Spices in the Apiaceae Family Represent the Healthiest Fatty Acid Profile: A Systematic Comparison of 34 Widely Used Spices and Herbs
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Reagents, and Standards
2.2. Extraction of Crude Lipid Compounds
2.3. Preparation of Fatty Acid Methyl Esters (FAMEs)
2.4. GC-FID and GC-MS Analysis of FAMEs
2.5. Calculation of Fat Quality Indices
2.6. Statistical Analysis and Quality Control
3. Results and Discussion
3.1. Fatty Acids Composition
3.2. Black Pepper and White Pepper
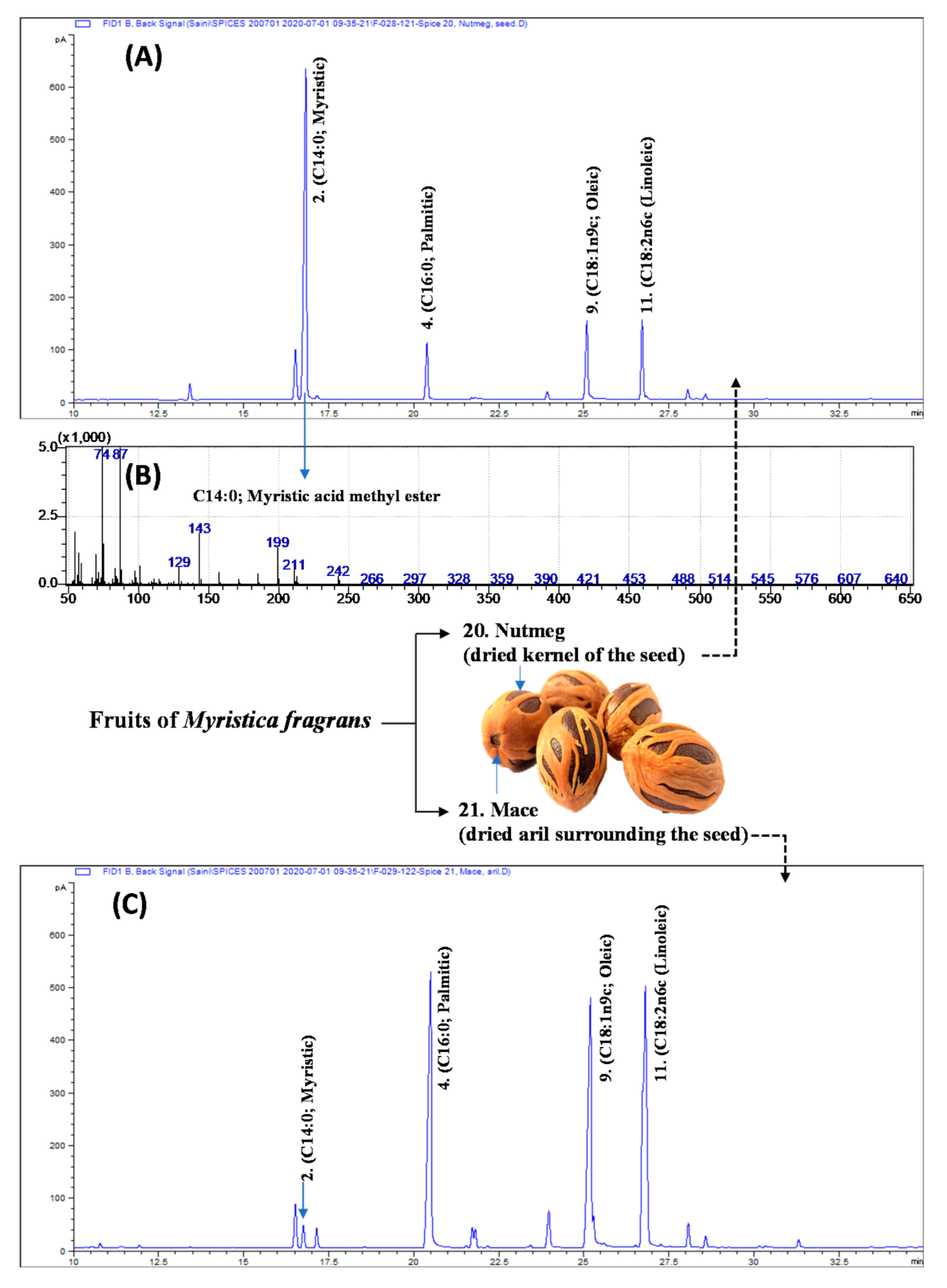
3.3. Nutmeg and Mace
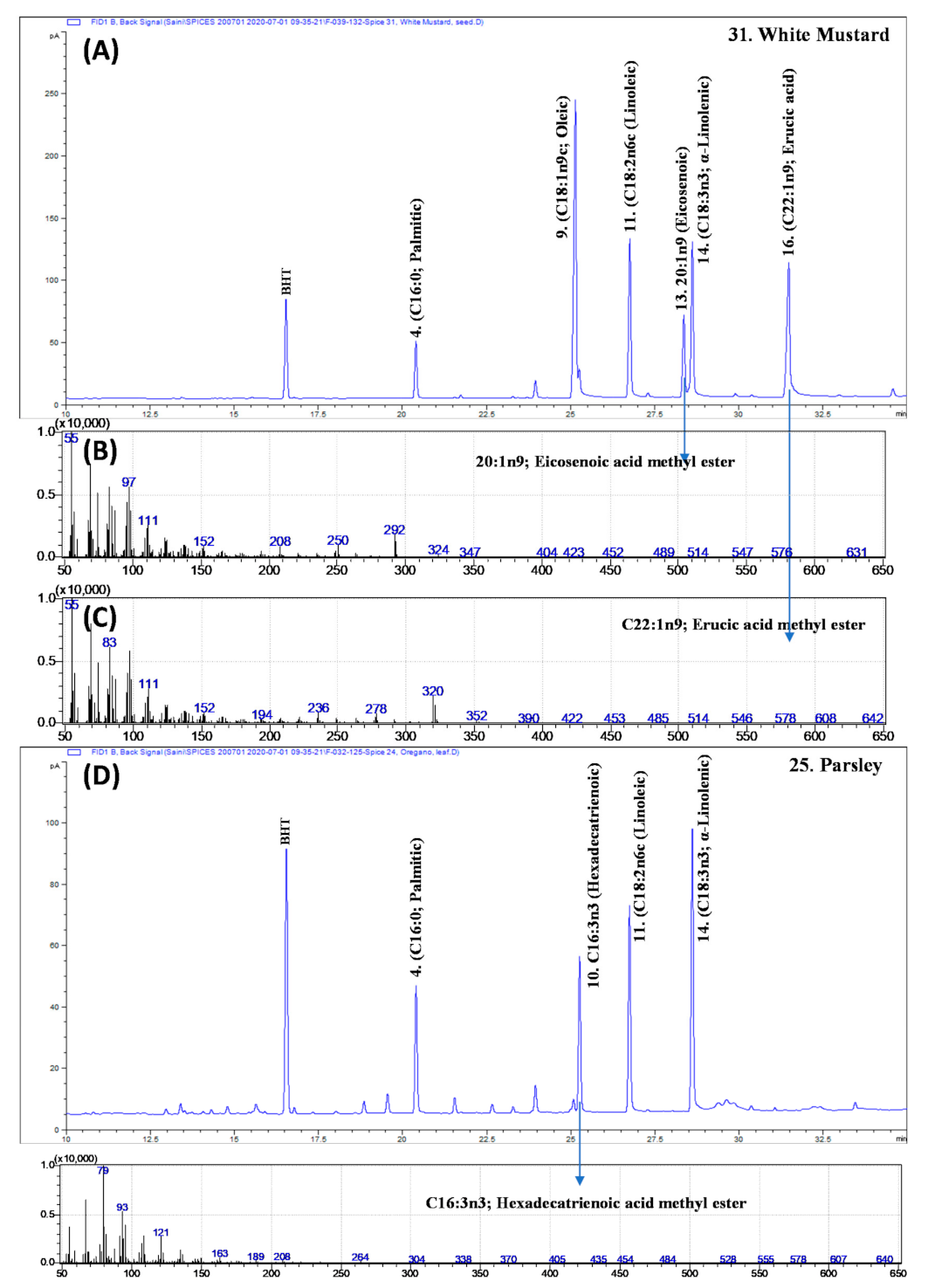
3.4. Erucic Acid in White Mustard
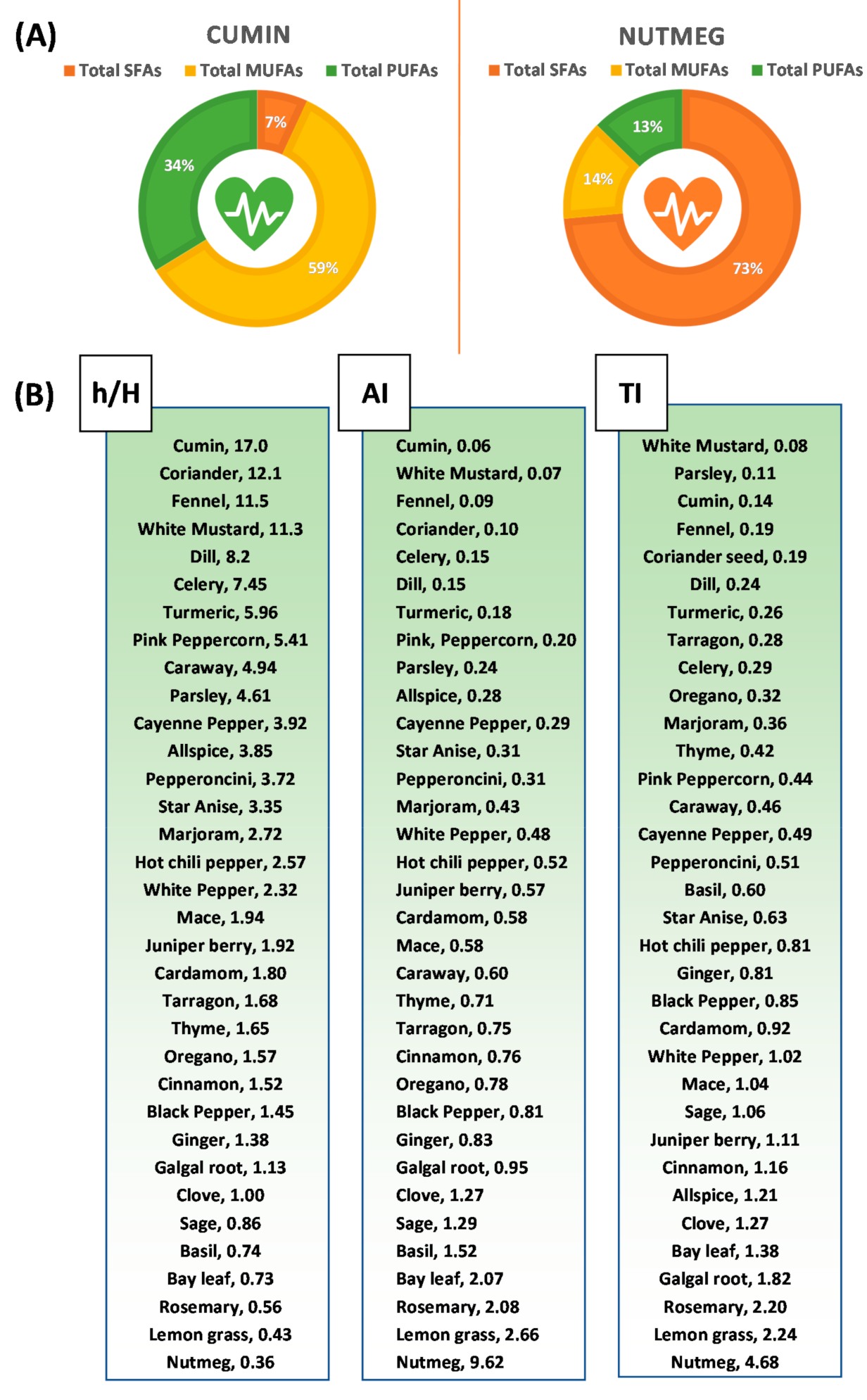
3.5. Fat Quality Indices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rubió, L.; Motilva, M.-J.; Romero, M.-P. Recent Advances in Biologically Active Compounds in Herbs and Spices: A Review of the Most Effective Antioxidant and Anti-Inflammatory Active Principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Graciá, C.; González-Bermúdez, C.A.; Cabellero-Valcárcel, A.M.; Santaella-Pascual, M.; Frontela-Saseta, C. Use of herbs and spices for food preservation: Advantages and limitations. Curr. Opin. Food Sci. 2015, 6, 38–43. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Spices as functional foods. Crit. Rev. Food Sci. Nutr. 2010, 51, 13–28. [Google Scholar] [CrossRef]
- Jiang, T.A. Health Benefits of Culinary Herbs and Spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef]
- Alasvand, S.; Bridges, W.; Haley-Zitlin, V. Efficacy of Common Spices on Improving Serum Lipids in Individuals with Type 2 Diabetes: Systematic Review and Meta-Analysis of Clinical Trials. Curr. Dev. Nutr. 2020, 4, 1. [Google Scholar] [CrossRef]
- Yu, L.; Choe, U.; Li, Y.; Zhang, Y. Oils from fruit, spice, and herb seeds. Bailey Ind. Oil Fat Prod. 2005, 1–35. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Harnack, K.; Andersen, G.; Somoza, V. Quantitation of alpha-linolenic acid elongation to eicosapentaenoic and docosahexaenoic acid as affected by the ratio of n6/n3 fatty acids. Nutr. Metab. 2009, 6, 8. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of the dietary omega-6/omega-3 fatty acid Ratio: Medical implications. In Evolutionary Thinking in Medicine; Springer: Berlin/Heidelberg, Germany, 2016; pp. 119–134. [Google Scholar]
- Briggs, M.; Petersen, K.; Kris-Etherton, P. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef]
- Cruz, R.; Casal, S.; Mendes, E.; Costa, A.; Santos, C.; Morais, S. Validation of a Single-Extraction Procedure for Sequential Analysis of Vitamin E, Cholesterol, Fatty Acids, and Total Fat in Seafood. Food Anal. Methods 2013, 6, 1196–1204. [Google Scholar] [CrossRef]
- Saini, R.K.; Mahomoodally, M.F.; Sadeer, N.B.; Keum, Y.S.; Rr Rengasamy, K. Characterization of nutritionally important lipophilic constituents from brown kelp Ecklonia radiata (C. Ag.) J. Agardh. Food Chem. 2021, 340. [Google Scholar] [CrossRef]
- Saini, R.K.; Rengasamy, K.R.R.; Ko, E.Y.; Kim, J.T.; Keum, Y.S. Korean Maize Hybrids Present Significant Diversity in Fatty Acid Composition: An Investigation to Identify PUFA-Rich Hybrids for a Healthy Diet. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S.; Rengasamy, K.R. Profiling of nutritionally important metabolites in green/red and green perilla (Perilla frutescens Britt.) cultivars: A comparative study. Ind. Crop Prod. 2020, 151, 112441. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Parry, J.W.; Cheng, Z.; Moore, J.; Yu, L.L. Fatty Acid Composition, Antioxidant Properties, and Antiproliferative Capacity of Selected Cold-Pressed Seed Flours. J. Am. Oil Chem. Soc. 2008, 85, 457–464. [Google Scholar] [CrossRef]
- Parry, J.; Hao, Z.; Luther, M.; Su, L.; Zhou, K.; Yu, L. Characterization of cold-pressed onion, parsley, cardamom, mullein, roasted pumpkin, and milk thistle seed oils. J. Am. Oil Chem. Soc. 2006, 83, 847–854. [Google Scholar] [CrossRef]
- Anaduaka, E.G.; Uchendu, N.O.; Ezeanyika, L.U.S. Mineral, amino acid and fatty acid evaluations of Myristica fragrans seeds extracts. Sci. Afr. 2020, 10, e00567. [Google Scholar] [CrossRef]
- Lee, J.-G.; Chae, Y.; Shin, Y.; Kim, Y.-J. Chemical composition and antioxidant capacity of black pepper pericarp. Appl. Biol. Chem. 2020, 63. [Google Scholar] [CrossRef]
- Rema, J.; Krishnamoorthy, B. 22—Nutmeg and mace. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing: Abington, UK, 2012; pp. 399–416. [Google Scholar] [CrossRef]
- Playfair, L. XX. On a new fat acid in the butter of nutmegs. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1841, 18, 102–113. [Google Scholar] [CrossRef][Green Version]
- Al-Khatib, I.M.H.; Hanifa Moursi, S.A.; Mehdi, A.W.R.; Al-Shabibi, M.M. Gas-liquid chromatographic determination of fatty acids and sterols of selected Iraqi foods. J. Food Compos. Anal. 1987, 1, 59–64. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczyńska, E.; Ścibisz, I.; Rudzińska, M. Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem. 2016, 213, 450–456. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G. Safety assessment of myristic acid as a food ingredient. Food Chem. Toxicol. 2007, 45, 517–529. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Erucic acid in feed and food. EFSA J. 2016, 14. [Google Scholar] [CrossRef]
- Ksouda, G.; Hajji, M.; Sellimi, S.; Merlier, F.; Falcimaigne-Cordin, A.; Nasri, M.; Thomasset, B. A systematic comparison of 25 Tunisian plant species based on oil and phenolic contents, fatty acid composition and antioxidant activity. Ind. Crop Prod. 2018, 123, 768–778. [Google Scholar] [CrossRef]
- Reiter, B.; Lechner, M.; Lorbeer, E. The fatty acid profiles—Including petroselinic and cis-vaccenic acid-of different Umbelliferae seed oils. Lipid/Fett 1998, 100, 498–502. [Google Scholar] [CrossRef]
- Alaluf, S.; Green, M.; Powell, J.; Rogers, J.; Watkinson, A.; Cain, F.; Hu, H.; Rawlings, A. Petroselinic Acid and Its Use in Food. U.S. Patent No. 6,365,175 B1, 2 April 2002. [Google Scholar]
- Mongrand, S.; Bessoule, J.-J.; Cabantous, F.; Cassagne, C. The C16:3\C18:3 fatty acid balance in photosynthetic tissues from 468 plant species. Phytochemistry 1998, 49, 1049–1064. [Google Scholar] [CrossRef]
- Wołoszyn, J.; Haraf, G.; Okruszek, A.; Wereńska, M.; Goluch, Z.; Teleszko, M. Fatty acid profiles and health lipid indices in the breast muscles of local Polish goose varieties. Poult. Sci. 2020, 99, 1216–1224. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Assefa, A.D.; Keum, Y.S.; Saini, R.K. A comprehensive study of polyphenols contents and antioxidant potential of 39 widely used spices and food condiments. J. Food Meas. Charact. 2018, 12, 1548–1555. [Google Scholar] [CrossRef]
| Sample No. | Common Name | Botanical Name | Family | Part |
|---|---|---|---|---|
| 1 | Galangal root | Alpinia galanga (L.) Willd. | Zingiberaceae | Rhizomes |
| 2 | Dill | Anethum graveolens L. | Apiaceae | Seeds |
| 3 | Celery | Apium graveolens L. | Apiaceae | Seeds |
| 4 | Tarragon | Artemisia dracunculus L. | Asteraceae | Leaves |
| 5 | Cayenne pepper | Capsicum annuum L. | Solanaceae | Pods |
| 6 | Pepperoncini | Capsicum annuum L. var. annuum | Solanaceae | Pods |
| 7 | Hot chili pepper | Capsicum frutescens L. | Solanaceae | Pods |
| 8 | Caraway | Carum carvi L. | Apiaceae | Fruits |
| 9 | Cinnamon | Cinnamomum verum J.Presl | Lauraceae | Bark |
| 10 | Coriander seed | Coriandrum sativum L. | Apiaceae | Seeds |
| 11 | Cumin | Cuminum cyminum L. | Apiaceae | Seeds |
| 12 | Turmeric | Curcuma longa L. | Zingiberaceae | Rhizomes |
| 13 | Lemongrass | Cymbopogon microstachys (J. D. Hooker) Soenarko | Poaceae | Leaves |
| 14 | Cardamom | Elettaria cardamomum (L.) Maton | Zingiberaceae | Pods |
| 15 | Fennel | Foeniculum vulgare Mill. | Apiaceae | Seeds |
| 16 | Star anise | Illicium verum Hook.f. | Schisandraceae | Fruits |
| 17 | Allspice | Pimenta dioica (L.) Merr. | Myrtaceae | Fruits |
| 18 | Juniper berry | Juniperus communis L. | Cupressaceae | Fruits |
| 19 | Bay leaf | Laurus nobilis L. | Lauraceae | Leaves |
| 20 | Nutmeg | Myristica fragrans Houtt. | Myrtaceae | Seeds |
| 21 | Mace | Myristica fragrans Houtt. | Myrtaceae | Aril |
| 22 | Basil | Ocimum basilicum L. | Lamiaceae | Leaves |
| 23 | Marjoram | Origanum majorana L. | Lamiaceae | Leaves |
| 24 | Oregano | Origanum vulgare L. | Lamiaceae | Leaves |
| 25 | Parsley | Petroselinum crispum (Mill.) Fuss | Apiaceae | Leaves |
| 26 | Black pepper | Piper nigrum L. | Piperaceae | Fruits (unripe) |
| 27 | White pepper | Piper nigrum L. | Piperaceae | Seeds |
| 28 | Rosemary | Rosmarinus officinalis L. | Lamiaceae | Leaves |
| 29 | Sage | Salvia officinalis L. | Lamiaceae | Leaves |
| 30 | Pink peppercorn | Schinus mole L. | Anacardiaceae | Fruits |
| 31 | White mustard | Sinapis alba L. | Brassicaceae | Seeds |
| 32 | Clove | Syzygium aromaticum (L.) Merr. and L. M. Perry | Myrtaceae | Flower buds |
| 33 | Thyme | Thymus vulgaris L. | Lamiaceae | Leaves |
| 34 | Ginger | Zingiber officinale Roscoe | Zingiberaceae | Rhizomes |
| Peak No | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Component (FAME) | C12:0 (Lauric) | C14:0 (Myristic) | C15:0 (Pentadecanoic) | C16:0 (Palmitic) | C16:1 (Palmitoleic) | C17:0 (Heptadecanoic) | C18:0 (Stearic) | C18:1n12c (Petroselinic) | C18:1n9c (Oleic) | C16:3n3 (Hexadecatrienoic) | C18:2n6c (Linoleic) | C20:0 (Arachidic) | 20:1n9 (Eicosenoic) | C18:3n3 (α-Linolenic) | C22:0 (Behenic) | C22:1n9 (Erucic) | C24:0 (Lignoceric) | C24:1n9 (Nervonic) |
| RT | 13.44 | 16.78 | 18.58 | 20.42 | 21.74 | 22.20 | 23.96 | 25.03 | 25.10 | 25.20 | 26.81 | 27.29 | 28.36 | 28.60 | 30.38 | 31.48 | 33.46 | 34.57 |
| S1 | 0.62 | 0.90 | 1.18 a | 37.65 | nd | nd | 13.06 | nd | 28.21 | nd | 13.59 | nd | nd | 2.45 | 1.01 | nd | 1.33 | nd |
| S2 | 1.20 | 0.90 | nd | 8.48 | 0.34 | nd | 1.54 | 50.37 | 15.26 | nd | 20.49 | 0.23 | nd | 0.85 | 0.23 | nd | 0.12 | nd |
| S3 | nd | 0.44 | nd | 11.09 | 0.21 | nd | 1.91 | 49.42 | 7.48 | nd | 27.55 | 0.23 | nd | 1.32 | 0.21 | nd | 0.15 | nd |
| S4 | 6.95 | 2.90 | nd | 24.86 | nd | nd | 3.03 | nd | 2.23 | nd | 24.10 | 2.21 | nd | 31.84 | 1.16 | nd | 0.72 | nd |
| S5 | 0.27 | 0.95 | nd | 18.25 | 0.64 | 0.13 | 2.73 | nd | 12.31 | nd | 61.46 | 0.38 | nd | 2.53 | 0.22 | nd | 0.12 | nd |
| S6 | 0.27 | 1.19 | nd | 18.71 | 0.86 | nd | 3.03 | nd | 12.68 | nd | 59.65 | 0.45 | nd | 2.75 | 0.27 | nd | 0.16 | nd |
| S7 | 1.18 | 3.22 | nd | 21.98 | 0.85 | nd | 3.87 | 21.76 | 17.43 | nd | 27.99 | 0.48 | nd | 0.68 | 0.36 | nd | 0.20 | nd |
| S8 | nd | 10.62 | nd | 5.55 | 0.13 | nd | 2.95 | 34.09 | 11.70 | nd | 33.70 | 0.50 | nd | 0.47 | 0.21 | nd | 0.07 | nd |
| S9 | Nd | 1.77 | nd | 33.97 | nd | nd | 7.49 | nd | 23.25 | nd | 26.99 | nd | nd | 4.04 | 1.13 | nd | 1.36 | nd |
| S10 | 0.10 | 0.45 | 0.14 | 6.91 | 0.28 | nd | 1.36 | 62.07 | 7.13 | nd | 20.85 | nd | nd | 0.35 | 0.23 | nd | 0.12 | nd |
| S11 | 0.08 | 0.10 | 0.16 | 5.28 | 0.29 | nd | 1.08 | 49.89 | 9.21 | nd | 33.34 | nd | nd | 0.39 | 0.12 | nd | 0.07 | nd |
| S12 | 2.81 | 0.40 | nd | 10.51 | 0.76 | nd | 2.67 | nd | 4.48 | nd | 72.86 a | nd | nd | 4.50 | 0.32 | nd | 0.67 | nd |
| S13 | 5.68 | 2.74 | nd | 47.82 a | nd | nd | 9.46 | nd | 6.20 | nd | 12.29 | 3.89 | nd | 5.70 | 3.64 a | nd | 2.57 | nd |
| S14 | nd | 0.75 | nd | 32.84 | 1.58 a | nd | 3.19 | nd | 41.81 a | nd | 15.07 | 0.67 | nd | 3.44 | 0.26 | nd | 0.38 | nd |
| S15 | 0.46 | 0.17 | nd | 7.25 | 0.21 | nd | 1.24 | 63.33 a | 6.88 | nd | 19.60 | 0.16 | nd | 0.53 | 0.10 | nd | 0.07 | nd |
| S16 | 1.47 | 0.26 | nd | 20.26 | 0.17 | nd | 3.74 | nd | 33.75 | nd | 39.27 | nd | nd | 0.71 | 0.29 | nd | 0.08 | nd |
| S17 | nd | 0.29 | nd | 14.27 | nd | 0.21 a | 27.16 a | nd | 13.90 | nd | 39.58 | 1.39 | nd | 2.53 | 0.47 | nd | 0.20 | nd |
| S18 | 0.61 | 0.92 | nd | 26.01 | nd | nd | 12.37 | nd | 22.41 | nd | 26.98 | 4.40 a | nd | 3.56 | 1.50 | nd | 1.24 | nd |
| S19 | 4.88 | 8.60 | nd | 37.43 | nd | nd | 5.43 | nd | 20.33 | nd | 9.55 | 1.49 | nd | 7.19 | 1.51 | nd | 3.58 a | nd |
| S20 | 2.19 | 60.81 a | nd | 8.94 | 0.39 | nd | 1.26 | nd | 13.36 | nd | 11.94 | 0.08 | nd | 0.76 | 0.14 | nd | 0.13 | nd |
| S21 | 0.08 | 1.59 | nd | 30.63 | 1.36 | 0.14 | 3.29 | nd | 28.00 | nd | 33.72 | 0.14 | nd | 0.81 | 0.13 | nd | 0.11 | nd |
| S22 | 17.47 a | 1.97 | nd | 29.85 | nd | nd | 7.81 | nd | 7.56 | nd | 10.18 | 4.18 | nd | 18.57 | 1.63 | nd | 0.79 | nd |
| S23 | 1.20 | 1.33 | nd | 21.60 | nd | nd | 5.39 | nd | 33.65 | nd | 13.64 | 2.63 | nd | 18.23 | 1.33 | nd | 0.99 | nd |
| S24 | 4.91 | 2.63 | nd | 26.85 | nd | nd | 7.89 | nd | 6.42 | nd | 12.49 | 1.83 | nd | 35.08 a | 1.04 | nd | 0.87 | nd |
| S25 | 1.15 | 0.72 | nd | 15.01 | nd | nd | 3.38 | nd | 2.14 | 17.74 a | 24.49 | 0.24 | nd | 33.40 | 0.64 | nd | 1.09 | nd |
| S26 | 4.71 | 1.93 | nd | 28.57 | nd | nd | 11.35 | nd | 14.95 | nd | 26.61 | 0.44 | nd | 9.32 | 0.81 | nd | 1.31 | nd |
| S27 | 2.62 | 0.95 | nd | 22.55 | nd | nd | 11.25 | nd | 41.64 a | nd | 17.59 | nd | nd | 1.49 | 0.81 | nd | 1.11 | nd |
| S28 | nd | 2.93 | nd | 47.85 a | nd | nd | 11.39 | nd | 15.62 | nd | 7.74 | 3.65 | nd | 5.31 | 3.05 | nd | 2.45 | nd |
| S29 | nd | 1.59 | nd | 42.71 | nd | nd | 10.67 | nd | 14.51 | nd | 10.90 | 3.09 | nd | 12.62 | 2.76 | nd | 1.14 | nd |
| S30 | nd | 0.35 | nd | 14.29 | 0.63 | nd | 4.59 | nd | 21.11 | nd | 56.56 | 0.43 | nd | 1.54 | 0.26 | nd | 0.24 | nd |
| S31 | 0.12 | 0.14 | nd | 5.53 | 0.30 | nd | 1.77 | nd | 32.97 | nd | 15.78 | 0.43 | 7.96 a | 16.59 | 0.23 | 17.28 a | 0.09 | 0.82 a |
| S32 | 7.95 | 3.44 | nd | 27.93 | nd | nd | 12.60 | nd | 4.47 | nd | 28.69 | 2.29 | nd | 5.99 | 2.39 | nd | 4.26 a | nd |
| S33 | 0.91 | 1.83 | nd | 29.78 | nd | nd | 10.11 | nd | 11.03 | nd | 14.62 | 1.52 | nd | 28.15 | 1.18 | nd | 0.87 | nd |
| S34 | 3.85 | 1.79 | nd | 31.47 | nd | nd | 10.01 | nd | 7.49 | nd | 32.47 | nd | nd | 11.10 | 0.75 | nd | 1.07 | nd |
| Sample No | Total FA (mg/g DW) | Total SFAs | Total MUFAs | Total PUFAs | PUFAs: SFAs | PUFAs: MUFAs | n−3 PUFA | n−6 PUFA | n−6/n−3 | h/H | AI | TI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 2.30b | 55.75 | 28.21 | 16.04 | 0.29 | 0.57 | 2.45 | 13.59 | 5.55 | 1.13 | 0.95 | 1.82 |
| S2 | 23.30 | 12.70 | 65.97 | 21.34 | 1.68 | 0.32 b | 0.85 | 20.49 | 24.02 | 8.22 | 0.15 | 0.24 |
| S3 | 27.60 | 14.02 | 57.11 | 28.88 | 2.06 | 0.51 | 1.32 | 27.55 | 20.82 | 7.45 | 0.15 | 0.29 |
| S4 | 8.30 | 41.83 | 2.23b | 55.94 | 1.34 | 25.13 | 31.84 | 24.10 | 0.76 b | 1.68 | 0.75 | 0.28 |
| S5 | 120.83 | 23.06 | 12.96 | 63.99 | 2.78 | 4.94 | 2.53 | 61.46 | 24.30 | 3.92 | 0.29 | 0.49 |
| S6 | 48.72 | 24.07 | 13.54 | 62.40 | 2.59 | 4.61 | 2.75 | 59.65 | 21.71 | 3.72 | 0.31 | 0.51 |
| S7 | 42.00 | 31.29 | 40.04 | 28.67 | 0.92 | 0.72 | 0.68 | 27.99 | 41.12 | 2.57 | 0.52 | 0.81 |
| S8 | 103.38 | 19.90 | 45.93 | 34.17 | 1.72 | 0.74 | 0.47 b | 33.70 | 71.19 | 4.94 | 0.60 | 0.46 |
| S9 | 2.78 | 45.73 | 23.25 | 31.03 | 0.68 | 1.33 | 4.04 | 26.99 | 6.68 | 1.52 | 0.76 | 1.16 |
| S10 | 57.06 | 9.30 | 69.49 a | 21.21 | 2.28 | 0.31 b | 0.35 b | 20.85 | 58.96 | 12.12 | 0.10 | 0.19 |
| S11 | 36.37 | 6.88 b | 59.39 | 33.73 | 4.90 a | 0.57 | 0.39 b | 33.34 | 85.99 a | 17.01 a | 0.06 b | 0.14 |
| S12 | 7.01 | 17.39 | 5.24 | 77.36 a | 4.45 | 14.75 | 4.50 | 72.86 a | 16.20 | 5.96 | 0.18 | 0.26 |
| S13 | 2.75 | 75.80 a | 6.20 | 17.99 | 0.24 | 2.90 | 5.70 | 12.29 | 2.16 | 0.43 b | 2.66 | 2.24 |
| S14 | 8.87 | 38.10 | 43.39 | 18.50 | 0.49 | 0.43 b | 3.44 | 15.07 | 4.38 | 1.80 | 0.58 | 0.92 |
| S15 | 53.18 | 9.45 | 70.42 a | 20.13 | 2.13 | 0.29 b | 0.53 b | 19.60 | 37.30 | 11.47 | 0.09 | 0.19 |
| S16 | 29.96 | 26.11 | 33.92 | 39.98 | 1.53 | 1.18 | 0.71 | 39.27 | 55.10 | 3.35 | 0.31 | 0.63 |
| S17 | 22.49 | 43.98 | 13.90 | 42.11 | 0.96 | 3.03 | 2.53 | 39.58 | 15.62 | 3.85 | 0.28 | 1.21 |
| S18 | 5.98 | 47.05 | 22.41 | 30.54 | 0.65 | 1.36 | 3.56 | 26.98 | 7.58 | 1.92 | 0.57 | 1.11 |
| S19 | 5.39 | 62.92 | 20.33 | 16.74 | 0.27 | 0.82 | 7.19 | 9.55 | 1.33 | 0.73 | 2.07 | 1.38 |
| S20 | 61.04 | 73.56 | 13.74 | 12.70 b | 0.17 b | 0.92 | 0.76 | 11.94 | 15.73 | 0.36 b | 9.62 a | 4.68 a |
| S21 | 130.32 a | 36.12 | 29.36 | 34.53 | 0.96 | 1.18 | 0.81 | 33.72 | 41.69 | 1.94 | 0.58 | 1.04 |
| S22 | 6.23 | 63.69 | 7.56 | 28.75 | 0.45 | 3.80 | 18.57 | 10.18 | 0.55 b | 0.74 | 1.52 | 0.60 |
| S23 | 8.09 | 34.48 | 33.65 | 31.87 | 0.92 | 0.95 | 18.23 | 13.64 | 0.75 b | 2.72 | 0.43 | 0.36 |
| S24 | 8.84 | 46.01 | 6.42 | 47.57 | 1.03 | 7.41 | 35.08 | 12.49 | 0.36 b | 1.57 | 0.24 | 0.32 |
| S25 | 14.05 | 22.23 | 2.14b | 75.63 | 3.40 | 35.37 a | 51.14 a | 24.49 | 0.48 b | 4.61 | 0.78 | 0.11 |
| S26 | 5.38 | 49.12 | 14.95 | 35.93 | 0.73 | 2.40 | 9.32 | 26.61 | 2.86 | 1.45 | 0.81 | 0.85 |
| S27 | 3.75 | 39.28 | 41.64 | 19.08 | 0.49 | 0.46 | 1.49 | 17.59 | 11.80 | 2.32 | 0.48 | 1.02 |
| S28 | 4.38 | 71.32 | 15.62 | 13.06 b | 0.18 b | 0.84 | 5.31 | 7.74 b | 1.46 | 0.56 | 2.08 | 2.20 |
| S29 | 6.74 | 61.96 | 14.51 | 23.53 | 0.38 | 1.62 | 12.62 | 10.90 | 0.86 b | 0.86 | 1.29 | 1.06 |
| S30 | 32.31 | 20.16 | 21.74 | 58.10 | 2.88 | 2.67 | 1.54 | 56.56 | 36.78 | 5.41 | 0.20 | 0.44 |
| S31 | 43.00 | 8.30 | 59.33 | 32.37 | 3.90 | 0.55 | 16.59 | 15.78 | 0.95 b | 11.29 | 0.07 b | 0.08 b |
| S32 | 7.13 | 60.86 | 4.47 | 34.68 | 0.57 | 7.76 | 5.99 | 28.69 | 4.79 | 1.00 | 1.27 | 1.27 |
| S33 | 5.95 | 46.20 | 11.03 | 42.77 | 0.93 | 3.88 | 28.15 | 14.62 | 0.52 b | 1.65 | 0.71 | 0.42 |
| S34 | 5.26 | 48.94 | 7.49 | 43.57 | 0.89 | 5.81 | 11.10 | 32.47 | 2.93 | 1.38 | 0.83 | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini, R.K.; Assefa, A.D.; Keum, Y.-S. Spices in the Apiaceae Family Represent the Healthiest Fatty Acid Profile: A Systematic Comparison of 34 Widely Used Spices and Herbs. Foods 2021, 10, 854. https://doi.org/10.3390/foods10040854
Saini RK, Assefa AD, Keum Y-S. Spices in the Apiaceae Family Represent the Healthiest Fatty Acid Profile: A Systematic Comparison of 34 Widely Used Spices and Herbs. Foods. 2021; 10(4):854. https://doi.org/10.3390/foods10040854
Chicago/Turabian StyleSaini, Ramesh Kumar, Awraris Derbie Assefa, and Young-Soo Keum. 2021. "Spices in the Apiaceae Family Represent the Healthiest Fatty Acid Profile: A Systematic Comparison of 34 Widely Used Spices and Herbs" Foods 10, no. 4: 854. https://doi.org/10.3390/foods10040854
APA StyleSaini, R. K., Assefa, A. D., & Keum, Y.-S. (2021). Spices in the Apiaceae Family Represent the Healthiest Fatty Acid Profile: A Systematic Comparison of 34 Widely Used Spices and Herbs. Foods, 10(4), 854. https://doi.org/10.3390/foods10040854







