Are Peach Cultivars Used in Conventional Long Food Supply Chains Suitable for the High-Quality Short Markets?
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphological Fruit Traits
2.2. Analysis of Physico-Chemical Measures
2.3. Rapid Detection of Volatile Compounds Using PTR-ToF-MS
2.4. Sensory Measurements
2.4.1. Panel Test
2.4.2. Consumer Acceptability
2.5. Correlation between Aroma Descriptors, Physico-Chemical Parameters and Volatile Compounds
3. Methodology
3.1. Fruit Collection
3.2. Analysis of Physicochemical Measures
3.3. Rapid Detection of Volatile Compounds Using PTR-ToF-MS
3.4. Sensory Measurements
3.5. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SFSCs | Short Food Supply Chains |
| LFSCs | Long Food Supply Chains |
| SSCPs | Short Supply Chain Peaches |
| LSCPs | Long Supply Chain Peaches |
Appendix A
| Compounds Code | m/z | Peach and Neactarine Varieties | Chemical Formula | Tentative Identification | Literature | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1° | 2° | 3° | 4° | 5° | 6° | 7° | 8° | 9° | 10° | 11° | 12° | 13° | |||||
| Big Top | Romagna Big | Maillard Magic | Maria Marta | Rome Star | Lady Erica | Sweet Dream | Alma | Venus | Alma2 | Nectaross | Guglielmina | Regina di Londa | |||||
| 1 | 27.022 | + | + | + | + | + | + | + | + | + | + | + | + | + | C2H3+ | Acetylene | |
| 2 | 30.049 | + | + | + | + | + | + | + | + | + | + | + | + | + | C2H7+ | Ethylene (isotope) | [53] |
| 3 | 31.018 | + | + | + | + | + | + | + | + | + | + | + | + | + | CH3O+ | Formaldehyde | [53] |
| 4 | 33.033 | + | + | + | + | + | + | + | + | + | + | + | + | + | CH5O+ | Methanol | [53] |
| 5 | 41.038 | + | + | + | + | + | + | + | + | + | + | + | + | + | C3H5+ | Fragment (alcohol, ester) | [53] |
| 6 | 43.018 | + | + | + | + | + | + | + | + | + | + | + | + | + | C2H3O+ | Fragment (ester) | [53] |
| 7 | 43.050 | + | + | + | + | + | + | + | + | + | + | + | + | + | C3H7+ | Fragment (alcohol, ester) | [53] |
| 8 | 45.033 | + | + | + | + | + | + | + | + | + | + | + | + | + | C2H5O+ | Acetaldehyde | [53] |
| 9 | 47.049 | + | + | + | + | + | + | + | + | + | + | + | + | + | C2H7O+ | Ethanol | [53] |
| 10 | 51.040 | − | − | − | + | + | + | − | − | − | − | − | + | + | C3H5+ | C4 fragment | [53] |
| 11 | 53.038 | + | + | + | + | + | + | + | + | + | + | + | + | + | C4H5+ | Fragment (ester) | [53] |
| 12 | 55.054 | + | + | + | + | + | + | + | + | + | + | + | + | + | C4H7+ | C4 aldehydes fragment (aldehyde) | [53] |
| 13 | 57.033 | + | + | + | + | + | + | + | + | + | + | + | + | + | C3H5O+ | Fragment (hexanal/1-butanol) | |
| 14 | 57.069 | + | + | + | + | + | + | + | + | + | + | + | + | + | C4H9+ | Alkyl Fragment (alcohol, ester) | [53] |
| 15 | 59.049 | + | + | + | + | + | + | + | + | + | + | + | + | + | C3H7O+ | Acetone | [53] |
| 16 | 61.028 | + | + | + | + | + | + | + | + | + | + | + | + | + | C2H5O2+ | Acetic acid | [53] |
| 17 | 65.038 | − | + | + | − | − | − | − | − | − | − | − | + | + | C5H5+ | N.I. | |
| 18 | 67.050 | + | + | + | + | + | + | + | + | + | + | + | + | + | C5H7+ | N.I. | |
| 19 | 69.069 | + | + | + | + | + | + | + | + | + | + | + | + | + | C5H9+ | Isoprene | [53] |
| 20 | 71.049 | + | + | + | + | + | + | + | + | + | + | + | + | + | C4H7O+ | 2-Butenal | [54] |
| 21 | 73.028 | + | + | + | + | + | + | + | + | + | + | + | + | + | C3H5O2+ | Vinyl formate | [55] |
| 22 | 73.064 | + | + | + | + | + | + | + | + | + | + | + | + | + | C4H9O+ | N.I. | |
| 23 | 75.044 | − | + | + | + | + | + | + | − | + | − | + | + | + | C3H7O2+ | Methyl acetate | [56] |
| 24 | 77.050 | + | + | + | + | + | + | + | + | + | + | + | + | + | C6H5+ | N.I. | |
| 25 | 79.049 | + | + | + | + | + | + | + | + | + | + | + | + | + | C6H7+ | Benzene | [57] |
| 26 | 81.069 | + | + | + | + | + | + | + | + | + | + | + | + | + | C6H9+ | Terpene and C6 fragments | |
| 27 | 83.086 | + | + | + | + | + | + | + | + | + | + | + | + | + | C6H11+ | Hydrocarbon fragment | [53] |
| 28 | 85.065 | − | + | + | + | + | + | + | + | + | + | + | − | − | C5H9O+ | (E)-2-Pentenal | [58] |
| 29 | 85.101 | + | + | + | + | + | + | + | + | + | + | + | + | + | C6H13+ | Fragment (alcohol) | [53] |
| 30 | 87.044 | + | + | + | + | + | − | − | + | + | − | + | − | − | C4H7O2+ | 2,3-Butanedione | [59] |
| 31 | 87.080 | + | + | + | + | + | + | + | + | + | + | − | + | + | C5H11O+ | Pentanal/1-Penten-3-ol | [58] |
| 32 | 89.059 | + | + | + | + | + | + | + | + | + | + | + | + | + | C4H9O2+ | Ethyl acetate | [55] |
| 33 | 91.050 | + | + | + | + | + | + | + | + | + | + | + | + | + | C4H11O2+ | 2,3-Butanediol | [59] |
| 34 | 93.069 | + | + | + | + | + | + | + | + | + | + | + | + | + | C7H9+ | Terpenes and C6 fragment | |
| 35 | 95.049 | + | − | − | − | − | − | − | − | − | − | − | − | − | C6H7O+ | Phenol | [58] |
| 36 | 95.086 | + | + | + | + | + | + | + | + | + | + | + | + | + | C7H11+ | Terpenes and C6 fragment | |
| 37 | 97.069 | + | + | + | + | + | + | + | + | + | + | + | + | + | C6H9O+ | 2,4-Hexadienal/2-Ethylfuran | [58] |
| 38 | 99.080 | + | + | + | + | + | + | + | + | + | + | + | + | + | C6H11O+ | 2-Hexenal | [58] |
| 39 | 101.060 | + | − | − | + | + | + | − | − | − | − | − | − | − | C5H9O2+ | γ-Valerolactone | [54] |
| 40 | 101.096 | + | + | + | + | + | + | + | + | + | + | + | + | + | C6H13O+ | Hexanal/3-Hexen-1-ol | [58] |
| 41 | 103.075 | + | + | + | + | + | + | + | + | + | + | + | + | + | C6H15O+ | 1-Hexanol/Hexyl alcohol | [58] |
| 42 | 107.049 | − | + | + | + | + | − | − | + | − | + | − | − | − | C7H7O+ | Benzaldehyde | [55] |
| 43 | 107.085 | + | + | + | + | + | + | + | + | + | + | + | + | + | C8H11+ | Xylene | [60] |
| 44 | 109.070 | + | + | + | + | + | + | + | + | + | + | + | + | + | C7H9O+ | Benzyl alcohol | [60] |
| 45 | 111.090 | + | + | + | + | + | + | + | + | + | + | + | + | + | C7H11O+ | 2,4-Heptadienal | [53] |
| 46 | 115.075 | − | + | + | + | + | + | + | + | + | + | + | + | + | C6H11O2+ | c-Hexalactone/ε-Caprolactone | [58] |
| 47 | 117.091 | − | − | − | + | + | + | + | + | + | + | + | + | + | C6H13O2+ | Butyl acetate/Hexanoic acid | [58] |
| 48 | 121.065 | + | + | + | + | + | + | + | + | + | + | + | + | + | C8H9O+ | Benzeneacetaldehyde | [53] |
| 49 | 129.091 | + | + | + | + | + | + | + | + | + | − | − | + | + | C7H13O2+ | c-Heptalactone | [58] |
| 50 | 131.106 | − | − | − | + | − | + | − | − | − | − | − | − | − | C7H15O2+ | Pentyl acetate | [58] |
| 51 | 133.122 | + | − | − | − | − | − | − | + | + | − | − | − | − | C7H17O2+ | N.I. | |
| 52 | 135.118 | + | + | + | + | + | + | + | + | + | − | − | − | − | C10H15+ | α-Cymene | [57] |
| 53 | 137.132 | + | + | + | − | + | + | − | − | + | + | + | + | + | C10H17+ | Monoterpene compounds | [58] |
| 54 | 143.107 | + | + | + | + | + | + | + | + | + | + | + | + | + | C8H15O2+ | Hexenyl acetate | [57] |
| 55 | 145.122 | − | − | + | + | − | + | − | + | + | + | + | + | + | C8H17O2+ | Ethyl hexanoate/Hexyl acetate | [58] |
| 56 | 145.159 | − | − | − | +(Tr) | − | +(Tr) | − | − | − | − | − | +(Tr) | − | C9H21O+ | Nonanol | [58] |
| 57 | 151.075 | + | − | − | − | − | − | − | − | − | − | − | − | − | C9H11O2+ | Ethyl benzoate | [58] |
| 58 | 153.127 | − | + | + | + | + | + | − | + | + | − | − | + | + | C10H17O+ | Terpenoid-like compound(e.g., camphor) or 2,4-Decadienal | [58] |
| 59 | 155.107 | − | − | − | − | + | − | − | − | − | − | − | − | − | C9H15O2+ | 2-Nonen-4-olide | [53] |
| 60 | 155.143 | − | + | − | − | − | − | − | − | + | − | − | − | − | C10H19O+ | Linalool/a-Terpineol/Eucalyptol | [58] |
| 61 | 159.140 | − | + | + | + | − | + | + | + | + | − | − | + | + | C9H19O2+ | Methyl octanoate | [57] |
| 62 | 167.107 | − | − | − | + | + | + | + | + | + | − | − | + | + | C10H15O2+ | 6-Pentyl-2H-pyran-2-one | [60] |
| 63 | 171.137 | − | − | − | + | + | + | + | − | − | − | − | − | − | C10H19O2+ | c-Decalactone | [58] |
| 64 | 177.185 | − | − | − | − | − | − | − | − | − | − | − | + | + | C10H25O2+ | Ethyl acetate cluster (x2) | [61] |
| 65 | 193.160 | − | − | + | − | − | − | − | − | − | − | − | + | + | C13H20O+ | á-Ionone | [58] |
| 66 | 205.195 | − | − | − | + | + | − | − | − | − | − | − | − | − | C15H25+ | Sesquiterpenes | |
| Total VOCs emission (ppbv) | 44,017 | 118,161 | 109,515 | 186,039 | 153,685 | 170,393 | 51,200 | 129,716 | 110,722 | 89,636 | 148,900 | 194,809 | 149,945 | ||||
| Total VOCs numbers | 47 | 51 | 52 | 57 | 55 | 55 | 48 | 51 | 53 | 45 | 45 | 53 | 52 | ||||
References
- Reynolds, C.; Buckley, J.; Weinstein, P.; Boland, J. Are the dietary guidelines for meat, fat, fruit and vegetable consumption appropriate for environmental sustainability? A review of the literature. Nutrients 2014, 6, 2251–2265. [Google Scholar] [CrossRef]
- Yahia, E.M.; García-Solís, P.; Celis, M.E.M. Contribution of fruits and vegetables to human nutrition and health. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Cambridge, UK, 2018; Chapter 1; pp. 19–45. [Google Scholar]
- Giarè, F.; Giuca, S. Agricoltori e Filiera Corta: Profili Giuridici e Dinamiche Socio-Economiche; INEA: Roma, Italy, 2012; pp. 12–15. [Google Scholar]
- Bartolini, S.; Ducci, E.; Viti, R. Local fruit varieties for sustainable cultivations: Pomological, nutraceutical and sensory characterization. Agrochim. Pisa 2015, 59, 281–294. [Google Scholar]
- Raffaelli, R.; Coser, L.; Gios, G. Esperienze di filiera corta nell’agro-alimentare: Un’indagine esplorativa in provincia di Trento. Econ. Agro-Aliment. 2009. [Google Scholar] [CrossRef]
- Darnhofer, I. Organic farming and rural development: Some evidence from Austria. Sociol. Ruralis 2005, 45, 308–323. [Google Scholar] [CrossRef]
- Robinson, G.M. Towards sustainable agriculture: Current debates. Geogr. Compass 2009, 3, 1757–1773. [Google Scholar] [CrossRef]
- Galli, F.; Brunori, G. Short food supply chains as drivers of sustainable development. Evid. Doc. 2013. [Google Scholar] [CrossRef]
- Little, J.; Ilbery, B.; Watts, D. Gender, consumption and the relocalisation of food: A research agenda. Sociol. Ruralis 2009, 49, 201–217. [Google Scholar] [CrossRef]
- Bernelli, M.; Marini, G. L’altra Spesa: Consumare Come il Mercato Non Vorrebbe; Ambiente: Milan, Italty, 2010. [Google Scholar]
- Mundler, P.; Laughrea, S. The contributions of short food supply chains to territorial development: A study of three Quebec territories. J. Rural Stud. 2016, 45, 218–229. [Google Scholar] [CrossRef]
- Jakobek, L.; García-Villalba, R.; Tomás-Barberán, F.A. Polyphenolic characterisation of old local apple varieties from Southeastern European region. J. Food Comp. Anal. 2013, 31, 199–211. [Google Scholar] [CrossRef]
- Migliore, G.; Schifani, G.; Cembalo, L. Opening the black box of food quality in the short supply chain: Effects of conventions of quality on consumer choice. Food Qual. Pref. 2015, 39, 141–146. [Google Scholar] [CrossRef]
- Caruso, T.; Sottile, F. La peschicoltura autunnale in Sicilia: Aspetti ambientali, varietali e colturali. Frutticoltura 1999, 2, 39–46. [Google Scholar]
- Badenes, M.L.; Llácer, G.; Crisosto, C.H. Mejoragenética de la Calidad en Plantas; Sociedad Española de Ciencias Hortícolas y Sociedad Española de Genética: Valencia, Spain, 2006; pp. 551–578. [Google Scholar]
- Hilaire, C.; Giauque, P. Le Pêcher; Centre Technique Interprofessionnel des Fruits et Légumes: Paris, France, 2003. [Google Scholar]
- El Hadi, M.A.M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Crisosto, C.; Garner, D.; Cid, L.; Day, K. Peach size affects storage, market life. Calif. Agric. 1999, 53, 33–36. [Google Scholar] [CrossRef]
- Miceli, C.; Infante, R.; Inglese, P. Instrumental and sensory evaluation of eating quality of peaches and nectarines. Eur. J. Hort. Sci. 2010, 75, 97–102. [Google Scholar] [CrossRef]
- Della Casa, R. In calo i consumi e l’export di pesche e nettarine italiane. Rivista di Frutticoltura e di Ortofloricoltura 2005, 67, 19–20. [Google Scholar]
- Taiti, C.; Costa, C.; Menesatti, P.; Caparrotta, S.; Bazihizina, N.; Azzarello, E.; Petrucci, A.W.; Pandolfi, C.; Giordani, E. Use of volatile organic compounds and physicochemical parameters for monitoring the post-harvest ripening of imported tropical fruits. Eur. Food Res. Technol. 2015, 241, 91–102. [Google Scholar] [CrossRef]
- Esti, M.; Messia, M.C.; Sinesio, F.; Nicotra, A.; Conte, L.; La Notte, E.; Palleschi, G. Quality evaluation of peaches and nectarines by electrochemical and multivariate analyses: Relationships between analytical measurements and sensory attributes. Food. Chem. 1997, 60, 659–666. [Google Scholar] [CrossRef]
- Wu, B.; Quilot, B.; Kervella, J.; Génard, M.; Li, S. Analysis of genotypic variation of sugar and acid contents in peaches and nectarines through the principle component analysis. Euphytica 2003, 132, 375–384. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Lu, M.T.; Song, C.W.; Huang, C.C.; Ou, S.K. Changes in flesh firmness and ethylene production of different peach types during fruit ripening. Acta Hortic. 2008, 768, 153–159. [Google Scholar]
- Dirlewanger, E.; Moing, A.; Rothan, C.; Svanella, L.; Pronier, V.; Guye, A.; Plomion, C.; Monet, R. Mapping QTLs controlling fruit quality in peach (Prunus persica (L.) Batsch). Theor. Appl. Genet. 1999, 98, 18–31. [Google Scholar] [CrossRef]
- Kader, A.A. Fruit maturity, ripening, and quality relationships. Acta Hortic. 1999, 485, 203–208. [Google Scholar] [CrossRef]
- Bellini, E. Il Germoplasma della Toscana: Tutela e Valorizzazione Delle Specie Legnose da Frutto; Il Germoplasma della Toscana: Tutela e valorizzazione; ARSIA: Firenzetel, Italy, 2000; Volume 33. [Google Scholar]
- Aubert, C.; Milhet, C. Distribution of the volatile compounds in the different parts of a white-fleshed peach (Prunus persica L. Batsch). Food Chem. 2007, 102, 375–384. [Google Scholar] [CrossRef]
- Kader, A.A. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Klee, H.J. Improving the flavor of fresh fruits: Genomics, biochemistry, and biotechnology. New Phytol. 2010, 187, 44–56. [Google Scholar] [CrossRef]
- Carrari, F.; Fernie, A.R. Metabolic regulation underlying tomato fruit development. J. Exp. Bot. 2006, 57, 1883–1897. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J. Sci. Food Agric. 2010, 90, 1146–1154. [Google Scholar] [CrossRef]
- Sánchez, G.; Venegas-Calerón, M.; Salas, J.J.; Monforte, A.; Badenes, M.L.; Granell, A. An integrative “omics” approach identifies new candidate genes to impact aroma volatiles in peach fruit. BMC Genom. 2013, 14, 343. [Google Scholar] [CrossRef]
- Contador, R.; González-Cebrino, F.; García-Parra, J.; Lozano, M.; Ramírez, R. Effect of hydrostatic high pressure and thermal treatments on two types of pumpkin purée and changes during refrigerated storage. J. Food Process Preserv. 2014, 38, 704–712. [Google Scholar] [CrossRef]
- Xi, W.; Zhang, Q.; Lu, X.; Wei, C.; Yu, S.; Zhou, Z. Improvement of flavour quality and consumer acceptance during postharvest ripening in greenhouse peaches by carbon dioxide enrichment. Food Chem. 2014, 164, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Colaric, M.; Veberic, R.; Stampar, F.; Hudina, M. Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes. J. Sci. Food Agric. 2005, 85, 2611–2616. [Google Scholar] [CrossRef]
- Cirilli, M.; Bassi, D.; Ciacciulli, A. Sugars in peach fruit: A breeding perspective. Hortic. Res. 2016, 3, 1–12. [Google Scholar] [CrossRef]
- Krawitzkyab, M.; Ariasa, E.; Peiroac, J.M.; Negueruelaa, A.I.; Valb, J.; Oriaa, R. Determination of color, antioxidant activity, phenolic profile of different fruit tissue of Spanish ‘Verde Doncella’ apple cultivar. Int. J. Food Prop. 2014, 17, 2298–2311. [Google Scholar] [CrossRef]
- Mennone, C.; Bellini, E.; Nencetti, V.; Natarelli, L.; Liverani, A.; Insero, O. Liste Varietali in Frutticoltura: Pesco. Terra e Vita 2007, 26, 48–75. [Google Scholar]
- Abbott, J.A. Quality measurement of fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 207–225. [Google Scholar] [CrossRef]
- Nencetti, V.; Liverani, A.; Sartori, A. Liste varietali Pesco 2015. Terra Vita 2015, 38, 1–5. [Google Scholar]
- Giovannini, D.; Liverani, A.; Bassi, D.; Lateur, M. ECPGR Priority descriptors for peach [Prunus persica (L.) Batsch]. Eur. Coop. Programme Plant Genet. Resour. 2013, 9–15. [Google Scholar]
- AOAC. Official Methods of Analysis; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Taiti, C.; Marone, E.; Lanza, M.; Azzarello, E.; Masi, E.; Pandolfi, C.; Giordani, E.; Mancuso, S. Nashi or Williams pear fruits? Use of volatile organic compounds, physicochemical parameters, and sensory evaluation to understand the consumer’s preference. Eur. Food Res. Technol. 2017, 243, 1917–1931. [Google Scholar] [CrossRef]
- Cappellin, L.; Biasioli, F.; Granitto, P.B.; Schuhfried, E.; Soukoulis, C.; Costa, F.; Tillman, M.D.; Gasperi, F. On data analysis in PTR-TOF-MS: From raw spectra to data mining. Sens. Actuators B Chem. 2011, 155, 183–190. [Google Scholar] [CrossRef]
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food. Int. J. Mass Spectrom. Ion Processes 1998, 173, 191–241. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Carr, B.T.; Civille, G.V. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Practices and Principals; Chapman and Hall: New York, NY, USA, 1998. [Google Scholar]
- Sgarbossa, A.; Costa, C.; Menesatti, P.; Antonucci, F.; Pallottino, F.; Zanetti, M.; Grigolato, S.; Cavalli, R. Colorimetric patterns of wood pellets and their relations with quality and energy parameters. Fuel 2014, 137, 70–76. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Bianchi, T.; Weesepoel, Y.; Koot, A.; Iglesias, I.; Eduardo, I.; Gratacós-Cubarsí, M.; Guerrero, L.; Hortos, M.; van Ruth, S. Investigation of the aroma of commercial peach (Prunus persica L. Batsch) types by Proton Transfer Reaction-Mass Spectrometry (PTR-MS) and sensory analysis. Food Res. Int. 2017, 99, 133–146. [Google Scholar] [CrossRef]
- Narain, N.; Hsieh, T.C.Y.; Johnson, C.E. Dynamic headspace concentration and gas chromatography of volatile flavor components in peach. J. Food Sci. 1990, 55, 1303–1307. [Google Scholar] [CrossRef]
- İmrak, B.; Küden, A.B.; Tanriver, E.; Kafkas, E. Volatile and some fruit quality characteristics of new promising peach genotypes. Acta Sci. Pol. Hortorum Cultus 2015, 14, 3–12. [Google Scholar]
- Rizzolo, A.; Bianchi, G.; Vanoli, M.; Lurie, S.; Spinelli, L.; Torricelli, A. Electronic nose to detect volatile compound profile and quality changes in ‘Spring Belle’ peach (Prunus persica L.) during cold storage in relation to fruit optical properties measured by time-resolved reflectance spectroscopy. J. Agric. Food Chem. 2012, 61, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Kralj, M.B.; Jug, T.; Komel, E.; Fajt, N.; Jarni, K.; Zivkovic, J.; Mujic, I. Aromatic compound in different peach cultivars and effect of preservatives on the final aroma of cooked fruits. Hem. Ind. 2014, 68, 767–780. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Li, S.; Yang, L.; Wang, Y.; Zhao, J.; Jiang, Q. Volatile characteristics of 50 peaches and nectarines evaluated by HP–SPME with GC–MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]
- Dabbou, S.; Lussiana, C.; Maatallah, S.; Gasco, L.; Hajlaoui, H.; Flamini, G. Changes in biochemical compounds in flesh and peel from Prunus persica fruits grown in Tunisia during two maturation stages. Plant Physiol. Biochem. 2016, 100, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Horvat, R.J.; Chapman, G.W., Jr.; Robertson, J.A.; Meredith, F.I.; Scorza, R.; Callahan, A.M.; Morgens, P. Comparison of the volatile compounds from several commercial peach cultivars. J. Agric. Food Chem. 1990, 38, 234–237. [Google Scholar] [CrossRef]
- Fiches, G.; Déléris, I.; Saint-Eve, A.; Brunerie, P.; Souchon, I. Modifying PTR-MS operating conditions for quantitative headspace analysis of hydro-alcoholic beverages. 2. Brandy characterization and discrimination by PTR-MS. Int. J. Mass Spectrom. 2014, 360, 15–23. [Google Scholar] [CrossRef]
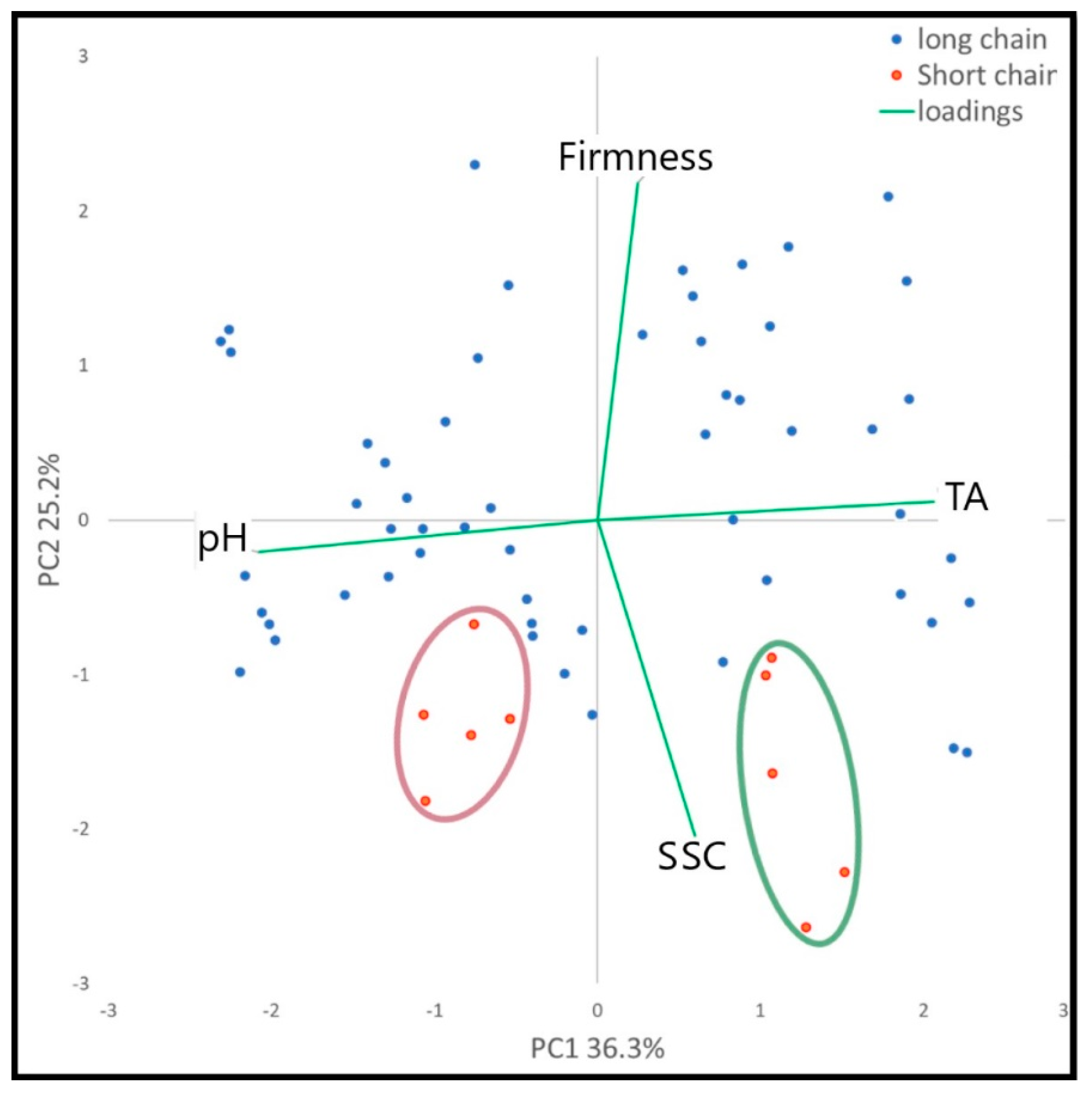


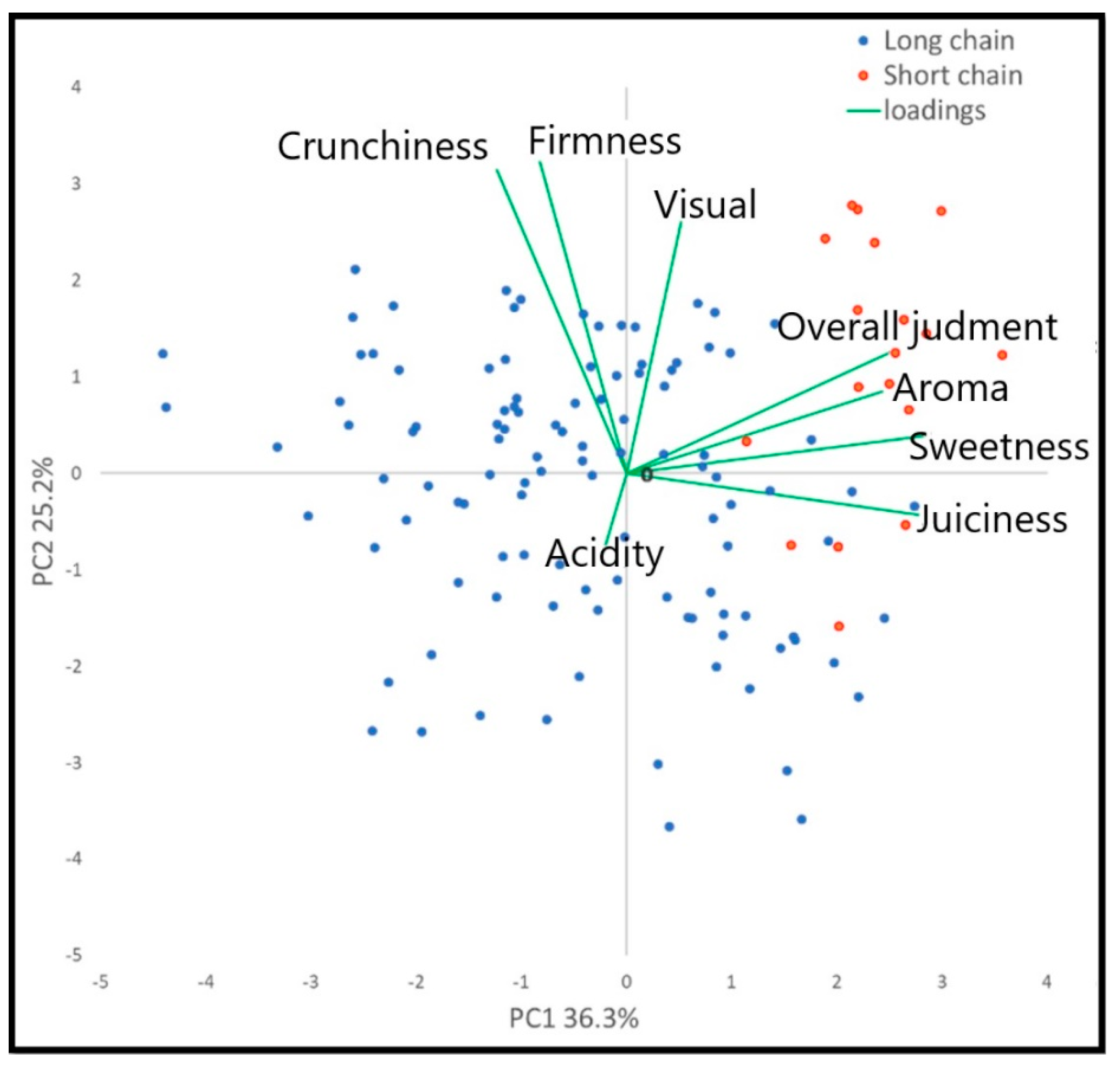


| Cultivar | Typology | Morphological Traits | Skin Color | ||||
|---|---|---|---|---|---|---|---|
| Average Fruits Weight (g) | Ø Max (mm) | Height (mm) | L | a | b | ||
| Alma | Nectarine | 295.5 (±24.8) | 80.4 (±2.1) | 76.3 (±5.3) | 80.2 (±2.6) | −3.7 (±1.8) | 52.3 (±3.5) |
| Alma2 | Nectarine | 254.1 (±28.3) | 79.2 (±3.6) | 75.0 (±3.7) | 75.3 (±3.2) | −1.8 (±3.8) | 54 (±2.3) |
| Big top | Nectarine | 226.0 (±4.6) | 91.7 (±13.7) | 64.7 (±6.9) | 47.5 (±10.9) | 13.8 (±1.7) | 31.9 (±9.3) |
| Guglielmina | Peach | 242.0 (±15.2) | 74.6 (±7.6) | 67.2 (±5.5) | 55.4 (±5.0) | 20.7 (±2.5) | 44.1 (±4.5) |
| Maillard Magic | Nectarine | 209.2 (±26.7) | 73.7 (±4.7) | 68.8 (±3.1) | 52.1 (±62.8) | 24.3 (±8.2) | 26.1 (±2.3) |
| Maria Marta | Peach | 209.0 (±15.1) | 74.7 (±2.5) | 64.1 (±2.3) | 57.1 (±5.5) | 23.1 (±4.8) | 41.9 (±4.9) |
| Nectaross | Nectarine | 170.0 (±10.2) | 69.0 (±2.4) | 64.0 (±3.2) | 77.5 (±3.5) | −2.4 (±2.9) | 54.7 (±1.8) |
| Regina di Londa | Peach | 256.0 (±16.4) | 79.3 (±5.4) | 72.0 (±2.2) | 52.1 (±3.2) | 23.3 (±3.4) | 31.9 (±9.3) |
| Romagna Big | Nectarine | 213.0 (±25.9) | 73.4 (±3.4) | 73.1 (±3.5) | 65.0 (±5.4) | 10.3 (±8.0) | 46.0 (±3.9) |
| Rome Star | Peach | 224.0 (±21.8) | 76.0 (±3.4) | 69.1 (±4.2) | 40.2 (±7.9) | 22.3 (±5.1) | 20.2 (±5.8) |
| Lady Erica | Nectarine | 312.0 (±15.1) | 83.0 (±1.9) | 80.6 (±4.8) | 48.8 (±9.4) | 27.7 (±5.2) | 30.9 (±10.4) |
| Sweet Dream | Peach | 277.5 (±26.6) | 84.2 (±3.2) | 73.0 (±2.2) | 44.2 (±9.3) | 18.8 (±2.3) | 21.1 (±10.2) |
| Venus | Nectarine | 260.0 (±27.9) | 77.0 (±2.4) | 76.3 (±5.8) | 76.6 (±3.3) | −2.5 (±3.4) | 55.4 (±1.6) |
| Nectarines average | 250.5 (±48.4) a | 79.0 (±4.5) a | 73.0 (±3.7) a | ||||
| Peaches average | 244.5 (±48.4) a | 78.7 (±8.3) a | 70.5 (±6.1) a | ||||
| Cultivar | Typology | Pulp Firmness (kgf) | SSC (°Brix) | pH | Titratable Acidity (meq/100 g Pulp FW) | SSC/TA Ratio |
|---|---|---|---|---|---|---|
| Alma | Nectarine | 4.20 (±0.4) | 13.3 (±0.9) | 3.3 (±0.05) | 42.3 (±0.3) | 0.31 |
| Alma2 | Nectarine | 4.10 (±0.6) | 14.3 (±1.0) | 4.5 (±0.1) | 13.2 (±1.1) | 1.08 |
| Big top | Nectarine | 4.30 (±0.5) | 13.3 (±1.5) | 3.1 (±0.4) | 29.1 (±0.7) | 0.46 |
| Guglielmina | Peach | 2.22 (±0.5) | 14.6 (±1.5) | 4.5 (±0.1) | 17.4 (±0.5) | 0.83 |
| Maillard Magic | Nectarine | 2.90 (±0.7) | 12.5 (±0.8) | 4.5 (±0.4) | 17.7 (±2.4) | 0.71 |
| Maria Marta | Peach | 1.6 (±0.5) | 11.9 (±0.8) | 4.2 (±0.1) | 26.2 (±0.5) | 0.45 |
| Nectaross | Nectarine | 3.4 (±0.3) | 16.0 (±1.2) | 3.5 (±0.1) | 47.8 (±2.7) | 0.34 |
| Regina di Londa | Peach | 1.5 (±0.5) | 16.4 (±1.4) | 3.5 (±0.1) | 31.6 (±0.5) | 0.52 |
| Romagna Big | Nectarine | 4.1 (±0.5) | 15.2 (±1.2) | 4.2 (±0.2) | 25.4 (±2.5) | 0.60 |
| Rome Star | Peach | 3.3 (±0.7) | 13.1 (±0.6) | 3.6 (±0.1) | 32.8 (±0.7) | 0.40 |
| Lady Erica | Nectarine | 2.5 (±0.4) | 14.1 (±0.8) | 4.9 (±0.1) | 11.4 (±0.3) | 1.24 |
| Sweet Dream | Peach | 3.9 (±0.6) | 11.7 (±0.6) | 5.2 (±0.3) | 8.6 (±0.6) | 1.36 |
| Venus | Nectarine | 4.0 (±0.5) | 11.9 (±0.6) | 3.5 (±0.05) | 35.5 (±1.1) | 0.33 |
| Nectarines average | 3.7 (±0.9) a | 13.5 (±1.5) a | 4.1 (±0.6) a | 25.6 (±11.9) a | 0.66 | |
| Peaches average | 2.5 (±1.1) b | 14.0 (±1.9) a | 3.9 (±0.6) a | 27.0 (±8.4) a | 0.55 | |
| Variety | Degree of Liking * (1–9) (Average Value) | Acceptance (%) | Neither Like nor Dislike (%) | Dislike (%) |
|---|---|---|---|---|
| Alma | 6.40 | 58% | 24% | 18% |
| Alma2 | 6.14 | 50% | 28% | 22% |
| Big Top | 6.65 | 68% | 32% | 0% |
| Guglielmina | 8.73 | 100% | 0% | 0% |
| Maillard Magic | 6.93 | 71% | 21% | 8% |
| Maria Marta | 7.00 | 59% | 37% | 4% |
| Nectaross | 7.43 | 86% | 14% | 0% |
| Regina di Londa | 8.36 | 100% | 0% | 0% |
| Romagna Big | 6.94 | 74% | 26% | 0% |
| Rome Star | 6.55 | 52% | 46% | 12% |
| Lady Erica | 6.79 | 78% | 22% | 0% |
| Sweet Dream | 6.08 | 50% | 29% | 21% |
| Venus | 6.60 | 64% | 28% | 8% |
| Varieties | Fruit Type | Fruit Flesh | Fruit Shape a (Longitudinal Section) | Diffusion Area | Ripening Time (±Days of Collection Compared to “Big Top”) ** | Perceived Acidity | Length of Supply Chain |
|---|---|---|---|---|---|---|---|
| Alma | Nectarine | Yellow | Round | Italy | +20 | B | LSCP |
| Alma2 | Nectarine | Yellow | Round | Italy | +32 | S | LSCP |
| Big Top | Nectarine | Yellow | Round | International | / | S | LSCP |
| Guglielmina | Peach | Orange yellow | Round | Italy | +40 | B | SSCP |
| Maillard Magic | Nectarine | White | Round | International | +5 | S | LSCP |
| Maria Marta | Peach | Light yellow | Round | Italy | +15 | B | LSCP |
| Nectaross | Nectarine | Yellow | Ovate | Italy | +20 | A | LSCP |
| Regina di Londa | Peach | White | Round | Local—Tuscany | +50 | B | SSCP |
| Romagna Big | Nectarine | Orange yellow | Round | Italy | +20 | B | LSCP |
| Rome Star | Peach | Light yellow | Ovate | International | +20 | B | LSCP |
| Lady Erica | Nectarine | Yellow | Round | Italy | +50 | S | LSCP |
| Sweet Dream | Peach | Light yellow | Round | Italy | +40 | S | LSCP |
| Venus | Nectarine | Light yellow | Ovate | Italy | +35 | A | LSCP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taiti, C.; Costa, C.; Petrucci, W.A.; Luzzietti, L.; Giordani, E.; Mancuso, S.; Nencetti, V. Are Peach Cultivars Used in Conventional Long Food Supply Chains Suitable for the High-Quality Short Markets? Foods 2021, 10, 1253. https://doi.org/10.3390/foods10061253
Taiti C, Costa C, Petrucci WA, Luzzietti L, Giordani E, Mancuso S, Nencetti V. Are Peach Cultivars Used in Conventional Long Food Supply Chains Suitable for the High-Quality Short Markets? Foods. 2021; 10(6):1253. https://doi.org/10.3390/foods10061253
Chicago/Turabian StyleTaiti, Cosimo, Corrado Costa, William Antonio Petrucci, Laura Luzzietti, Edgardo Giordani, Stefano Mancuso, and Valter Nencetti. 2021. "Are Peach Cultivars Used in Conventional Long Food Supply Chains Suitable for the High-Quality Short Markets?" Foods 10, no. 6: 1253. https://doi.org/10.3390/foods10061253
APA StyleTaiti, C., Costa, C., Petrucci, W. A., Luzzietti, L., Giordani, E., Mancuso, S., & Nencetti, V. (2021). Are Peach Cultivars Used in Conventional Long Food Supply Chains Suitable for the High-Quality Short Markets? Foods, 10(6), 1253. https://doi.org/10.3390/foods10061253








