Analysis of Cultivable Microbial Community during Kimchi Fermentation Using MALDI-TOF MS
Abstract
1. Introduction
2. Materials and Methods
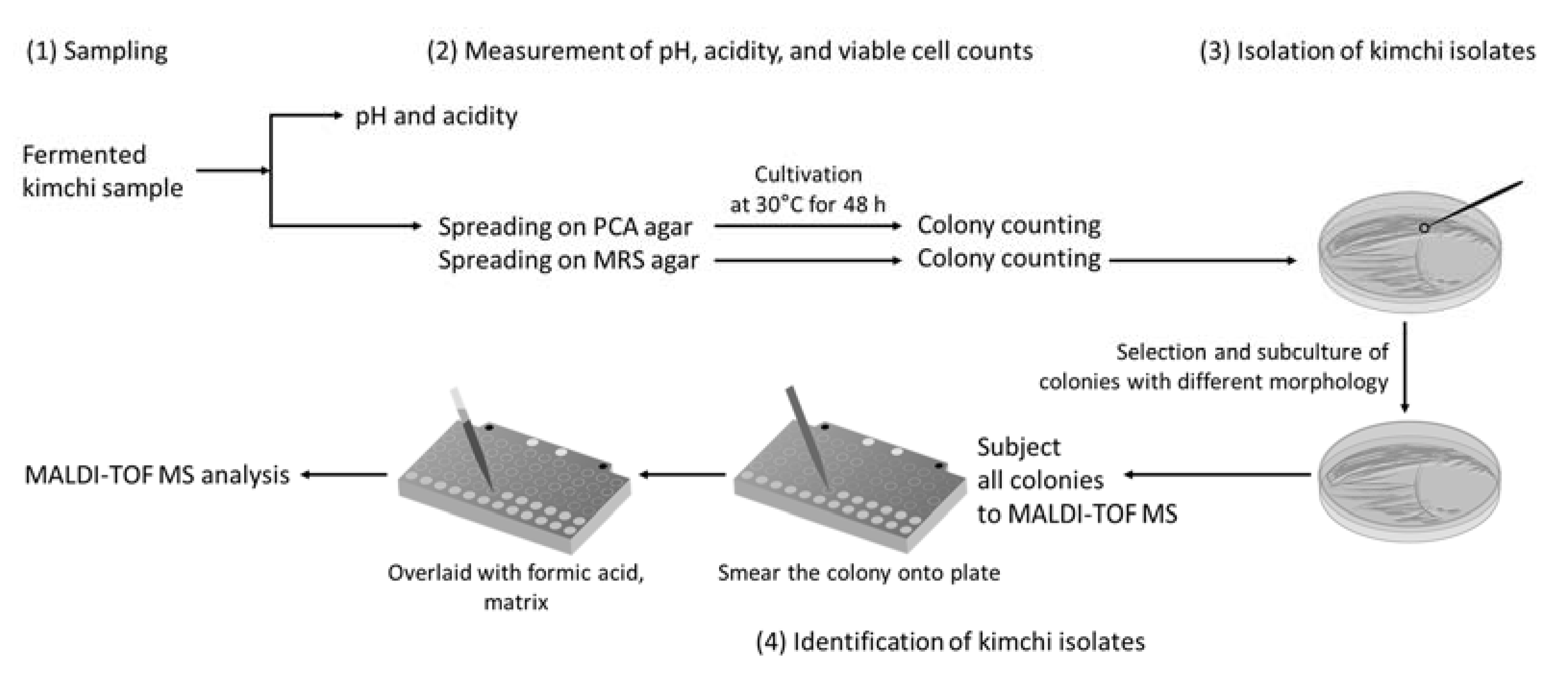
2.1. Sample Preparation and Sampling
2.2. Measurement of Viable Cell Counts, pH, and Acidity
2.3. Analysis of Microbial Community in Fermented Kimchi by MALDI-TOF MS
2.4. Statistical Analysis
3. Results and Discussion
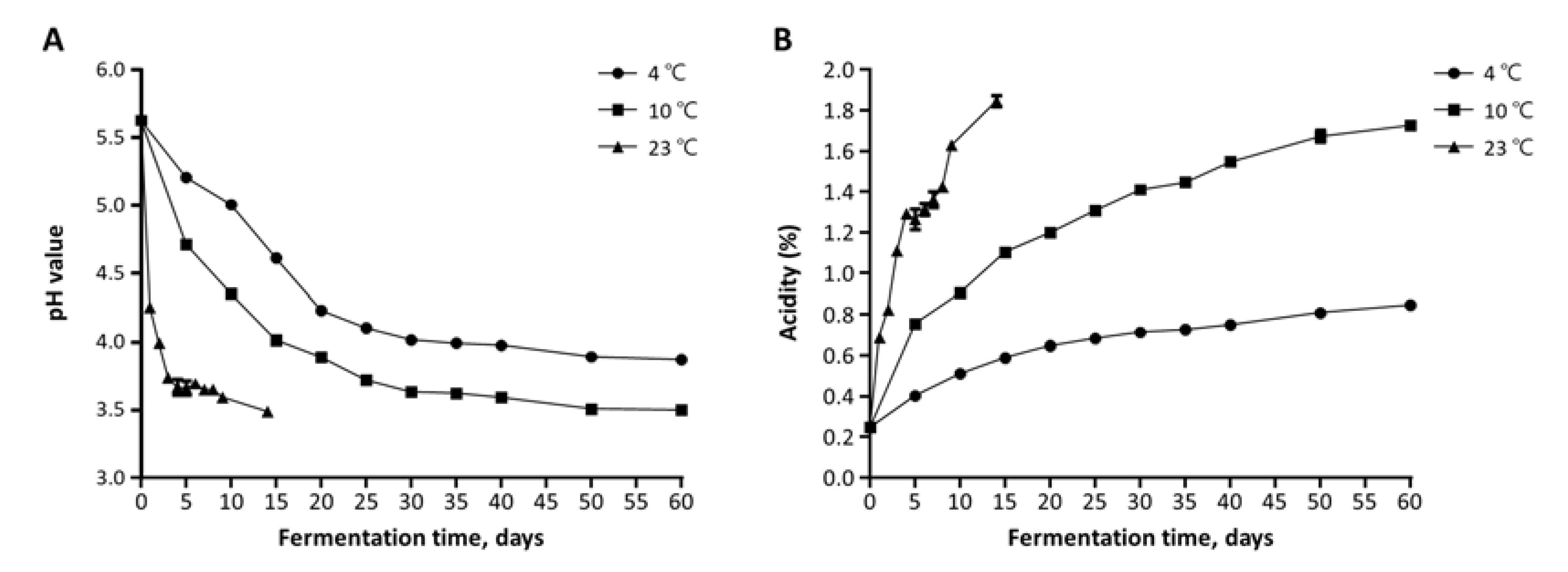
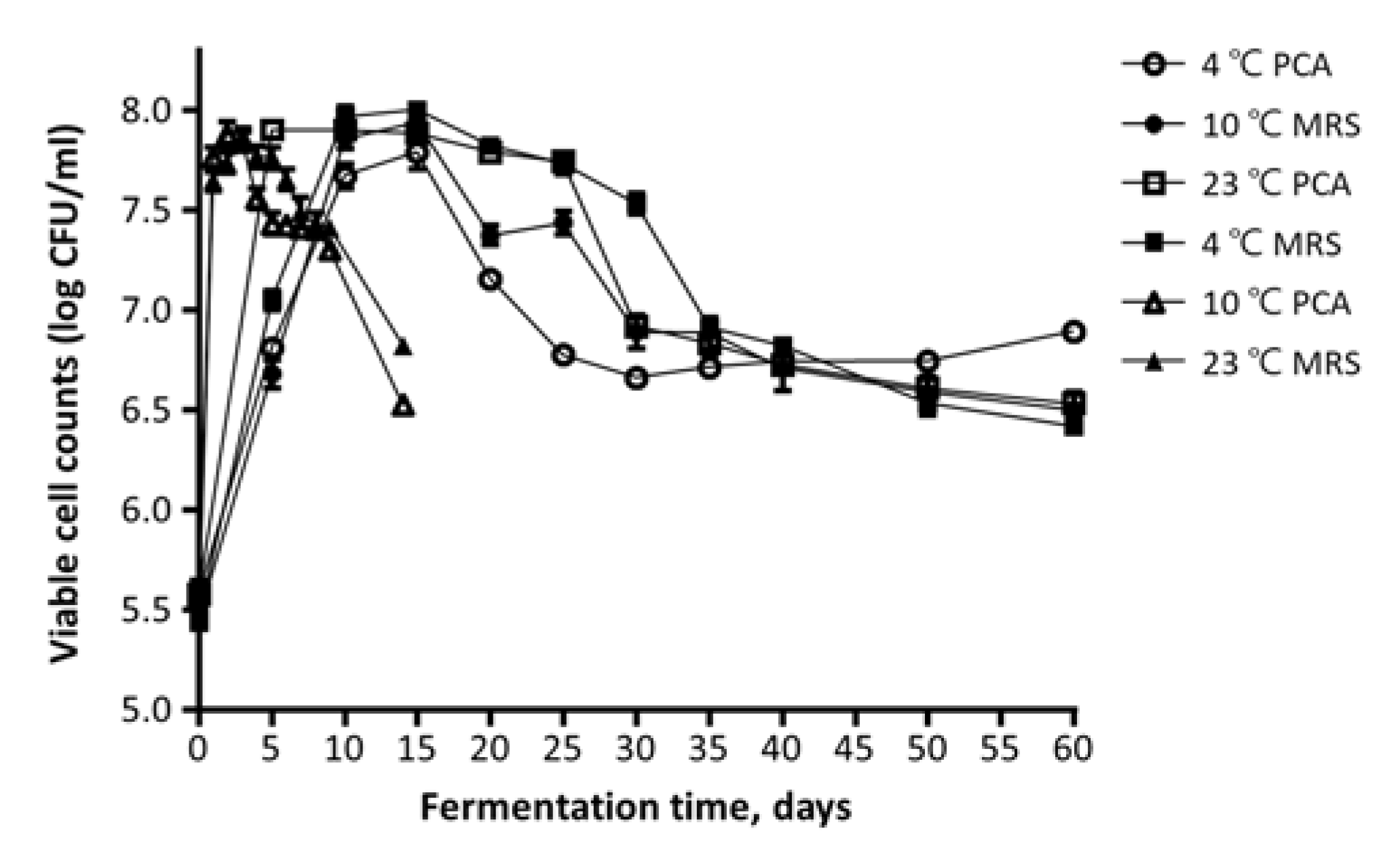
3.1. Changes in Viable Cell Count, pH, and Acidity
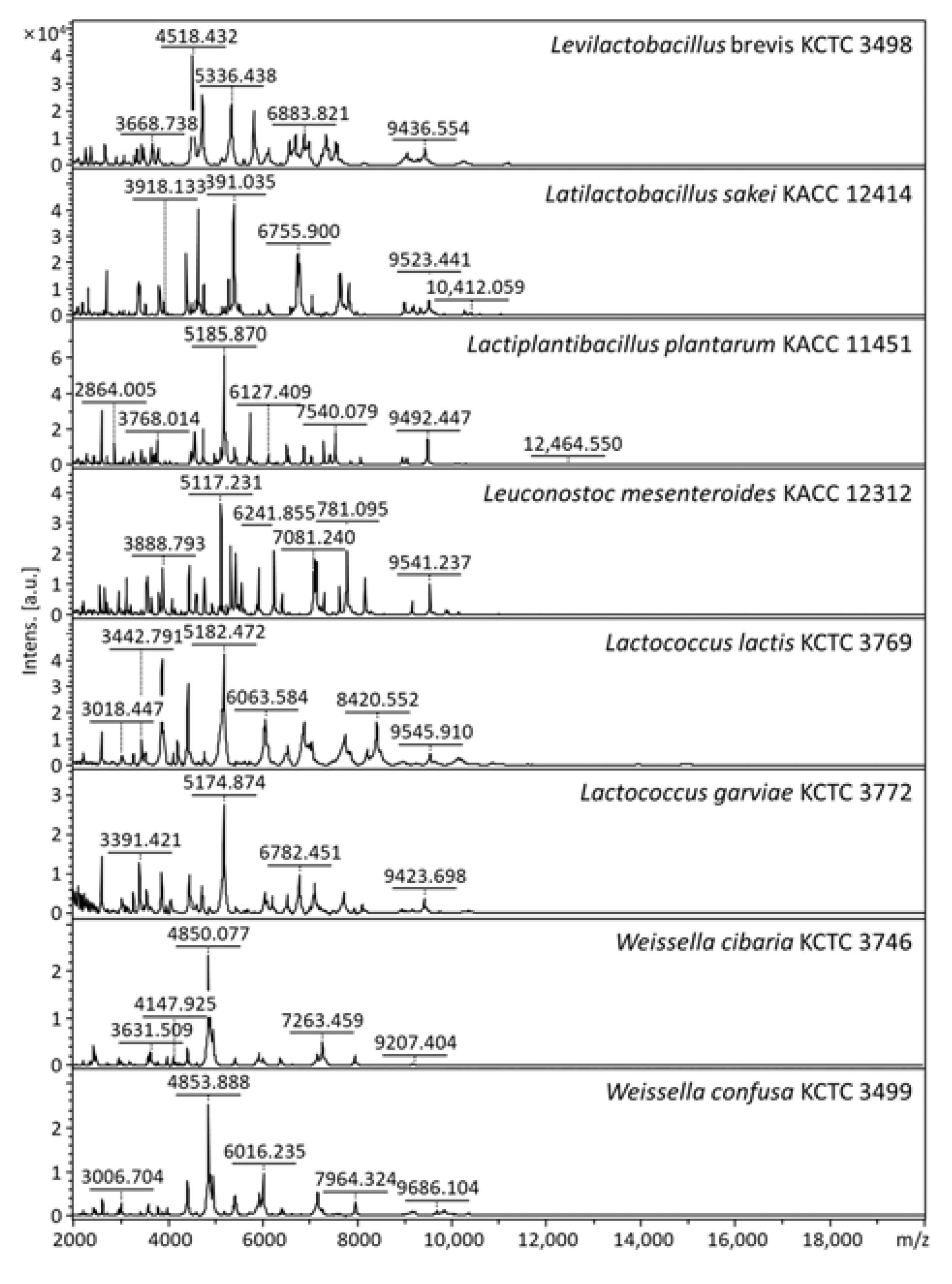
3.2. Identification of Kimchi Isolates with an In-House Database
3.3. Changes in Microbial Community during Kimchi Fermentation
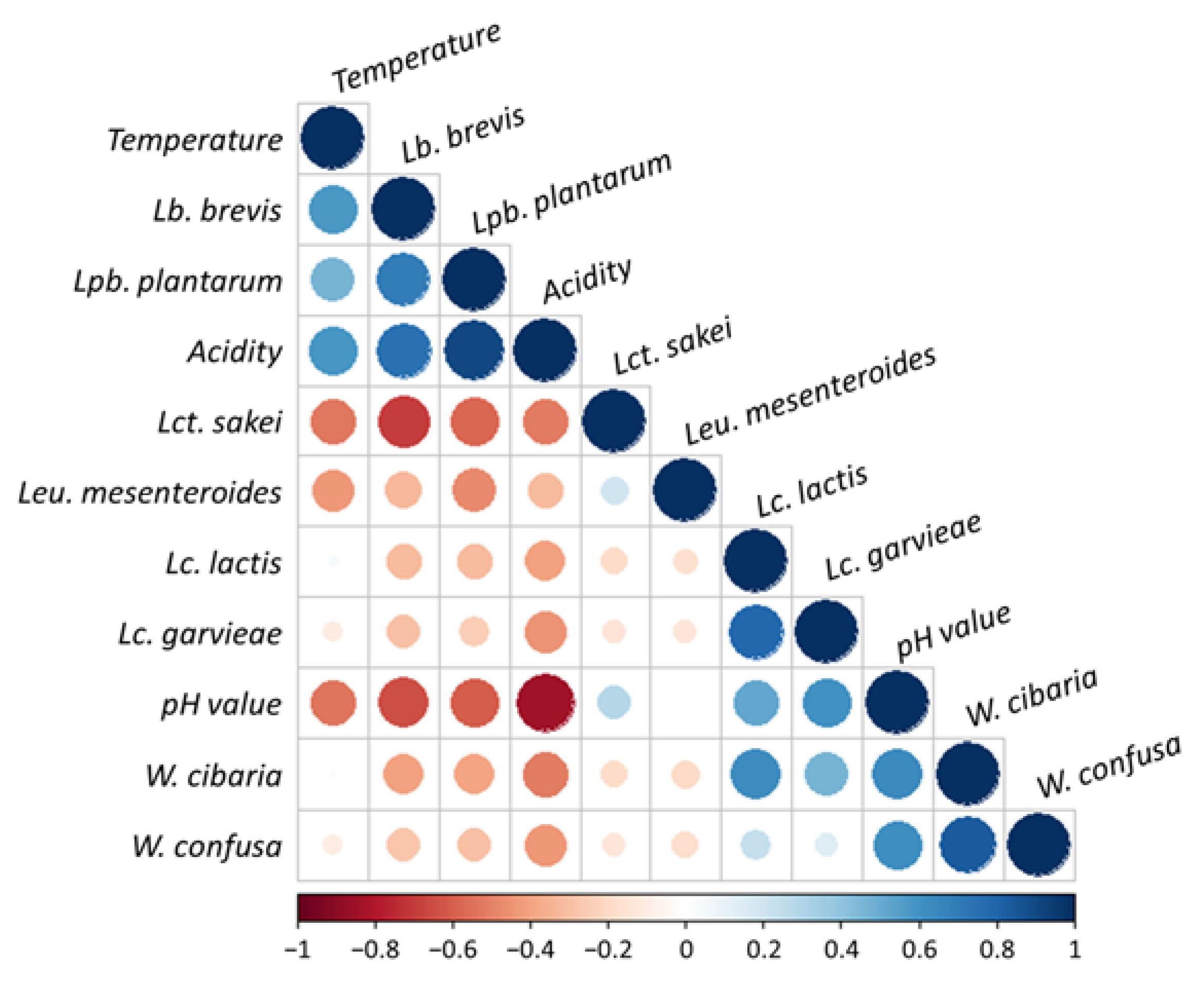
3.4. Relationships between Microbial Composition and Environmental Factors
3.5. Greenness Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, M.; Song, J.H.; Park, J.M.; Chang, J.Y. Bacterial diversity in Korean temple kimchi fermentation. Food Res. Int. 2019, 126, 108592. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, K.Y.; Kim, H.Y.; Ahn, S.C.; Cho, E.J. Anti-aging effects and mechanisms of kimchi during fermentation under stress-induced premature senescence cellular system. Food Sci. Biotechnol. 2011, 20, 643–649. [Google Scholar] [CrossRef]
- Kwak, S.-H.; Cho, Y.-M.; Noh, G.-M.; Om, A.-S. Cancer Preventive Potential of Kimchi Lactic Acid Bacteria (Weissella cibaria, Lactobacillus plantarum). J. Cancer Prev. 2014, 19, 253–258. [Google Scholar] [CrossRef]
- Park, Y.K.; Lee, J.H.; Mah, J.-H. Occurrence and Reduction of Biogenic Amines in Kimchi and Korean Fermented Seafood Products. Foods 2019, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Song, J.H.; Jung, M.Y.; Lee, S.H.; Chang, J.Y. Large-scale targeted metagenomics analysis of bacterial ecological changes in 88 kimchi samples during fermentation. Food Microbiol. 2017, 66, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Hong, Y.; Yang, H.S.; Chang, H.C.; Kim, H.Y. Distribution of lactic acid bacteria in garlic (Allium sativum) and green onion (Allium fistulosum) using SDS-PAGE whole cell protein pattern comparison and 16S rRNA gene sequence analysis. Food Sci. Biotechnol. 2012, 21, 1457–1462. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, J.Y.; Song, H.S.; Park, J.H.; Ji, G.E.; Kim, H.Y. Isolation and identification of Weissella kimchii from green onion by cell protein pattern analysis. J. Microbiol. Biotechnol. 2004, 14, 105–109. [Google Scholar]
- Mun, S.Y.; Chang, H.C. Characterization of Weissella koreensis sk isolated from kimchi fermented at low temperature (Around 0 °C) based on complete genome sequence and corresponding phenotype. Microorganisms 2020, 8, 1147. [Google Scholar] [CrossRef]
- Hong, Y.; Yang, H.S.; Chang, H.C.; Kim, H.Y. Comparison of bacterial community changes in fermenting Kimchi at two different temperatures using a denaturing gradient gel electrophoresis analysis. J. Microbiol. Biotechnol. 2013, 23, 76–84. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Mills, D.A. Differentiation of mixed lactic acid bacteria communities in beverage fermentations using targeted terminal restriction fragment length polymorphism. Food Microbiol. 2012, 31, 126–132. [Google Scholar] [CrossRef]
- Ercolini, D. PCR-DGGE fingerprinting: Novel strategies for detection of microbes in food. J. Microbiol. Methods 2004, 56, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Ahmadsah, L.S.F.; Min, S.G.; Han, S.K.; Hong, Y.; Kim, H.Y. Effect of low salt concentrations on microbial changes during Kimchi fermentation monitored by PCR-DGGE and their sensory acceptance. J. Microbiol. Biotechnol. 2015, 25, 2049–2057. [Google Scholar] [CrossRef]
- Kim, E.; Cho, E.J.; Yang, S.M.; Kim, M.J.; Kim, H.Y. Novel approaches for the identification of microbial communities in kimchi: MALDI-TOF MS analysis and high-throughput sequencing. Food Microbiol. 2021, 94, 103641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Che, Z.; Xu, W.; Yue, P.; Li, R.; Li, Y.; Pei, X.; Zeng, P. Dynamics of physicochemical factors and microbial communities during ripening fermentation of Pixian Doubanjiang, a typical condiment in Chinese cuisine. Food Microbiol. 2020, 86, 103342. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.A.; Kim, E.; Kim, M.J.; Lee, S.; Yoon, S.R.; Ryu, J.G.; Kim, H.Y. Physicochemical Characteristics and Microbial Communities in Gochujang, a Traditional Korean Fermented Hot Pepper Paste. Front. Microbiol. 2021, 11, 620478. [Google Scholar] [CrossRef]
- Höll, L.; Behr, J.; Vogel, R.F. Identification and growth dynamics of meat spoilage microorganisms in modified atmosphere packaged poultry meat by MALDI-TOF MS. Food Microbiol. 2016, 60, 84–91. [Google Scholar] [CrossRef]
- Kraková, L.; Šoltys, K.; Otlewska, A.; Pietrzak, K.; Purkrtová, S.; Savická, D.; Puškárová, A.; Bučková, M.; Szemes, T.; Budiš, J.; et al. Comparison of methods for identification of microbial communities in book collections: Culture-dependent (sequencing and MALDI-TOF MS) and culture-independent (Illumina MiSeq). Int. Biodeterior. Biodegrad. 2018, 131, 51–59. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Prescott, H.C.; Martinez, F.J.; Curtis, J.L.; Lama, V.N.; Huffnagle, G.B. Analysis of culture-dependent versus culture-independent techniques for identification of bacteria in clinically obtained bronchoalveolar lavage fluid. J. Clin. Microbiol. 2014, 52, 3605–3613. [Google Scholar] [CrossRef]
- Kim, E.J.; Seo, S.H.; Park, S.E.; Lim, Y.W.; Roh, S.W.; Son, H.S. Initial storage of kimchi at room temperature alters its microbial and metabolite profiles. LWT 2020, 134, 110160. [Google Scholar] [CrossRef]
- Konrad, R.; Berger, A.; Huber, I.; Boschert, V.; Hörmansdorfer, S.; Busch, U.; Hogardt, M.; Schubert, S.; Sing, A. Matrix-assisted laser desorption/ionisation time-offlight (MALDI-TOF) mass spectrometry as a tool for rapid diagnosis of potentially toxigenic Corynebacterium species in the laboratory management of diphtheriaassociated bacteria. Eurosurveillance 2010, 15, 1–5. [Google Scholar] [CrossRef]
- Nacef, M.; Chevalier, M.; Chollet, S.; Drider, D.; Flahaut, C. MALDI-TOF mass spectrometry for the identification of lactic acid bacteria isolated from a French cheese: The Maroilles. Int. J. Food Microbiol. 2017, 247, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Mörtelmaier, C.; Panda, S.; Robertson, I.; Krell, M.; Christodoulou, M.; Reichardt, N.; Mulder, I. Identification performance of MALDI-ToF-MS upon mono- and bi-microbial cultures is cell number and culture proportion dependent. Anal. Bioanal. Chem. 2019, 411, 7027–7038. [Google Scholar] [CrossRef] [PubMed]
- Hausdorf, L.; Mundt, K.; Winzer, M.; Cordes, C.; Fröhling, A.; Schlüter, O.; Klocke, M. Characterization of the cultivable microbial community in a spinach-processing plant using MALDI-TOF MS. Food Microbiol. 2013, 34, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Stephan, R.; Ziegler, D.; Pflüger, V.; Vogel, G.; Lehner, A. Rapid genus- and species-specific identification of Cronobacter spp. by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2010, 48, 2846–2851. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-A.; Kim, E.; Yang, S.-M.; Lee, S.; Yoon, S.-R.; Jang, K.-S.; Kim, H.-Y. High-throughput sequencing of the microbial community associated with the physicochemical properties of meju (dried fermented soybean) and doenjang (traditional Korean fermented soybean paste). LWT 2021, 146, 111473. [Google Scholar] [CrossRef]
- Kim, E.; Kim, H.J.; Yang, S.M.; Kim, C.G.; Choo, D.W.; Kim, H.Y. Rapid identification of staphylococcus species isolated from food samples by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Microbiol. Biotechnol. 2019, 29, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Quéro, L.; Courault, P.; Cellière, B.; Lorber, S.; Jany, J.L.; Puel, O.; Girard, V.; Vasseur, V.; Nodet, P.; Mounier, J. Application of MALDI-TOF MS to species complex differentiation and strain typing of food related fungi: Case studies with Aspergillus section Flavi species and Penicillium roqueforti isolates. Food Microbiol. 2020, 86, 103311. [Google Scholar] [CrossRef]
- Mo, L.; Yu, J.; Jin, H.; Hou, Q.; Yao, C.; Ren, D.; An, X.; Tsogtgerel, T.; Zhang, H. Investigating the bacterial microbiota of traditional fermented dairy products using propidium monoazide with single-molecule real-time sequencing. J. Dairy Sci. 2019, 102, 3912–3923. [Google Scholar] [CrossRef]
- Li, R.; Tun, H.M.; Jahan, M.; Zhang, Z.; Kumar, A.; Dilantha Fernando, W.G.; Farenhorst, A.; Khafipour, E. Comparison of DNA-, PMA-, and RNA-based 16S rRNA Illumina sequencing for detection of live bacteria in water. Sci. Rep. 2017, 7, 5752. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.H.; Lee, H.J.; Seo, H.Y.; Park, W.S.; Jeon, C.O. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during kimchi fermentation. Int. J. Food Microbiol. 2012, 153, 378–387. [Google Scholar] [CrossRef]
- Kim, E.; Cho, Y.; Lee, Y.; Han, S.K.; Kim, C.G.; Choo, D.W.; Kim, Y.R.; Kim, H.Y. A proteomic approach for rapid identification of Weissella species isolated from Korean fermented foods on MALDI-TOF MS supplemented with an in-house database. Int. J. Food Microbiol. 2017, 243, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, B.; Brodner, K.; Bloemberg, G.V.; Zbinden, R.; Böttger, E.C.; Hombach, M. Identification of Gram-positive cocci by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry: Comparison of different preparation methods and implementation of a practical algorithm for routine diagnostics. J. Clin. Microbiol. 2013, 51, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bang, J.; Beuchat, L.R.; Kim, H.; Ryu, J.H. Controlled fermentation of kimchi using naturally occurring antimicrobial agents. Food Microbiol. 2012, 32, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Björkroth, K.J.; Schillinger, U.; Geisen, R.; Weiss, N.; Hoste, B.; Holzapfel, W.H.; Korkeala, H.J.; Vandamme, P. Taxonomic study of Weissella confusa and description of Weissella cibaria sp. nov., detected in food and clinical samples. Int. J. Syst. Evol. Microbiol. 2002, 52, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zabat, M.A.; Sano, W.H.; Wurster, J.I.; Cabral, D.J.; Belenky, P. Microbial community analysis of sauerkraut fermentation reveals a stable and rapidly established community. Foods 2018, 7, 77. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.M.; Cho, G.S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M.A.P. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 155. [Google Scholar] [CrossRef]
- Zagorec, M.; Champomier-Vergès, M.-C. Lactobacillus sakei: A Starter for Sausage Fermentation, a Protective Culture for Meat Products. Microorganisms 2017, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Kawamoto, J.; Sato, S.B.; Iki, T.; Watanabe, I.; Kudo, K.; Esaki, N.; Kurihara, T. Alkyl hydroperoxide reductase enhances the growth of Leuconostoc mesenteroides lactic acid bacteria at low temperatures. AMB Express 2015, 5, 11. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Mohamed, D.; Fouad, M.M. Application of NEMI, Analytical Eco-Scale and GAPI tools for greenness assessment of three developed chromatographic methods for quantification of sulfadiazine and trimethoprim in bovine meat and chicken muscles: Comparison to greenness profile of reported HPLC methods. Microchem. J. 2020, 157, 104873. [Google Scholar] [CrossRef]

| Fermentation Temperature (No. of Isolates) | No. of Isolates with Results 1 | ||
|---|---|---|---|
| ≥2.000 | 1.700–1.999 | ≤1.699 | |
| 4 °C (1733) | 1466 | 267 | 0 |
| 10 °C (1733) | 1490 | 243 | 0 |
| 23 °C (1738) | 1511 | 227 | 0 |
| Total isolates (5204) | 4467 | 737 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Yang, S.-M.; Kim, H.-Y. Analysis of Cultivable Microbial Community during Kimchi Fermentation Using MALDI-TOF MS. Foods 2021, 10, 1068. https://doi.org/10.3390/foods10051068
Kim E, Yang S-M, Kim H-Y. Analysis of Cultivable Microbial Community during Kimchi Fermentation Using MALDI-TOF MS. Foods. 2021; 10(5):1068. https://doi.org/10.3390/foods10051068
Chicago/Turabian StyleKim, Eiseul, Seung-Min Yang, and Hae-Yeong Kim. 2021. "Analysis of Cultivable Microbial Community during Kimchi Fermentation Using MALDI-TOF MS" Foods 10, no. 5: 1068. https://doi.org/10.3390/foods10051068
APA StyleKim, E., Yang, S.-M., & Kim, H.-Y. (2021). Analysis of Cultivable Microbial Community during Kimchi Fermentation Using MALDI-TOF MS. Foods, 10(5), 1068. https://doi.org/10.3390/foods10051068







