kefir on casein as starter culture. Process. Biochem. 2008, 43, 1323–1329. [Google Scholar] [CrossRef]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef]
- Kopsahelis, N.; Panas, P.; Kourkoutas, Y.; Koutinas, A.A. Evaluation of the Thermally Dried Immobilized Cells of Lactobacillus delbrueckii subsp.bulgaricuson Apple Pieces as a Potent Starter Culture. J. Agric. Food Chem. 2007, 55, 9829–9836. [Google Scholar] [CrossRef]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2016, 23, 57–91. [Google Scholar] [CrossRef]
- Khorasani, A.C.; Shojaosadati, S.A. Bacterial nanocellulose-pectin bionanocomposites as prebiotics against drying and gastrointestinal condition. Int. J. Biol. Macromol. 2016, 83, 9–18. [Google Scholar] [CrossRef]
- Nwagu, T.N.; Ugwuodo, C.J. Stabilizing bromelain for therapeutic applications by adsorption immobilization on spores of probiotic Bacillus. Int. J. Biol. Macromol. 2019, 127, 406–414. [Google Scholar] [CrossRef]
- Ugwuodo, C.J.; Nwagu, T.N.T.; Ugwu, T.T.; Onwosi, C.O. Enhancement of the Anti-inflammatory Effect of Bromelain by Its Immobilization on Probiotic Spore of Bacillus cereus. Probiotics Antimicrob. Proteins 2020, 1–15. [Google Scholar] [CrossRef]
- Ester, B.; Noelia, B.; Laura, C.-J.; Francesca, P.; Cristina, B.; Rosalba, L.; Marco, D.R. Probiotic survival and in vitro digestion of L. salivarius spp. salivarius encapsulated by high homogenization pressures and incorporated into a fruit matrix. LWT 2019, 111, 883–888. [Google Scholar] [CrossRef]
- Vitola, H.R.S.; Cruxen, C.; da Silva, F.T.; Thiel, P.R.; Marques, J.D.L.; da Silva, W.P.; Fiorentini, Â.M. Lactobacillus casei CSL3: Evaluation of supports for cell immobilization, viability during storage in Petit Suisse cheese and passage through gastrointestinal transit in vitro. LWT 2020, 127, 109381. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kanellaki, M.; Kopsahelis, N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT 2019, 105, 242–249. [Google Scholar] [CrossRef]
- Bhat, A.; Irorere, V.; Bartlett, T.; Hill, D.; Kedia, G.; Charalampopoulos, D.; Nualkaekul, S.; Radecka, I. Improving survival of probiotic bacteria using bacterial poly-γ-glutamic acid. Int. J. Food Microbiol. 2015, 196, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Fijałkowski, K.; Peitler, D.; Rakoczy, R.; Żywicka, A. Survival of probiotic lactic acid bacteria immobilized in different forms of bacterial cellulose in simulated gastric juices and bile salt solution. LWT 2016, 68, 322–328. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Chaikham, P.; Rattanasena, P. Survival of immobilized probiotics in chocolate during storage and with an in vitro gastrointestinal model. Food Biosci. 2016, 16, 37–43. [Google Scholar] [CrossRef]
- Terpou, A.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A.; Nigam, P. Enhanced probiotic viability and aromatic profile of yogurts produced using wheat bran (Triticum aestivum) as cell immobilization carrier. Process. Biochem. 2017, 55, 1–10. [Google Scholar] [CrossRef]
- Kyereh, E.; Sathivel, S. Viability of Lactobacillus plantarum NCIMB 8826 immobilized in a cereal-legume complementary food “weanimix” with simulated gastrointestinal conditions. Food Biosci. 2021, 40, 100848. [Google Scholar] [CrossRef]
- Moya, A.; Ferrer, M. Functional Redundancy-Induced Stability of Gut Microbiota Subjected to Disturbance. Trends Microbiol. 2016, 24, 402–413. [Google Scholar] [CrossRef]
- Ha, C.W.Y.; Lam, Y.Y.; Holmes, A.J. Mechanistic links between gut microbial community dynamics, microbial functions and metabolic health. World J. Gastroenterol. 2014, 20, 16498–16517. [Google Scholar] [CrossRef]
- Pérez-Cobas, A.E.; Gosalbes, M.J.; Friedrichs, A.; Knecht, H.; Artacho, A.; Eismann, K.; Otto, W.; Rojo, D.; Bargiela, R.; Von Bergen, M.; et al. Gut microbiota disturbance during antibiotic therapy: A multi-omic approach. Gut 2013, 62, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef]
- Ivanovska, T.P.; Mladenovska, K.; Zhivikj, Z.; Pavlova, M.J.; Gjurovski, I.; Ristoski, T.; Petrushevska-Tozi, L. Synbiotic loaded chitosan-Ca-alginate microparticles reduces inflammation in the TNBS model of rat colitis. Int. J. Pharm. 2017, 527, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Zubaidah, A.; Yuhana, M. Widanarni Encapsulated Synbiotic Dietary Supplementation at Different Dosages to Prevent Vibriosis in White Shrimp, Litopenaeus vannamei. HAYATI J. Biosci. 2015, 22, 163–168. [Google Scholar] [CrossRef]
- Singh, P.K.; Kaur, I.P. Synbiotic (probiotic and ginger extract) loaded floating beads: A novel therapeutic option in an experimental paradigm of gastric ulcer. J. Pharm. Pharmacol. 2012, 64, 207–217. [Google Scholar] [CrossRef]
| Bioactive Substance | Probiotic Strain | Co-Encapsulation Technique | Carrier Material | Highlights | Ref. |
|---|---|---|---|---|---|
| GTE (rich in polyphenols) | Lactobacillus helveticus R0052 | emulsification and internal gelation | whey protein and calcium pectinate | - with initial 0.5 mg/mL GTE concentration, 95.5% L. helveticus and 79% polyphenol EYs in MCs were observed - additional protection of L. helveticus during sGIC was significantly enhanced in MCs from pectin solutions coated with whey proteins and containing 1 mg/mL GTE | [137] |
| GTE (rich in polyphenols) | Lactobacillus rhamnosus GG | spray-drying | modified huauzontle’s starch and whey protein | - final count of the cells was 9.01 ± 0.03 log CFU/g within the MCs after spray-drying - with 0.1 mg/mL of ascorbic acid within MCs, 7.33 ± 0.16 CFU/mL of L. rhamnosus was maintained for five weeks of storage at 4 °C - 38.52 ± 0.72% of green tea polyphenols formed complex with at least one component of the MCs | [186] |
| Agar-based extract from Gelidium seaweed (rich in polyphenols) | Bifidobacterium pseudocatenulatum CECT 7765 | emulsification and internal gelation | agar/agarose/whey protein/gelatin/starch | - the presence of polyphenols and proteins in the unpurified agar MCs significantly improved the B. pseudocatenulatum viability both at ambient and refrigerated storage conditions | [118] |
| Blackberry juice (rich in polyphenols and anthocyanins) | Lactobacillus acidophilus DSM13241 | spray-drying | gum Arabic/maltodextrin/whey protein/50:50 blends | - 98.4 ± 1.0% total phenolic compounds and 99.0 ± 1.0% total monomeric anthocyanin content presented in gum Arabic and maltodextrin blend MCs - L. acidophilus survival was 81.2 ± 0.7% after ten weeks at 20 °C in whey protein MCs | [120] |
| Black currant extract (rich in anthocyanins, polyphenols, and flavonoids) | Lactobacillus casei ssp. paracasei (L. casei 431®) | freeze-drying | whey protein and inulin and chitosan | - 95.46% ± 1.30% EY for anthocyanins and 87.38% ± 0.48% EY for L. casei - viability after 90 days at 4 °C of the co-encapsulated cells with black currant extract ranged from 8.13 to 6.35 log CFU/g - anthocyanins were mostly released in the intestinal environment during sGIC | [136] |
| Apple skin extract (ASPE) (rich in polyphenols) | Lactobacillus acidophilus | co-extrusion | alginate | - EY for all the obtained alginate MCs was over 96%- the co-encapsulation of L. acidophilus with an aqueous or ethanolic ASPE protected cells in acidic conditions, with cell loss only 2.61 and 2.78 log CFU/g, respectively, in comparison with cell loss in MCs without ASPE (3.08 log CFU/g) and free cells (5.41 log CFU/g) | [187] |
| Cinnamon extract (PRCE) (rich in proanthocyanidin) | Lactobacillus paracasei (BGP1) and Bifidobacterium animalis subsp. lactis (BLC1) | complex coacervation followed by freeze drying | whey protein and gum Arabic | - the treatments with B. animalis and 5% PRCE presented greater EY for probiotic, phenolics, and proanthocyanids, with 98.59% ± 0.45, 119.49% ± 4.21, and 81.25% ± 1.9, respectively - higher viability of B. animalis (9.30 ± 0.16 log CFU/g) after 120 days of storage at 7 °C than L. paracasei (6.64 ± 0.10 log CFU/g) | [188] |
| Yellow onion skin extract (rich in flavonoids) | Lactobacillus casei ssp. paracasei (L. casei 431®) | freeze-drying | whey protein and inulin and maltodextrin | - EY of L. casei in MCs with flavonoids was 72.49 ± 0.11% - 85% of flavonoids in MCs were available after sGIC - stimulating effect on L. casei viability was observed after 21 days in soft cheese with MCs | [107] |
| Bioactive Substance | Probiotic Strain | Co-encapsulation Technique | Carrier Material | Highlights | Ref. |
|---|---|---|---|---|---|
| GOS (BiMunoTM) | Bifidobacterium breve NCIMB 8807 | fluid-bed drying | alginate and chitosan and poly(D,L-lactic-co-glycolic acid) | - 6.6 ± 0.5 log CFU/mL cells of encapsulated B. breve survived 1 h in sGIC in alginate and chitosan MCs - 8.0 ± 0.3 log CFU/mL cells survived in MCs with GOS/poly(d,l-lactic-co-glycolic acid) included | [209] |
| Sugar beet | Lactobacillus salivarius NRRL B-30514 | emulsification | sugar beet pectin | - 87% EY of L. salivarius in sugar beet pectin MCs prepared in sugar beet pectin/soybean oil/water emulsions - after 2 h incubation in sGIC, the lowest decrease in viability was observed in emulsion with CaCl2 - free L. salivarius became undetectable after 3 h in sGIC - cross-linking sugar beet pectin by Ca2+ ions additionally protected L. salivarius during sGIC | [117] |
| Lactitol, GOS, eight types of commercial prebiotics | Lactobacillus casei 28-2, Lactobacillus casei 30-1, Lactobacillus paracasei 6062, Lactobacillus plantarum 25-1 | extrusion | alginate and chitosan | - lacticol had a highest prebiotic score value for Lactobacillus strains - mechanical strength of MCs with different lacticol additions decreased constantly in sGIC - log reduction of cells after 120 min in sGIC was 7.37, 3.41, 3.13, and 2.97 for 0, 10, 20, and 25 g/L concentration of lacticol in MCs, respectively | [210] |
| Inulin, polydextrose | Lactobacillus acidophilus 04 | spray-chilling | lipid matrix | -free cells were not detectable after 210 min in sGIC -ca. 60% of the cells in the MCs with or without a prebiotic were viable after 300 min in sGIC | [122] |
| Inulin, GOS | Lactobacillus acidophilus 5 and Lactobacillus casei 01 | extrusion | alginate and chitosan | -the presence of 1.5% GOS in the MCs provided the best protection with only 3.1 and 2.9 log CFU/g reduction for L. acidophilus 5 and L. Casei 01, respectively, after incubation in sGIC | [211] |
| Inulin, polydextrose | Bifidobacterium BB-12 | spray-drying | sweet whey protein | -after sGIC, the free cell count showed a decrease of 1.18 log CFU/g, while the MCs showed decreases of 0.49, 0.97, and 2.45 log CFU/g for sweet whey, sweet whey and inulin, and sweet whey and polydextrose, respectively | [212] |
| Inulin | Lactobacillus casei 431 | extrusion | alginate and chitosan | - 5.7 log reduction for free cells, 3.9 log reduction for alginate MCs, 2.7–2.8 log reduction for alginate and inulin MCs, 0.7–0.9 log CFU/g reduction for alginate and inulin MCs coated with chitosan after exposition to sGIC | [213] |
| Inulin, hi-maize, trehalose | Lactobacillus acidophilus La-5 | spray-drying | gum Arabic and maltodextrin and inulin/hi-maize/trehalose | - MCs produced with hi-maize showed the greatest viability after sGIC, from 11.50 ± 0.09 to 10.49 ± 0.12 log CFU/g, followed by inulin, from 11.38 ± 0.11 to 10.16 ± 0.08 log CFU/g | [214] |
| Inulin | Lactococcus lactis subsp. lactis R7 | spray-drying | whey protein and inulin | - 94.61% EY of L. lactis in MCs - free cells exposed for 7 days to pH 2.0, 2.5, and 3.0 had 2.18, 1.00, and 1.78 log CFU/g reduction, respectively; in contrast, no significant decrease of co-encapsulated L. lactis was observed | [215] |
| Inulin, resistant starch | Lactobacillus plantarum ATCC 8014™ and Bifidobacterium animalis subsp. lactis | electro-hydrodynamic atomization | alginate and chitosan | - MCs containing resistant starch were better in maintaining the viability of probiotics under sGIC - viability of B. lactis in MCs with resistant starch was reduced from 8.77 ± 0.12 to only 7.19 ± 0.15 CFU/g | [127] |
| Inulin, hi-maize, rice bran | Lactobacillus acidophilus LA-5 | extrusion/external ionic gelation | alginate or blends with (rice bran/inulin/hi-maize) | - initial count of L. acidophilus was 13.85 ± 0.05, 13.94 ± 0.20, 14.24 ± 0.05, and 11.21 ± 0.09 log CFU/g for alginate, rice bran, inulin, and hi-maize, respectively, and after exposure to sGIC: 11.18 ± 0.13, 8.06 ± 0.01, 8.93 ± 0.09, and 9.47 ± 0.23 log CFU/g, respectively - the alginate, rice bran, and hi-maize MCs maintained viable probiotics for 120 days at 25 °C; rice bran and inulin preserved viable probiotics in MCs over the 120 days of storage at 7 °C; only in MCs with inulin did cells remain viable for 120 days at −18 °C | [42] |
| Inulin, hi-maize, rice bran | Lactobacillus acidophilus LA-5 | emulsification/internal ionic gelation | pectin | - the best EY was obtained in MCs with rice bran and inulin: 91.24% and 90.59%, respectively - 3.30 log reduction in viability of free cells after the sGIC; however, in co-encapsulated L. acidophilus, only 0.11, 0.9, 1.63, and 2.37 log CFU/g reductions were observed for the pectin MCs or in formations with hi-maize, inulin, and rice bran, respectively | [216] |
| Inulin, hi-maize, rice bran | Lactobacillus acidophilus LA-5 | emulsification/internal ionic gelation followed by freeze-drying | pectin | - the highest EY was obtained in MCs with inulin: 68.1%; 3.4 ± 0.1 log reduction in viability of free cells after sGIC and for co-encapsulated ones: 1.3 ± 0.2, 0.1 ± 0.0, 1.6 ± 0.2, and 1.0 ± 0.2 log CFU/g for pectin MCs or in formations with hi-maize, inulin, and rice bran, respectively, in relation to initial counts | [217] |
| Inulin | Lactobacillus rhamnosus ATCC 7469 | freeze-drying | whey protein and crystalline nanocellulose and inulin | - the highest EY was 89.60% for formulation: whey protein—57.22%, crystalline nanocellulose—25.00%, and inulin—17.78%; this composition significantly improved survival of the probiotics in the sGIC in comparison with free cells | [218] |
| Carrier Material | Probiotic Strain | Simulated Gastrointestinal Conditions | Ref. |
|---|---|---|---|
| Apple pieces | Lactobacillus casei ATCC 393 | - counts of immobilized L. casei were significantly higher after 120 min at pH 2.0 and after 30, 60, 90, and 120 min at pH 1.5 compared to free cells; cell immobilization resulted in significantly higher survival rates in pancreatic juices supplemented with 0.45% bile salts after 240 min and in bile salts after 120 min; reduced counts of staphylococci, enterobacteria, coliforms, and streptococci in rat feces after oral administration of free or immobilized L. casei contained in probiotic-fermented milk revealed modulation of gut microbiota | [54] |
| Apple disks | Lactobacillus salivarius spp. salivarius CECT 4063 | - dried apple with immobilized encapsulated L. salivarius was mainly affected by the acidic environment created (10 mL of pepsin (0.6% w/v) adjusted to pH 3 with HCl 4 M) and the addition of bile; survival of immobilized L. salivarius also decreased with storage time at different gastro-intestinal stages | [237] |
| Dehydrated fruits: pineapple, guava, and kiwi | Lactobacillus casei CSL3 | - the most appropriate support for immobilization of L. casei was pineapple, depending on viability and sensorial evaluation; sGIC did not affect viability of probiotics incorporated in cheese, either in its free or immobilized form | [238] |
| Sea buckthorn berries (Hippophae rhamnoides L.) | Lactobacillus casei ATCC 393 | - immobilized L. casei remained at concentration 7.47 log CFU/g, while the free cells remained at 6.01 ± 0.13 CFU/g after sGIC - 90 days of frozen storage did not affect viability of L. casei incorporated in frozen yogurt, either in its free or immobilized form | [239] |
| Poly-γ-glutamic acid (γ-PGA) | Bifidobacterium longum NCIMB 8809 and Bifidobacterium breve NCIMB 8807 | - both strains, protected with 2.5% γ-PGA, survived in simulated gastric juice (pH 2.0) with a slight reduction (<0.47 log CFU/mL) or no significant reduction after 4 h, while free cells died within 2 h - loss in viable cells of γ-PGA-immobilized B. breve and B. longum showed only around 0.5 log and 1.1 log CFU/mL reductions, respectively; however, around 4.0 log and 3.4 log CFU/mL reductions were observed in free B. breve and B. longum cells, respectively, after 13 days of storage in orange juice at 4 °C | [240] |
| Bacterial cellulose (BC) (produced by Gluconacetobacter xylinus) | Lactobacillus delbrueckii PKM 490, Lactobacillus plantarum DSM 13,273, and Lactobacillus casei ATCC 393 | - the immobilization of Lactobacillus in BC during co-culture with cellulose-synthetizing G. xylinus enabled almost full protection of the probiotic bacteria against the harmful environment of sGIC - co-cultures of G. xylinus and Lactobacillus strains did not adversely influence the BC biosynthesis | [241] |
| White, milk, and dark chocolate | Lactobacillus casei 01 and Lactobacillus acidophilus LA-5 | - the immobilized L. casei in different chocolates had higher levels of survivability after being exposed to sGIC, and they still remained to be viable at ~2 log CFU/mL after 6 h - the survival rate of L. casei was generally higher than that of L. acidophilus, regardless of the types of chocolate; however, storage conditions at 4 °C were suitable for retaining probiotic viability, no matter the probiotic strain or chocolate type used | [242] |
| Wheat bran | Lactobacillus casei ATCC 393 | - incubation for 2 h in the simulated gastric acid led to higher reduction of viability of free cells than immobilized ones - similarly, enhanced viability of immobilized L. casei incorporated into the yogurt samples during simulated gastric juice conditions (from 9.94 log CFU/g to 9.27 log CFU/g) compared to the free cells (from 9.50 log CFU/g to 8.22 log CFU/g) was observed | [243] |
| Wheat bran | Lactobacillus casei ATCC 393 | - incubation for 2 h in simulated gastric juice (pH 3) of cheese samples with the freeze-dried immobilized L. casei resulted in a low loss of cell viability (from 8.43 to 8.19 log CFU/g), while in the case of cheese containing free L. casei, the loss of cell viability was higher (from 8.24 to 7.82 log CFU/g) | [22] |
| MCPPM, MCP, MC | Lactobacillus plantarum NCIMB 8826 | - at an adjusted simulated gastric fluid (pH 3.0), reduction in the viability of free cells was 4.4 log CFU/g after 180 min, while the immobilized L. plantarum had reductions of 1.0, 1.1, and 1.6 log CFU/g for MCPPM, MCP, and MC, respectively - after 24 h exposure to 1% bile juice, 4.2, 1.9, 1.7, and 1.8 log CFU/g reductions were observed for free cells, MC, MCP, and MCPPM, respectively | [244] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kvakova, M.; Bertkova, I.; Stofilova, J.; Savidge, T.C. Co-Encapsulated Synbiotics and Immobilized Probiotics in Human Health and Gut Microbiota Modulation. Foods 2021, 10, 1297. https://doi.org/10.3390/foods10061297
Kvakova M, Bertkova I, Stofilova J, Savidge TC. Co-Encapsulated Synbiotics and Immobilized Probiotics in Human Health and Gut Microbiota Modulation. Foods. 2021; 10(6):1297. https://doi.org/10.3390/foods10061297
Chicago/Turabian StyleKvakova, Monika, Izabela Bertkova, Jana Stofilova, and Tor C. Savidge. 2021. "Co-Encapsulated Synbiotics and Immobilized Probiotics in Human Health and Gut Microbiota Modulation" Foods 10, no. 6: 1297. https://doi.org/10.3390/foods10061297
APA StyleKvakova, M., Bertkova, I., Stofilova, J., & Savidge, T. C. (2021). Co-Encapsulated Synbiotics and Immobilized Probiotics in Human Health and Gut Microbiota Modulation. Foods, 10(6), 1297. https://doi.org/10.3390/foods10061297