The Relationship between the Browning Index, Total Phenolics, Color, and Antioxidant Activity of Polish-Originated Honey Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. The Origin of Honey Samples
2.3. Determination of Browning Index
2.4. Determination of Color by CIE Lab System and Sensory Panel
2.5. Determination of Honey’s Antioxidant Activity by ACW and FRAP Assays
2.6. Determination of the Total Phenolic Content (TPC)
2.7. Statistical Analysis
3. Results and Discussion
3.1. The Browning Index of Honey Samples
3.2. Honey Color Determined by CIELAB System and Sensory Panel
3.3. Total Phenolic Content (TPC) in Different Honey Types
3.4. Antioxidant Capacity of Honey Samples Provided by ACW and FRAP Assays
3.5. Results of Principal Component Analysis (PCA) for Honey Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Kopała, E.; Kuźnicka, E.; Balcerak, M. Survey of consumer preferences on the bee product market. Part 1. Honey. Ann. Wars. Univ. Life Sci. SGGW Anim. Sci. 2019, 58, 153–158. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Owayss, A.A.; Mahmoud, A.A. Mineral content and physical properties of local and imported honeys in Saudi Arabia. J. Saudi Chem. Toxic. 2012, 50, 1508–1516. [Google Scholar] [CrossRef]
- Brudzynski, K.; Miotto, D. Honey melanoidins: Analysis of the compositions of the high molecular weight melanoidins exhibiting radical-scavenging activity. Food Chem. 2011, 127, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Qian, H.; Yao, W.-R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Brudzynski, K.; Sjaarda, C.; Maldonado-Alvarez, L. A new look on protein-polyphenol complexation during honey storage: Is this a random or organized event with the help of dirigent-like proteins? PLoS ONE 2013, 30, e72897. [Google Scholar] [CrossRef]
- Langner, E.; Rzeski, W. Biological Properties of melanoidins: A review. Int. J. Food Prop. 2014, 17, 344–353. [Google Scholar] [CrossRef]
- Brudzynski, K. Honey melanoidins: Emerging novel understanding on the mechanism of antioxidant and antibacterial action of honey. In Honey: Current Research Clinical Application; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012. [Google Scholar]
- Brudzynski, K.; Miotto, D. The relationship between the content of Maillard reaction-like products and bioactivity of Canadian honeys. Food Chem. 2011, 124, 869–874. [Google Scholar] [CrossRef]
- Baltršaitytė, V.; Rimantas Veskutonis, P.; Čeksterytė, V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem. 2007, 101, 502–514. [Google Scholar] [CrossRef]
- Wilczyńska, A. Phenolic content and antioxidant activity of different types of Polish honey—A short report. Pol. J. Food Nutr. Sci. 2010, 60, 309–313. [Google Scholar]
- Tomczyk, M.; Tarapatskyy, M.; Dżugan, M. The influence of geographical origin on honey composition studied by Polish and Slovak honeys. Czech J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [PubMed]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic profile and antioxidant properties of Polish honeys. Int. J. Food Sci. Techn. 2011, 46, 528–534. [Google Scholar] [CrossRef]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Waheed, M.; Javed, A.; Mushtaq, Z.; Hassan, S.; Shariati, M.A.; Khan, M.U.; Majeed, M.; Nigam, M.; Mishra, A.P.; Heydari, M. Honey and cancer: A mechanistic review. Clin. Nutr. 2019, 38, 2499–2503. [Google Scholar] [CrossRef]
- Ahmed, S.; Othman, N.H. Honey as a potential natural anticancer agent: A review of its mechanisms. Evid. Based Complementary Altern. Med. 2013, 2013, 829070. [Google Scholar] [CrossRef]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Cardetti, M.; Keen, C.L. Honey with high levels of antioxidants can provide protection to healthy human subjects. J. Agric. Food Chem. 2003, 51, 1732–1735. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Oliveira Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Gül, A.; Pehlivan, T. Antioxidant activities of some monofloral honey types produced across Turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Tech. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- ISO 8586:2012. Sensory Analysis-General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. Available online: https://www.iso.org/standard/45352.html (accessed on 30 March 2021).
- ISO 8589:2007. Sensory Analysis-General Guidance for the Design of Test Rooms. Available online: https://www.iso.org/standard/36385.html (accessed on 30 March 2021).
- Zieliński, H.; Zielińska, D.; Kostyra, H. Antioxidant capacity of a new crispy type food products determined by updated analytical strategies. Food Chem. 2012, 130, 1098–1104. [Google Scholar] [CrossRef]
- Horszwald, A.; Andlauer, W. Characterisation of bioactive compounds in berry juices by traditional photometric and modern microplate methods. J. Berry Res. 2011, 1, 189–199. [Google Scholar] [CrossRef]
- Nayik, G.A.; Nanda, V. Physico-chemical, enzymatic, mineral and colour characterization of three different varieties of honeys from Kashmir Valley of India with a multivariate approach. Pol. J. Food Nutr. Sci. 2015, 65, 101–108. [Google Scholar] [CrossRef]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Facino, R.M. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorometric assays and chemometrics. Anal. Chim. Acta 2005, 533, 180–191. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2011, 11, 340–346. [Google Scholar] [CrossRef]
- Codex Alimentarius Committee on Sugars. Codex standard 12, revised Codex Standard for Honey. Stand. Stand. Methods 2001, 11, 1–7. [Google Scholar]
- Šedík, P.; Prokeinová, R.B.; Horská, E. Consumption patterns and sensory perception of honey by young segment in Slovakia. Econ. Manag. Innov. EMI 2018, 10, 1299–1804. [Google Scholar]
- Karabagias, I.K.; Karabournioti, S. Discrimination of clover and citrus honeys from Egypt according to floral type using easily assessable physicochemical parameters and discriminant analysis: An external parameter of the chemometric approach. Foods 2018, 7, 70. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Guerra-Rodriguez, M.E.; Vazquez, M. Analytical methods used in the quality control of honey. J. Agric. Food Chem. 2017, 65, 690–703. [Google Scholar] [CrossRef]
- Sawicki, T.; Bączek, N.; Starowicz, M. Characterisation of the total phenolic, vitamins C and E content and antioxidant properties of the beebread and honey from the same batch. Czech J. Food Sci. 2020, 38, 158–163. [Google Scholar] [CrossRef]
- Attanzio, A.; Tesoriere, L.; Allegra, M.; Livrea, M.A. Monofloral honeys by Sicilian black honeybee (Apis mellifera ssp. sicula) have high reducing power and antioxidant capacity. Heliyon 2016, 2. [Google Scholar] [CrossRef]
- Wesołowska, M.; Dżugan, M. The use of PHOTOCHEM device in evaluation of antioxidant activity of Polish honey. Food Anal. Methods 2017, 10, 1568–1574. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Faso honeys as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Besco, E.; Braccioli, E.; Vertuani, S.; Ziosi, P.; Brazzo, F.; Bruni, R.; Sacchetti, G.; Manfredini, S. The use of photochemiluminescence for the measurement of the integral antioxidant capacity of baobab products. Food Chem. 2007, 102, 1352–1356. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Poliwoda, A.; Dereń, M.; Kafarski, P. Phenolic compounds and abscisic acid as potential markers for the floral origin of two Polish unifloral honeys. Food Chem. 2012, 131, 1149–1156. [Google Scholar] [CrossRef]
- Chua, L.S.; Rahaman, N.L.A.; Ardawati Adnan, N.; Tan, T.T.E. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013, 2013, 313798. [Google Scholar] [CrossRef] [PubMed]
- Bertoncejl, J.; Dobersek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Blasa, M.; Candiracci, M.; Accorosi, A.; Piacentini, M.P.; Albertini, M.C.; Piatti, E. Raw Millefiori honey is packed full of antioxidants. Food Chem. 2006, 97, 217–222. [Google Scholar] [CrossRef]
- Stagos, D.; Soulitsiotis, N.; Tsadila, C.; Papaeconomou, S.; Arvanitis, C.; Ntontos, A.; Karkanta, F.; Adamou-Androulaki, S.; Petrotos, K.; Spandidos, D.A.; et al. Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int. J. Mol. Med. 2018, 42, 726–734. [Google Scholar] [CrossRef]
- Kuś, P.M.; Congiu, F.; Teper, D.; Sroka, Z.; Jerković, I.; Tuberoso, C.I.G. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT Food Sci. Technol. 2014, 55, 124–130. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Tezcan, F.; Erim, F.B.; Yildiz, O.; Sahin, H.; Can, Z.; Kolayli, S. Characterization of Anatolian honeys based on minerals, bioactive components and principal component analysis. LWT Food Sci. Technol. 2016, 68, 273–279. [Google Scholar] [CrossRef]
- Vela, L.; de Lorenzo, C.; Pérez, R.A. Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. J. Sci. Food Agric. 2007, 87, 1069–1075. [Google Scholar] [CrossRef]
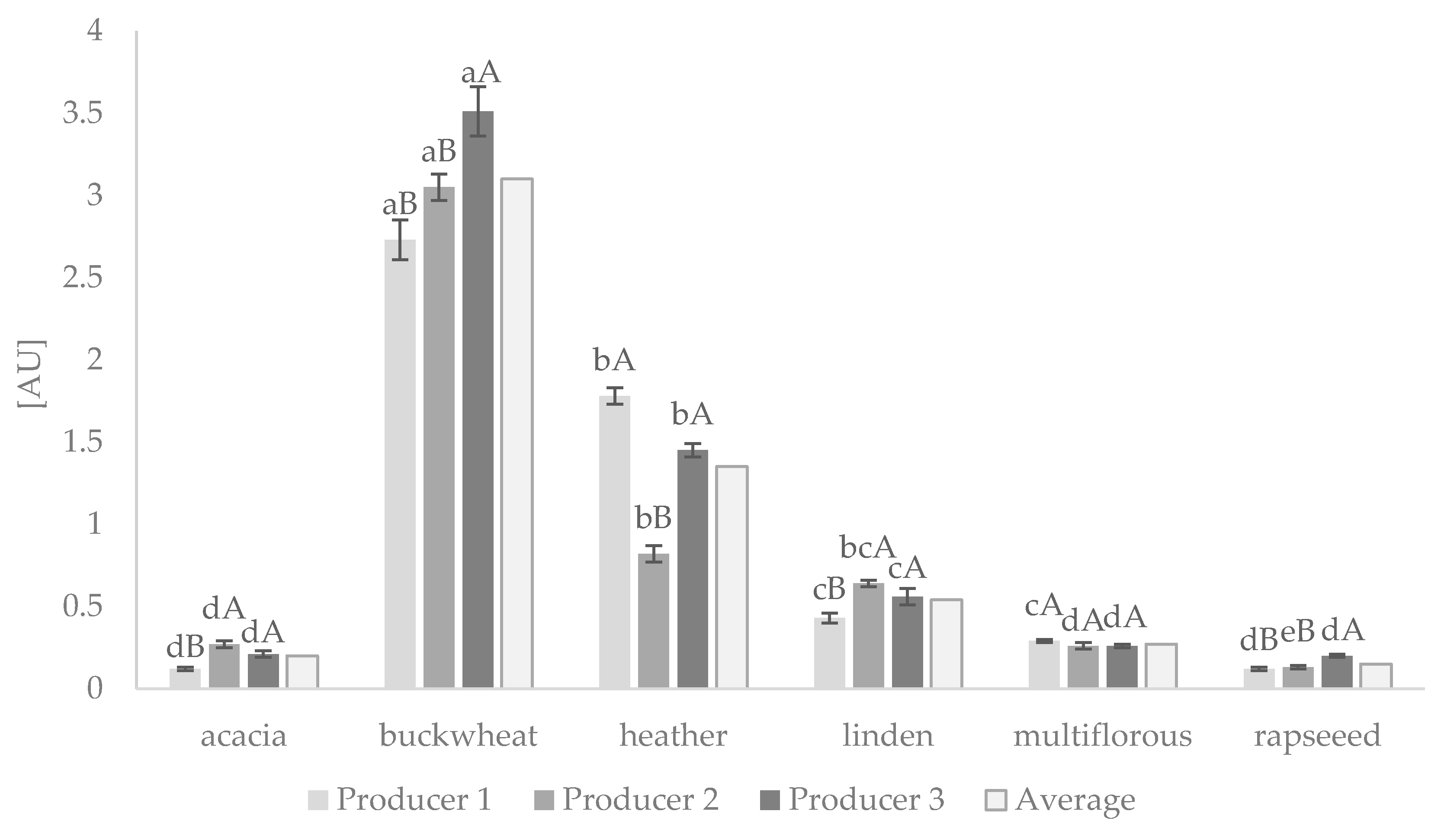
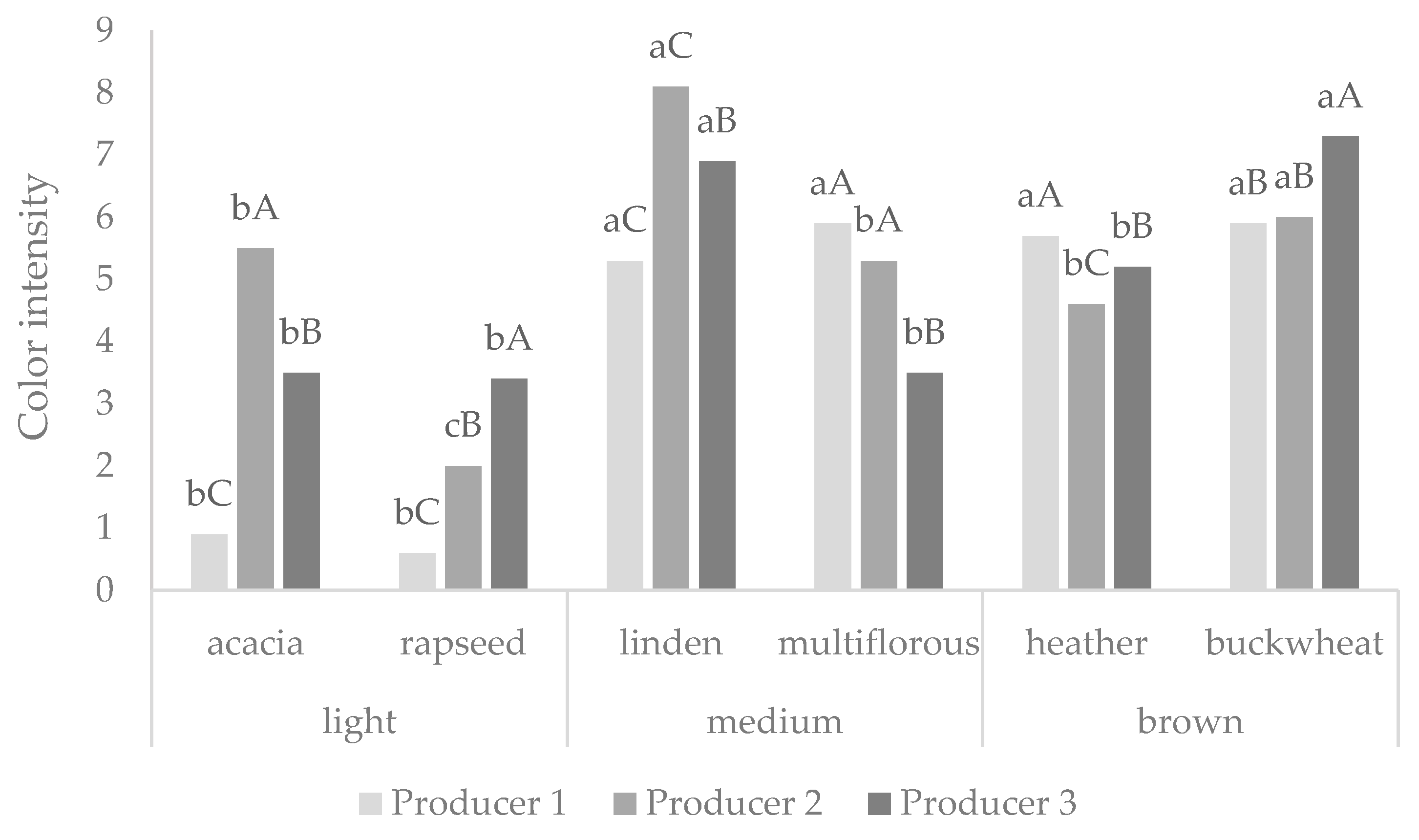
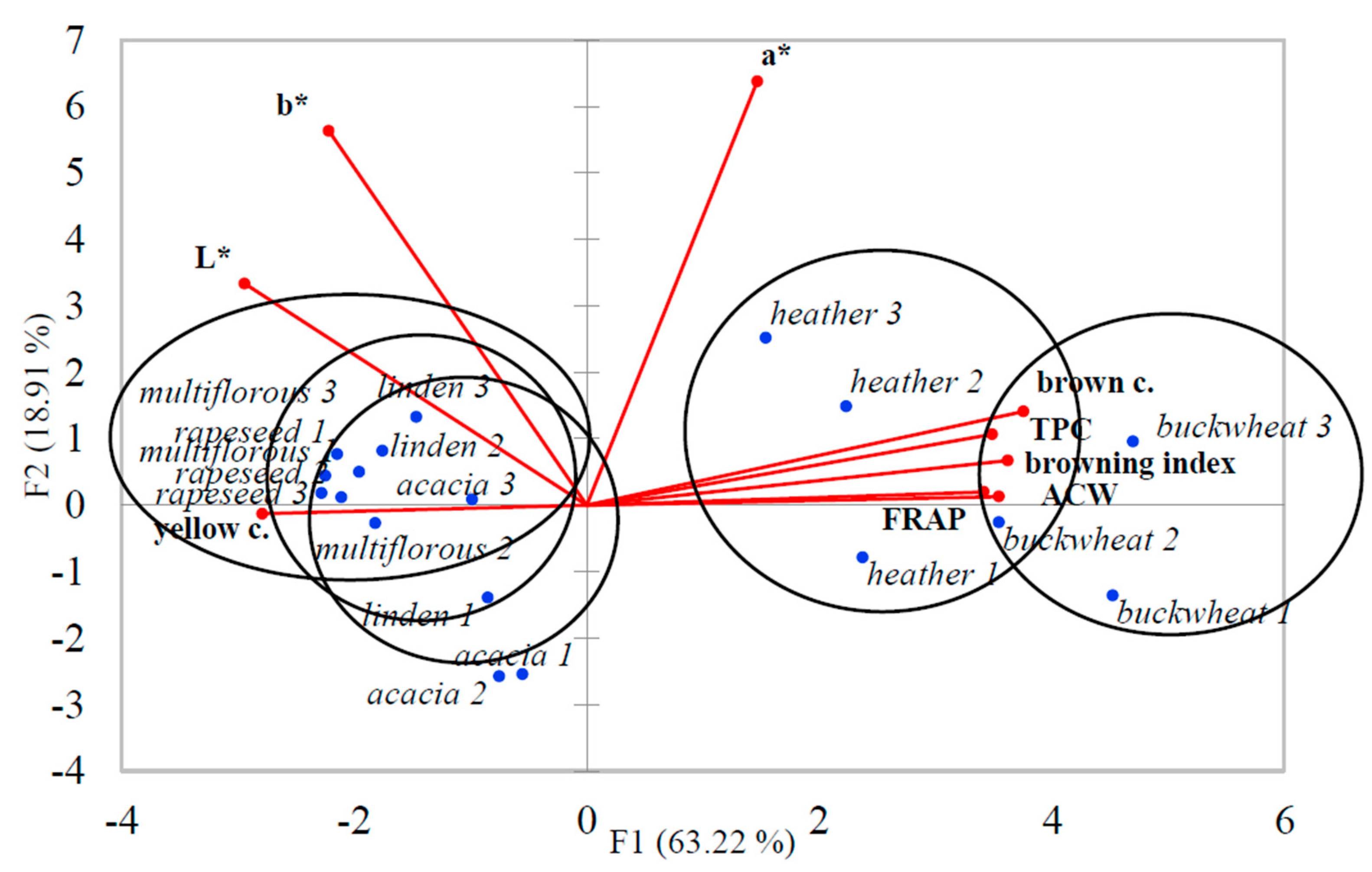
| Type of Honey | Producer 1 | Producer 2 | Producer 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | |
| Acacia | 8.70 ± 0.03 e | –2.12 ± 0.08 f | 6.87 ± 0.16 e | 9.17 ± 0.03 e | –1.38 ± 0.16 f | 3.08 ± 0.13 f | 47.52 ± 0.05 c | 4.02 ± 0.03 e | 31.51 ± 0.09 d |
| Buckwheat | 3.38 ± 0.07 f | 1.89 ± 0.19 d | 3.86 ± 0.07 f | 8.40 ± 0.24 f | 8.68 ± 0.08 b | 9.21 ± 0.30 e | 12.29 ± 0.10 f | 12.78 ± 0.26 b | 17.17 ± 0.11 f |
| Heather | 9.24 ± 0.01 d | 3.87 ± 0.10 c | 12.45 ± 0.09 d | 20.38 ± 0.12 d | 14.82 ± 0.12 a | 26.70 ± 0.15 d | 33.97 ± 0.07 d | 16.86 ± 0.04 a | 40.20 ± 0.08 b |
| Linden | 14.58 ± 0.18 c | 1.61 ± 0.19 e | 19.54 ± 0.28 c | 48.71 ± 0.04 b | 6.53 ± 0.09 c | 41.43 ± 0.31 a | 31.77 ± 0.06 e | 9.47 ± 0.03 c | 50.22 ± 0.23 a |
| Multiflorous | 57.29 ± 0.11 b | 5.12 ± 0.13 b | 34.90 ± 0.20 a | 40.51 ± 0.06 c | 3.50 ± 0.06 e | 29.94 ± 0.17 c | 56.26 ± 0.12 b | 6.56 ± 0.02 d | 37.75 ± 0.03 c |
| Rapeseed | 59.45 ± 0.01 a | 6.07 ± 0.02 a | 33.33 ± 0.02 b | 59.45 ± 0.01 a | 3.63 ± 0.05 d | 32.21 ± 0.08 b | 72.17 ± 0.20 a | 3.36 ± 0.06 f | 29.34 ± 0.11 e |
| Type of Honey | TPC | Average Content | ||
|---|---|---|---|---|
| (mg GAE 100 g−1) | ||||
| Producer 1 | Producer 2 | Producer 3 | ||
| Acacia | 42.3 ± 7.40 cC | 58.9 ± 2.56 cB | 65.4 ± 1.39 cA | 55.5 |
| Buckwheat | 170.3 ± 1.91 aA | 97.2 ± 5.12 bC | 155.7 ± 2.09 aB | 141.1 |
| Heather | 156.7 ± 4.61 bB | 195.1 ± 10.25 aA | 125.8 ± 5.20 bC | 159.2 |
| Linden | 24.7 ± 1.63 dB | 32.8 ± 0.55 dA | 25.9 ± 0.15 eB | 27.8 |
| Multiflorous | 37.7 ± 3.64 cA | 31.4 ± 0.12 eB | 45.0 ± 0.5 dA | 38.0 |
| Rapeseed | 13.5 ± 0.78 eC | 17.5 ± 0.50 eB | 28.9 ± 1.36 eA | 19.9 |
| Type of Honey | ACW | FRAP | Average | |||||
|---|---|---|---|---|---|---|---|---|
| (µmol Trolox g−1) | (mmol Trolox g−1) | |||||||
| Producer 1 | Producer 2 | Producer 3 | Average | Producer 1 | Producer 2 | Producer 3 | ||
| Acacia | 33.5 ± 0.40 eB | 36.0 ± 2.61 eA | 37.6 ± 1.93 eA | 35.70 | 18.5 ± 0.36 dC | 20.0 ± 0.01 dB | 35.1 ± 1.66 bA | 24.53 |
| Buckwheat | 289.8 ± 14.1 aA | 207.1 ± 22.9 aC | 269.3 ± 5.50 aB | 255.40 | 52.3 ± 1.02 aA | 40.9 ± 0.15 aC | 48.2 ± 0.09 aB | 47.13 |
| Heather | 94.1 ± 5.18 bB | 119.9 ± 2.51 bA | 72.5 ± 0.11 bC | 95.50 | 29.7 ± 0.01 fB | 32.0 ± 0.05 bA | 33.0 ± 0.01 aB | 31.58 |
| Linden | 61.6 ± 0.22 cB | 67.7 ± 0.12 cA | 64.5 ± 2.41 cB | 64.60 | 27.2 ± 0.89 cB | 25.4 ± 0.80 cA | 27.9 ± 0.04 cB | 26.83 |
| Multiflorous | 26.2 ± 1.32 fA | 27.7 ± 0.95 fA | 26.9 ± 0.23 fA | 26.94 | 10.8 ± 0.05 eB | 15.9 ± 0.04 eA | 11.6 ± 0.01 dB | 12.77 |
| Rapeseed | 40.3 ± 1.82 dB | 43.5 ± 0.72 dA | 46.1 ± 2.05 dA | 43.30 | 4.85 ± 0.08 eC | 5.33 ± 0.01 fB | 5.69 ± 0.02 eA | 5.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starowicz, M.; Ostaszyk, A.; Zieliński, H. The Relationship between the Browning Index, Total Phenolics, Color, and Antioxidant Activity of Polish-Originated Honey Samples. Foods 2021, 10, 967. https://doi.org/10.3390/foods10050967
Starowicz M, Ostaszyk A, Zieliński H. The Relationship between the Browning Index, Total Phenolics, Color, and Antioxidant Activity of Polish-Originated Honey Samples. Foods. 2021; 10(5):967. https://doi.org/10.3390/foods10050967
Chicago/Turabian StyleStarowicz, Małgorzata, Anita Ostaszyk, and Henryk Zieliński. 2021. "The Relationship between the Browning Index, Total Phenolics, Color, and Antioxidant Activity of Polish-Originated Honey Samples" Foods 10, no. 5: 967. https://doi.org/10.3390/foods10050967
APA StyleStarowicz, M., Ostaszyk, A., & Zieliński, H. (2021). The Relationship between the Browning Index, Total Phenolics, Color, and Antioxidant Activity of Polish-Originated Honey Samples. Foods, 10(5), 967. https://doi.org/10.3390/foods10050967







