Application of Thermosonication in Red Pitaya Juice Processing: Impacts on Native Microbiota and Quality Properties during Storage
Abstract
:1. Introduction
2. Material and Methods
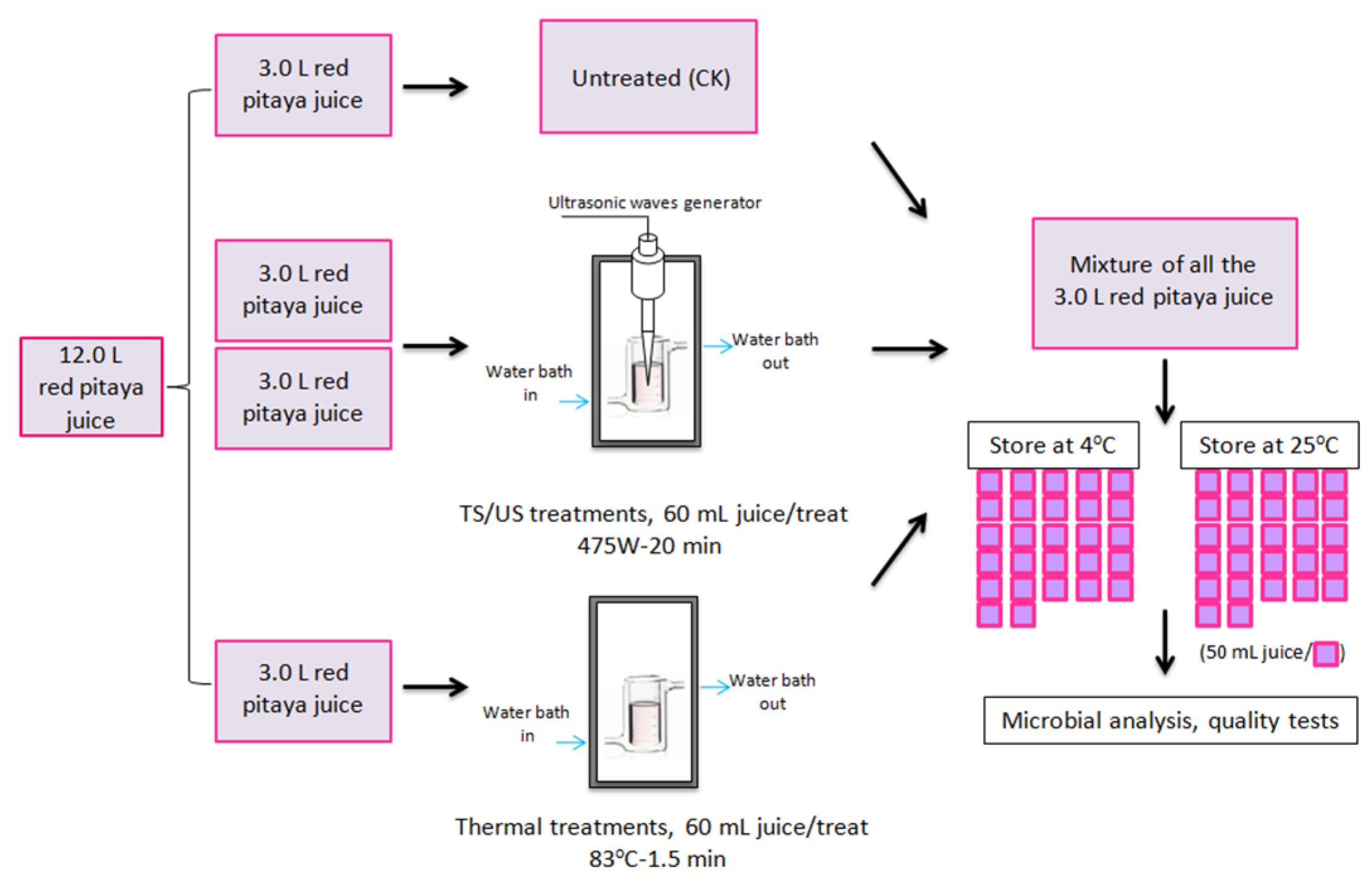
2.1. Preparation of Clear Red Pitaya Juice
2.2. Experimental Design
2.3. Thermosonication, Ultrasonication, and Thermal Treatments
2.4. Microbial Analysis and Quality Assay
2.4.1. Microbial Enumeration
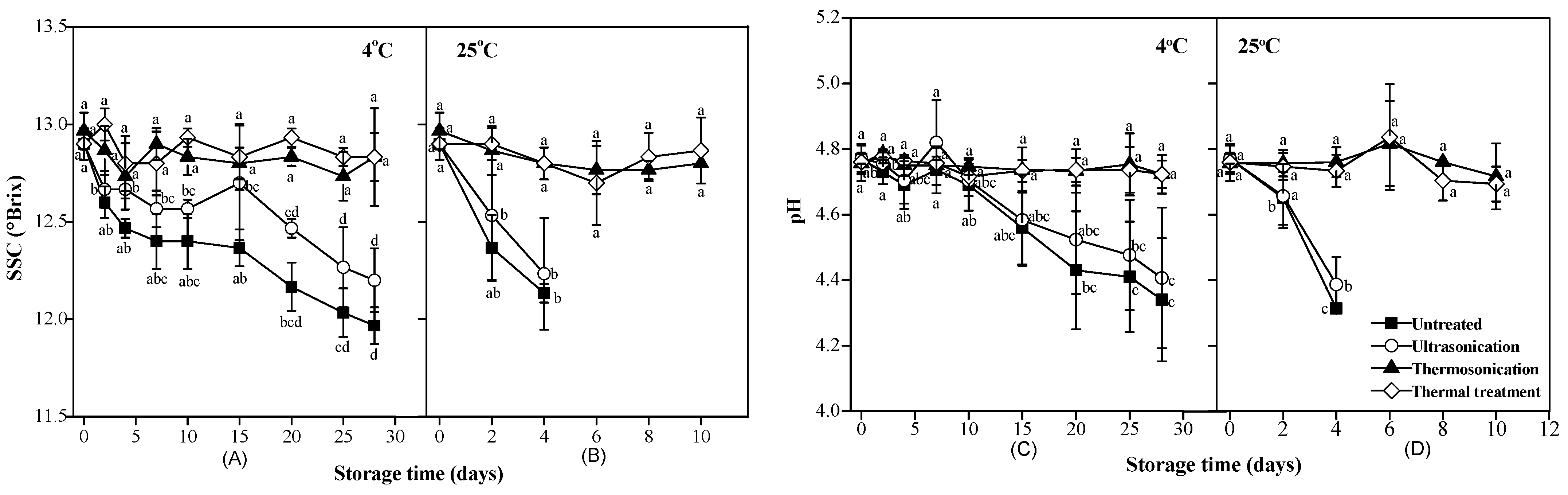
2.4.2. pH and Soluble Solid Content
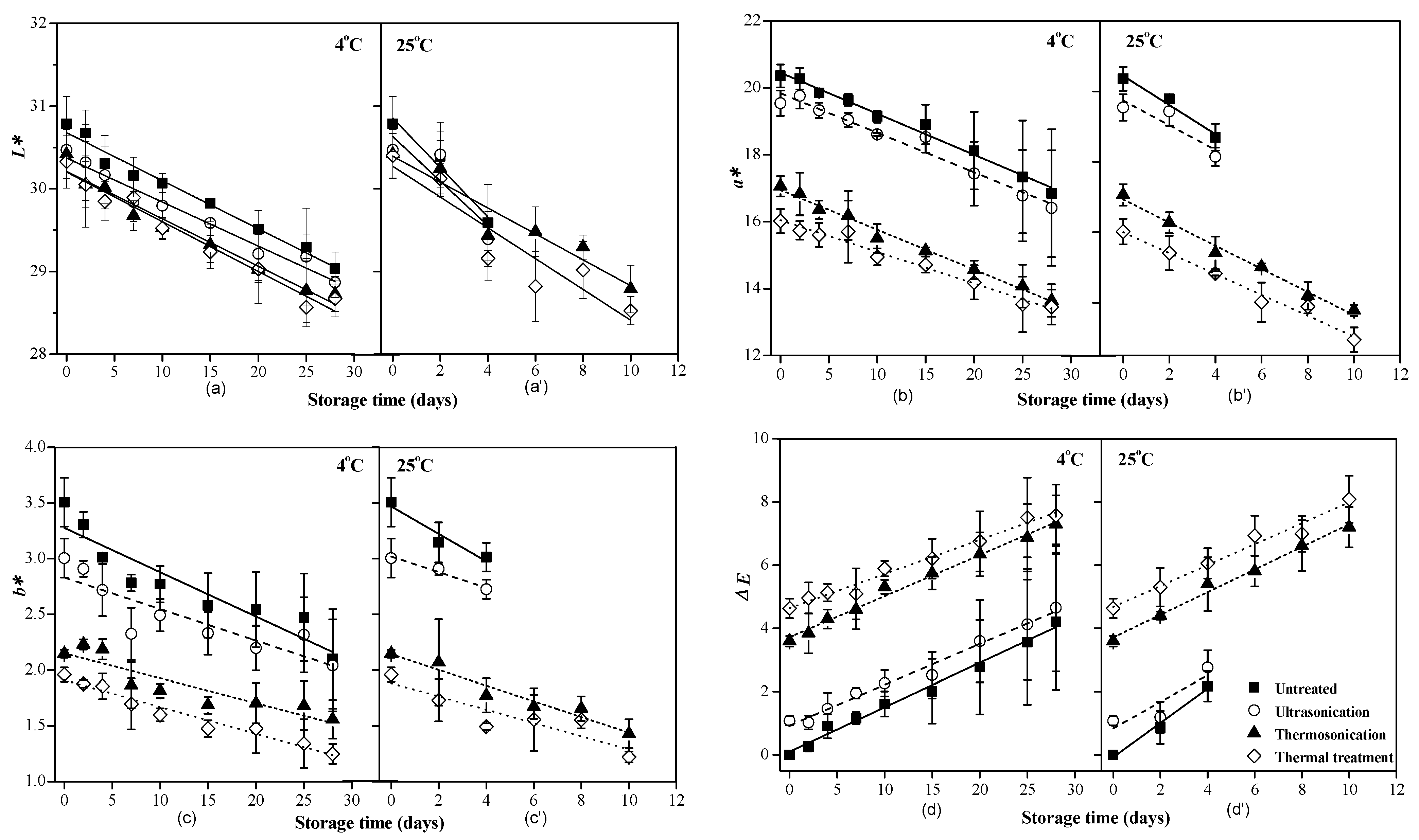
2.4.3. Color Indexes
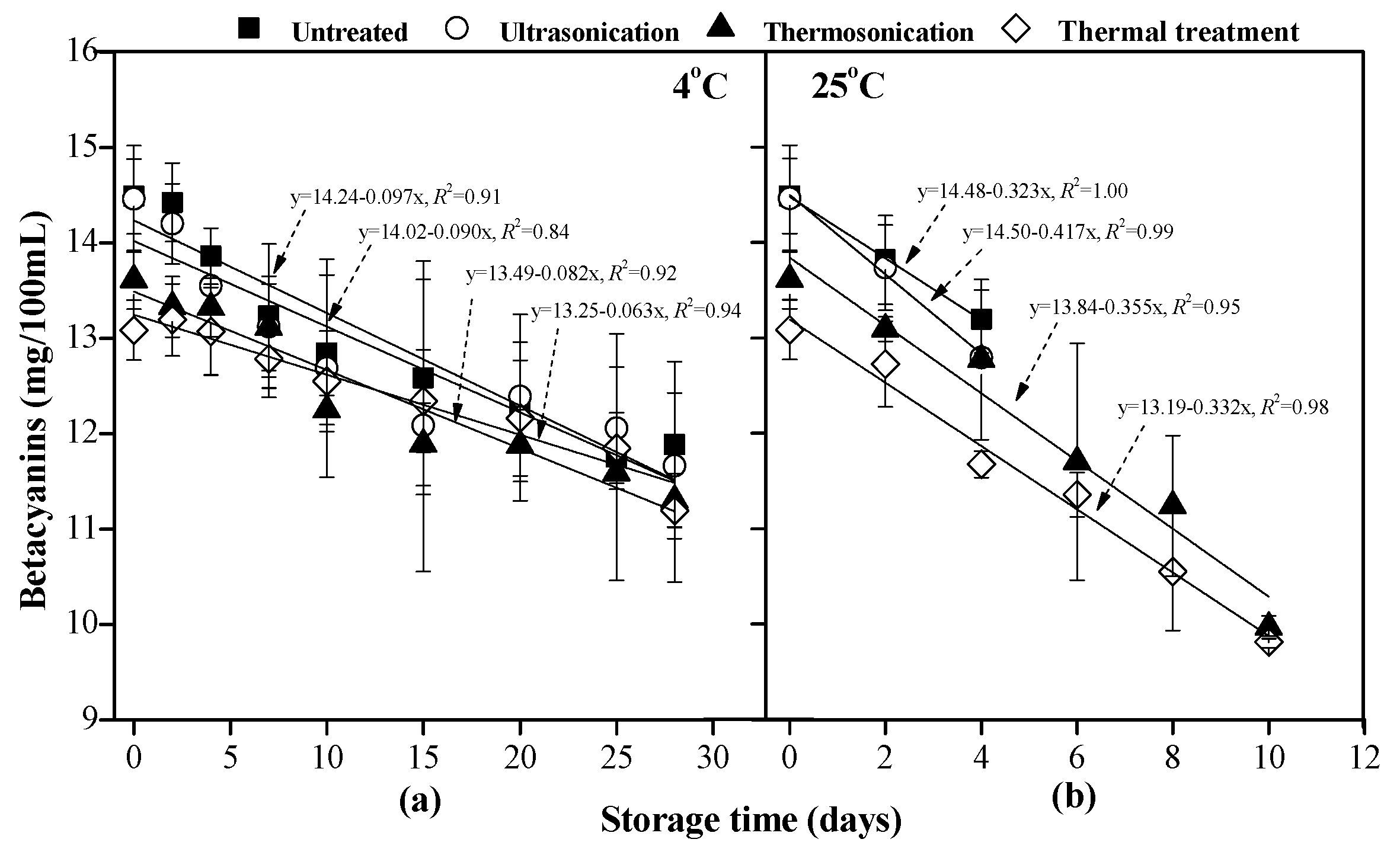
2.4.4. Betacyanins Content
2.4.5. Enzymes Activity
2.4.6. Browning Degree
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microbial Growth
3.2. SSC, pH and Color
3.3. Betacyanins Content
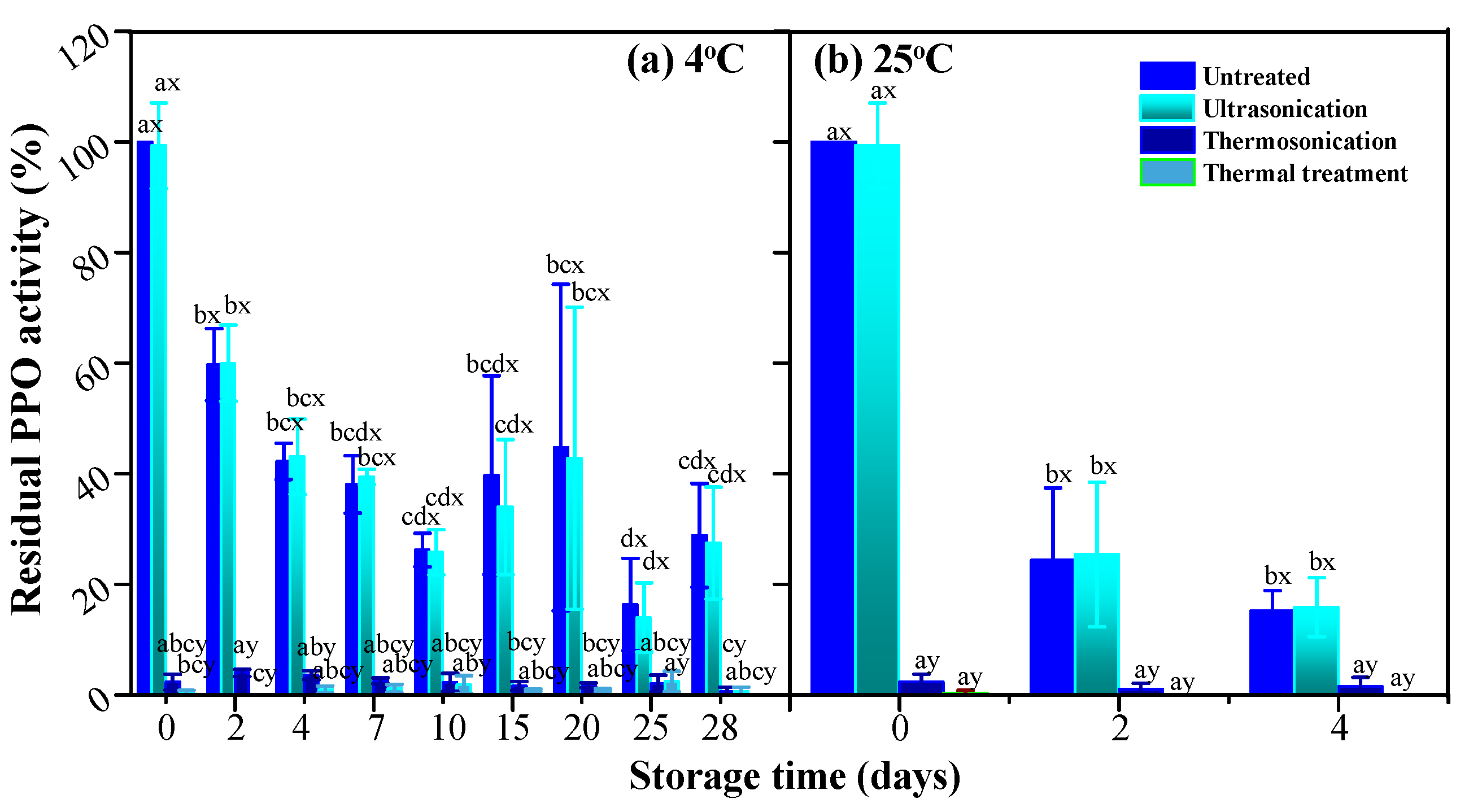
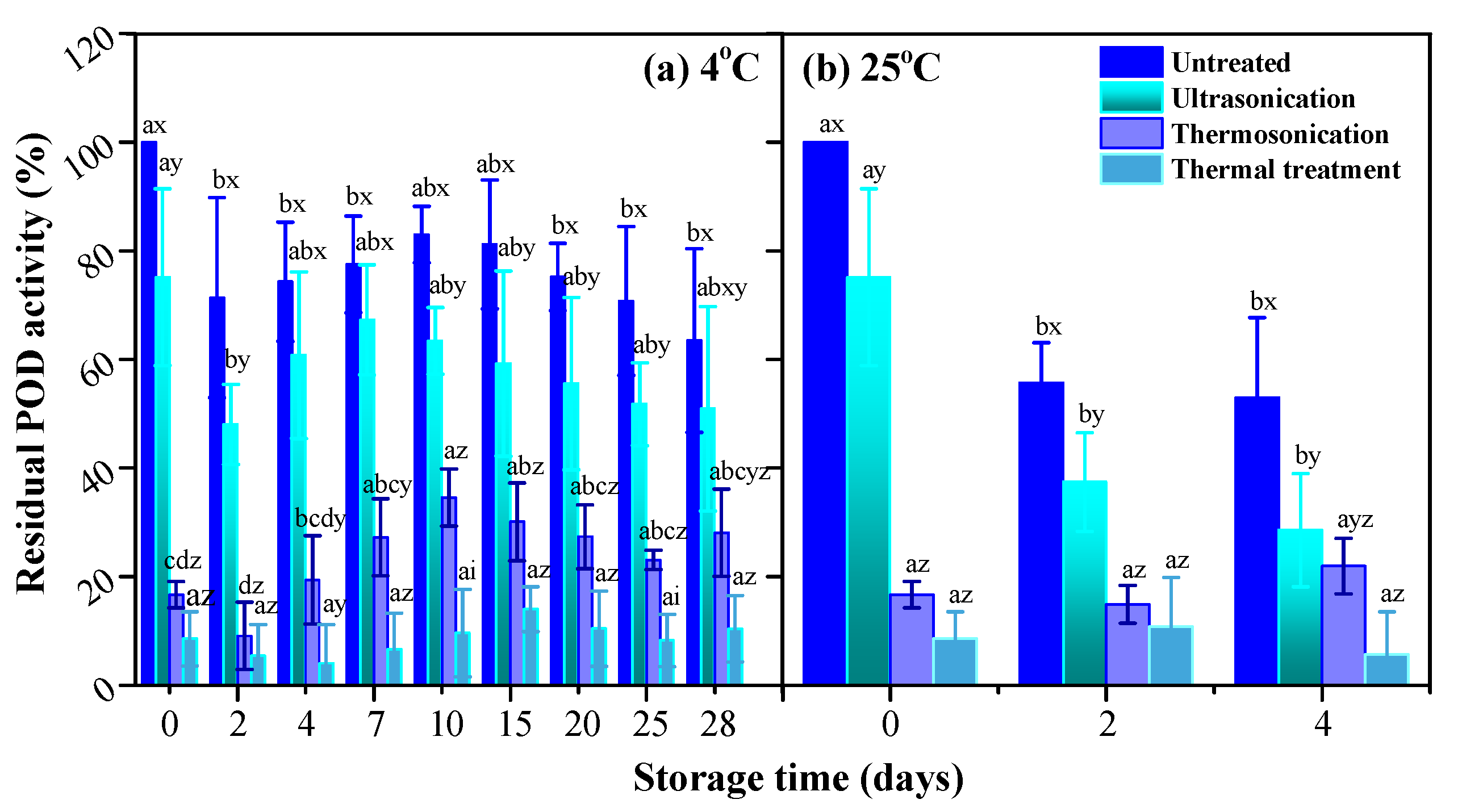
3.4. PPO and POD Activities
3.5. BD Value
3.6. Pearson Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dolas, R.; Saravanan, C.; Kaur, B.P. Emergence and era of ultrasonic’s in fruit juice preservation: A review. Ultrason. Sonochem. 2019, 57, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Cansino, N.C.; Carrera, G.P.; Rojas, Q.Z.; Olivares, L.D.; García, E.A.; Ramírez, E. Ultrasound processing on green cactus pear (Opuntia ficus Indica) juice:Physical, microbiological and antioxidant properties. J. Food Process. Technol. 2013, 4, 1–6. [Google Scholar]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation-structural and chromatic aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- García-Mateos, M.D.R.; Quiroz-González, B.; Corrales-García, J.; Ybarra-Moncada, M.C.; Leyva-Ruelas, G. Ozone-high hydrostatic pressure synergy for the stabilization of refrigerated pitaya (Stenocereus pruinosus) juice. Innov. Food Sci. Emerg. 2019, 56, 1–9. [Google Scholar]
- Liaotrakoon, W.; Clercq, N.; Hoed, V.V.; Walle, D.V.D.; Lewille, B.; Dewettinck, K. Impact of thermal treatment on physicochemical, antioxidative and rheological properties of white-flesh and red-flesh dragon fruit (Hylocereus spp.) purees. Food Bioproc. Technol. 2013, 6, 416–430. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. The effect of pH treatment and refrigerated storage on natural colourant preparations (Betacyanins) from red pitahaya and their potential application in yoghurt. LWT-Food Sci. Technol. 2017, 80, 437–445. [Google Scholar] [CrossRef]
- Halim, H.; Noranizan, M.; Sobhi, B.; Sew, C.C.; Karim, R.; Osman, A. Nonthermal pasteurization of pitaya (Hylocereus polyrhizus) juice using the hurdle concept. Int. Food Res. J. 2012, 19, 1457–1461. [Google Scholar]
- Quiroz-González, B.; Rodríguez-Martínez, V.; García-Mateos, M.D.R.; Torres, J.A.; Welti-Chanes, J. High hydrostatic pressure inactivation and recovery study of Listeria innocua and Saccharomyces cerevisiae in pitaya (Stenocereus pruinosus) juice. Innov. Food Sci. Emerg. Technol. 2018, 50, 169–173. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Hu, B.; Hashim, M.M.; Wu, T.; Lei, S.; Khan, M.A.; Zeng, X. Thermosonication as a potential quality enhancement technique of apple juice. Ultrason.Sonochem. 2014, 21, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, S.; Abid, M.; Bing, H.; Hashim, M.M.; Lei, S.; Wu, T.; Zeng, X. Exploring the potential of thermosonication in carrot juice processing. J. Food Sci. Technol. 2015, 52, 7002–7013. [Google Scholar] [CrossRef]
- Chitgar, M.F.; Aalam, M.; Kadkhodaee, R.; Maghsoudlou, Y.; Milani, E. Effect of thermosonication and thermal treatments on phytochemical stability of barberry juice copigmented with ferulic acid and licorice extract. Innov. Food Sci. Emerg. 2018, 50, 102–111. [Google Scholar] [CrossRef]
- Martínez-Flores, H.E.; Garnica-Romo, M.G.; Bermúdez-Aguirre, D.; Pokhrel, P.R.; Barbosa-Cánovas, G.V. Physico-chemical parameters, bioactive compounds and microbial quality of thermo-sonicated carrot juice during storage. Food Chem. 2015, 172, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Wahia, H.; Zhou, C.; Mustapha, A.T.; Amanor-Atiemoh, R.; Mo, L.; Fakayode, O.A.; Ma, H. Storage effects on the quality quartet of orange juice submitted to moderate thermosonication: Predictive modeling and odor fingerprinting approach. Ultrason. Sonochem. 2020, 64, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Zhu, W.; Zhong, K.; Liu, Y. Evaluation of colour stability of clear red pitaya juice treated by thermosonication. LWT-Food Sci. Technol. 2020, 121, 1–9. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Patras, A.; Brunton, N.; Cullen, P.J.; O’Donnell, C.P. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochem. 2010, 17, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.; Vervoort, L.; Tomic, J.; Santiago, J.S.; Lemmens, L.; Panozzo, A.; Grauwet, T.; Hendrickx, M.; Loey, A.V. Colour and carotenoid changes of pasteurised orange juice during storage. Food Chem. 2015, 171, 330–340. [Google Scholar] [CrossRef]
- Liao, H.; Jiang, L.; Cheng, Y.; Liao, X.; Zhang, R. Application of nisin-assisted thermosonication processing for preservation and quality retention of fresh apple juice. Ultrason. Sonochem. 2018, 42, 244–249. [Google Scholar] [CrossRef]
- Marszałek, K.; Krzyżanowska, J.; Skąpska, S. Kinetic modelling of polyphenol oxidase, peroxidase, pectin esterase, polygalacturonase, degradation of the main pigments and polyphenols in beetroot juice during high pressure carbon dioxide treatment. LWT-Food Sci. Technol. 2017, 85, 412–417. [Google Scholar] [CrossRef]
- Yi, J.; Kebede, B.T.; Dang, D.N.H.; Buve, C.; Grauwet, T.; Loey, A.V.; Hu, X.; Hendrickx, M. Quality change during high pressure processing and thermal processing of cloudy apple juice. LWT-Food Sci. Technol. 2017, 75, 85–92. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Y.; Zhang, F.; Wang, Y.; Yi, J.; Liao, X. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J. Sci. Food Agric. 2011, 91, 877–885. [Google Scholar] [CrossRef]
- Roig, M.G.; Bello, J.F.; Rivera, Z.S.; Kennedy, J.F. Studies on the occurrence of non-enzymic browning during storage of citrus juice. Food Res. Int. 1999, 32, 609–619. [Google Scholar] [CrossRef]
- Cruz-Cansino, N.D.S.; Ramírez-Moreno, E.; León-Rivera, J.E.; Delgado-Olivares, L.; Alanís-García, E.; Ariza-Ortega, J.A.; Manríquez-Torreset, J.D.J.; Jaramillo-Bustos, D.P. Shelf life, physicochemical, microbiological and antioxidant properties of purple cactus pear (Opuntia ficusindica) juice after thermoultrasound treatment. Ultrason. Sonochem. 2015, 27, 277–286. [Google Scholar] [CrossRef] [PubMed]
- GB 7101-2015: National Food Safety Standard Beverage. Available online: http://www.nssi.org.cn/nssi/front/listpage.jsp (accessed on 13 November 2015).
- Tomadoni, B.; Cassani, L.; Viacava, G.; Moreira, M.D.R.; Ponce, A. Effect of ultrasound and storage time on quality attributes of strawberry juice. J. Food Process Eng. 2017, 40, 1–8. [Google Scholar] [CrossRef]
- Adiamo, O.Q.; Ghafoor, K.; Al-Juhaimi, F.; Mohamed Ahmed, I.A.; Babiker, E.E. Effects of thermosonication and orange by-products extracts on quality attributes of carrot (Daucus carota) juice during storage. Int. J. Food. Sci. Technol. 2017, 52, 2115–2125. [Google Scholar] [CrossRef]
- Rivas, A.; Rodrigo, D.; Martínez, A.; Barbosa-Cánovas, G.V.; Rodrigo, M. Effect of PEF and heat pasteurization on the physical-chemical characteristics of blended orange and carrot juice. LWT-Food Sci. Technol. 2006, 39, 1163–1170. [Google Scholar] [CrossRef]
- Kwaw, E.; Tchabo, W.; Ma, Y.; Apaliya, M.T.; Sackey, A.S.; Mintah, B.K.; Farooq, M.; Ma, S. Effect of storage on quality attributes of lactic-acid-fermented mulberry juice subjected to combined pulsed light and ultrasonic pasteurization treatment. J. Food Meas. Charact. 2018, 12, 1763–1771. [Google Scholar] [CrossRef]
- Taira, J.; Tsuchida, E.; Katoh, M.C.; Uehara, M.; Ogi, T. Antioxidant capacity of betacyanins as radical scavengers for peroxyl radical and nitric oxide. Food Chem. 2015, 166, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Siow, L.F.; Wong, Y.M. Effect of juice concentration on storage stability, betacyanin degradation kinetics, and sensory acceptance of red-fleshed dragon fruit (Hylocereus polyrhizus) juice. Int. J. Food Prop. 2017, 20, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Bot, F.; Calligaris, S.; Cortella, G.; Nocera, F.; Peressini, D.; Anese, M. Effect of high pressure homogenization and high power ultrasound on some physical properties of tomato juices with different concentration levels. J. Food Eng. 2018, 221, 70–76. [Google Scholar] [CrossRef]
- Wybraniec, S.; Michałowski, T. New pathways of betanidin and betanin enzymatic oxidation. J. Agric. Food Chem. 2011, 59, 9612–9622. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M. Stabilization of betalains: A review. Food Chem. 2016, 197, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Mendel, F. Food browning and its prevention: An overview. J. Agric. Food Chem. 1996, 44, 631–653. [Google Scholar]
- Herceg, Z.; Lelas, V.; Jambrak, A.R.; Vukušić, T.; Levaj, B. Influence of thermo-sonication on microbiological safety, color and anthocyanins content of strawberry juice. J. Hyg. Eng. Des. 2013, 4, 26–37. [Google Scholar]

| ΔE | L* | a* | b* | Betacyanins | pH | SSC | PPO | POD | BD | AB | Y&M | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔE | 1 | −0.98 ** | −1.00 ** | −0.96 ** | −0.91 ** | −0.73 ** | −0.47 | 0.51 | 0.38 | 0.75 ** | 0.85 ** | 0.67 * |
| L* | −0.98 ** | 1 | 0.97 ** | 0.96 ** | 0.91 ** | 0.73 ** | 0.54 | −0.52 | −0.27 | −0.73 ** | −0.83 ** | −0.71 ** |
| a* | −1.00 ** | 0.97 ** | 1 | 0.96 ** | 0.91 ** | 0.73 ** | 0.45 | −0.50 | −0.38 | −0.75 ** | −0.85 ** | −0.65 * |
| b* | −0.94 ** | 0.93 ** | 0.93 ** | 1 | 0.94 ** | 0.81 ** | 0.55 | −0.45 | −0.41 | −0.78 ** | −0.89 ** | −0.77 ** |
| Betacyanins | −0.98 ** | 0.95 ** | 0.97 ** | 0.92 ** | 1 | 0.80 ** | 0.56 | −0.25 | −0.31 | −0.67 * | −0.90 ** | −0.61 * |
| pH | −0.75 ** | 0.68 * | 0.75 ** | 0.76 ** | 0.73 ** | 1 | 0.50 | −0.38 | −0.55 | −0.49 | −0.64 * | −0.66 * |
| SSC | −0.69 * | 0.71 * | 0.69 * | 0.53 | 0.65 * | 0.43 | 1 | −0.38 | −0.05 | −0.33 | −0.54 | −0.58 * |
| PPO | −0.59 * | 0.45 | 0.60 * | 0.68 * | 0.57 | 0.65 * | 0.05 | 1 | 0.10 | 0.42 | 0.21 | 0.41 |
| POD | 0.65 * | −0.66 * | −0.64 * | −0.79 ** | −0.69 * | −0.76 ** | −0.45 | −0.43 | 1 | 0.16 | 0.23 | 0.55 |
| BD | 0.77 ** | −0.81 ** | −0.77 ** | −0.80 ** | −0.67 * | −0.43 | −0.53 | −0.46 | 0.44 | 1 | 0.73 ** | 0.61 * |
| AB | 0.96 ** | −0.96 ** | −0.96 ** | −0.94 ** | −0.92 ** | −0.76 ** | −0.70 * | −0.56 | 0.74 ** | 0.83 ** | 1 | 0.59 * |
| Y&M | 0.85 ** | −0.79 ** | −0.86 ** | −0.79 ** | −0.83 ** | −0.65 * | −0.57 | −0.68 * | 0.49 | 0.69 * | 0.85 ** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Ai, Y.; Fang, F.; Liao, H. Application of Thermosonication in Red Pitaya Juice Processing: Impacts on Native Microbiota and Quality Properties during Storage. Foods 2021, 10, 1041. https://doi.org/10.3390/foods10051041
Zhu W, Ai Y, Fang F, Liao H. Application of Thermosonication in Red Pitaya Juice Processing: Impacts on Native Microbiota and Quality Properties during Storage. Foods. 2021; 10(5):1041. https://doi.org/10.3390/foods10051041
Chicago/Turabian StyleZhu, Wenxian, Yana Ai, Fang Fang, and Hongmei Liao. 2021. "Application of Thermosonication in Red Pitaya Juice Processing: Impacts on Native Microbiota and Quality Properties during Storage" Foods 10, no. 5: 1041. https://doi.org/10.3390/foods10051041
APA StyleZhu, W., Ai, Y., Fang, F., & Liao, H. (2021). Application of Thermosonication in Red Pitaya Juice Processing: Impacts on Native Microbiota and Quality Properties during Storage. Foods, 10(5), 1041. https://doi.org/10.3390/foods10051041







