Evolution of Food Safety Features and Volatile Profile in White Sturgeon Caviar Treated with Different Formulations of Salt and Preservatives during a Long-Term Storage Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
- NC series: sodium chloride (NaCl) was added at 3.8% by weight;
- Experimental OAM (organic acids mixture) series: an experimental preservative, made of a mixture of sodium chloride, sorbic acid (E200), potassium sorbate (E202), and isoascorbic acid (E315), was added at 4% by weight;
- BSM (borax and salt mixture) series: salt and sodium tetraborate (E285) mixture was added at 3.8% by weight.
2.2. PH and aw
2.3. Microbial Counts
2.4. Volatile Organic Compounds Profile
- Identification by mass spectra of authentic standards injected in the same analytical conditions, when available. When analyzing the chemical standards, the injection port of the GC system was set in the split mode (split ratio 1:100) and 1 µL was injected. A purge flow of 50 mL/min was set at 2 min to avoid an oversaturation of the MS ion source.
2.5. Data Analysis
3. Results and Discussion
3.1. PH, aw (Water Activity), and Microbial Counts
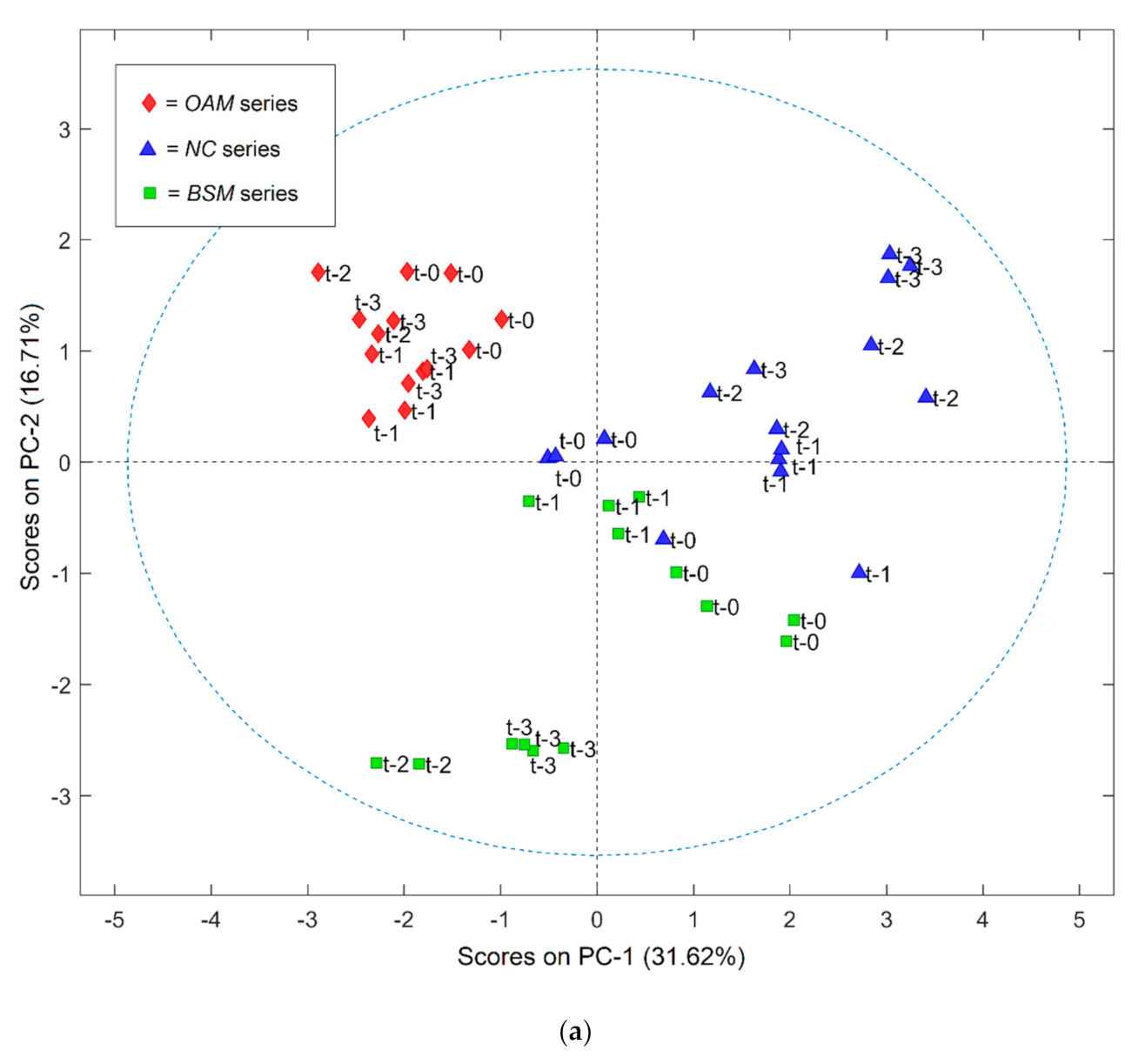
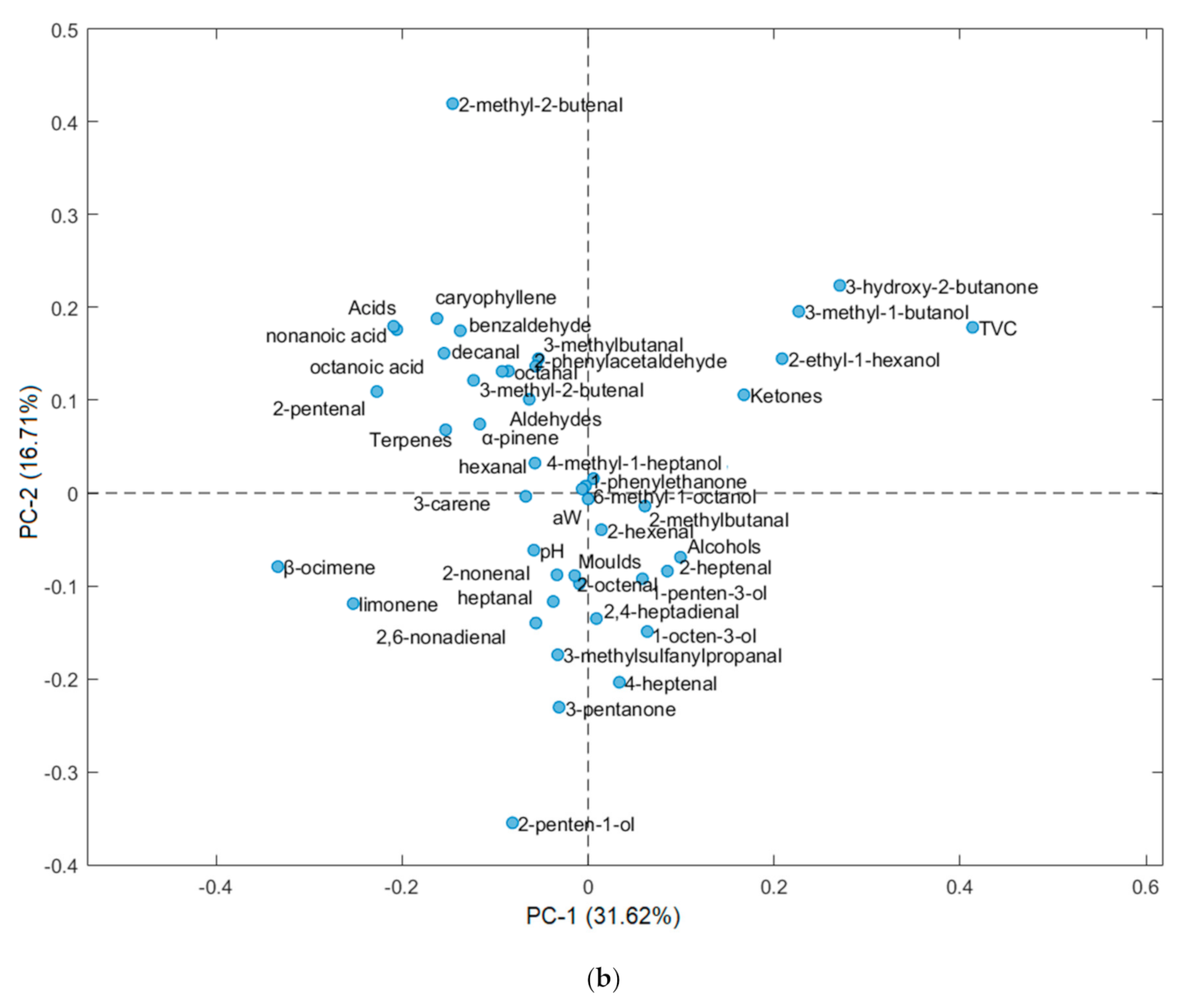
3.2. Volatile Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Uhm, D.; Siegel, D. The Illegal Trade in Black Caviar. Trends Organ. Crime 2016, 19, 67–87. [Google Scholar] [CrossRef]
- CITES Convention on International Trade in Endangered Species of Wild Fauna and Flora. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.645.3850&rep=rep1&type=pdf (accessed on 26 November 2020).
- Bronzi, P.; Chebanov, M.; Michaels, J.T.; Wei, Q.; Rosenthal, H.; Gessner, J. Sturgeon Meat and Caviar Production: Global Update 2017. J. Appl. Ichthyol. 2019, 35, 257–266. [Google Scholar] [CrossRef]
- Huss, H.H. Control of Indigenous Pathogenic Bacteria in Seafood. Food Control 1997, 8, 91–98. [Google Scholar] [CrossRef]
- Codex Standard for Sturgeon Caviar CXS 291-2010. 2013. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B291-2010%252FCXS_291e.pdf (accessed on 26 November 2020).
- Heshmati, M.K.; Hamdami, N.; Shahedi, M.; Hejazi, M.A.; Motalebi, A.A.; Nasirpour, A. Impact of Zataria Multiflora Essential Oil, Nisin, Potassium Sorbate and LDPE Packaging Containing Nano-ZnO On Shelf Life of Caviar. Food Sci. Technol. Res. 2013, 19, 749–758. [Google Scholar] [CrossRef][Green Version]
- Jubenot, J.L. Le Caviar. Ph.D. Thesis, Université Paul Sabatier de Toulouse, Toulouse, France, 1992. [Google Scholar]
- Gussoni, M.; Greco, F.; Vezzoli, A.; Paleari, M.A.; Moretti, V.M.; Beretta, G.; Caprino, F.; Lanza, B.; Zetta, L. Monitoring the Effects of Storage in Caviar from Farmed Acipenser Transmontanus Using Chemical, SEM, and NMR Methods. J. Agric. Food Chem. 2006, 54, 6725–6732. [Google Scholar] [CrossRef]
- Williot, P.; Sabeau, L.; Gessner, J.; Arlati, G.; Bronzi, P.; Gulyas, T.; Berni, P. Sturgeon Farming in Western Europe: Recent Developments and Perspectives. Aquat. Living Resour. 2001, 14, 367–374. [Google Scholar] [CrossRef]
- European Parliament; EU Comission. Regulation 1129/2011. 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32011R1129&from=EN (accessed on 26 November 2020).
- European Food Safety Authority (EFSA). Scientific Opinion on the Re-Evaluation of Boric Acid (E 284) and Sodium Tetraborate (Borax) (E 285) As Food Additives. EFSA J. 2013, 11, 1–52. [Google Scholar] [CrossRef]
- Brul, S.; Coote, P. Preservative Agents in Foods: Mode of Action and Microbial Resistance Mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef]
- Parke, D.V.; Lewis, D.F.V. Safety Aspects of Food Preservatives. Food Addit. Contam. 1992, 9, 561–577. [Google Scholar] [CrossRef]
- Oeleker, K.; Alter, T.; Kleer, J.; Pund, R.P.; Gölz, G.; Hildebrandt, G.; Huehn, S. Microbiological and Chemical Investigation of Caviar at Retail. J. Verbrauch. Lebensm. 2015, 10, 35–37. [Google Scholar] [CrossRef]
- Heude, C.; Elbayed, K.; Jezequel, T.; Fanuel, M.; Lugan, R.; Heintz, D.; Benoit, P.; Piotto, M. Metabolic Characterization of Caviar Specimens By 1h NMR Spectroscopy: Towards Caviar Authenticity and Integrity. Food Anal. Methods 2016, 9, 3428–3438. [Google Scholar] [CrossRef]
- Bonpunt, E. The Off-Flavors Management in The Production of Farmed Sturgeon. In The Siberian Sturgeon (Acipenser Baerii, Brandt, 1869); Williot, P., Nonnotte, G., Chebanov, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 2—Farming, pp. 175–180. ISBN 9783319616766. [Google Scholar]
- International Organization for Standardization (ISO). Microbiology of The Food Chain—Horizontal Method for The Enumeration of Microorganisms—Part 1: Colony Count At 30 Degrees by The Pour Plate Technique—ISO 4833-1:2013 2013. Available online: https://www.iso.org/obp/ui/#iso:std:iso:4833:-1:ed-1:v1:en (accessed on 26 November 2020).
- Lopez, A.; Vasconi, M.; Bellagamba, F.; Mentasti, T.; Pazzaglia, M.; Moretti, V.M. Volatile Organic Compounds Profile in White Sturgeon (Acipenser Transmontanus) Caviar at Different Stages of Ripening by Multiple Headspace Solid Phase Microextraction. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of The Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Kovàts, E. Characterization of Organic Compounds by Gas Chromatography. Part 1. Retention Indices of Aliphatic Halides, Alcohols, Aldehydes and Ketones. Helvetica 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Van Den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; Van Der Werf, M.J. Centering, Scaling, And Transformations: Improving the Biological Information Content of Metabolomics Data. BMC Genomics 2006, 7, 1–15. [Google Scholar] [CrossRef]
- Bledsoe, G.E.; Bledsoe, C.D.; Rasco, B. Caviars and Fish Roe Products. Crit. Rev. Food Sci. Nutr. 2003, 43, 317–356. [Google Scholar] [CrossRef]
- Altug, G.; Bayrak, Y. Microbiological Analysis of Caviar from Russia and Iran. Food Microbiol. 2003, 20, 83–86. [Google Scholar] [CrossRef]
- Handa, S.; Kimura, B.; Takahashi, H.; Koda, T.; Hisa, K.; Fujii, T. Incidence of Listeria Monocytogenes in Raw Seafood Products in Japanese Retail Stores. J. Food Prot. 2005, 68, 411–415. [Google Scholar] [CrossRef]
- Shin, J.H.; Oliveira, A.C.M.; Rasco, B.A. Quality Attributes and Microbial Storage Stability of Caviar from Cultivated White Sturgeon (Acipenser Transmontanus). J. Food Sci. 2010, 75, 3–8. [Google Scholar] [CrossRef]
- Vasconi, M.; Tirloni, E.; Stella, S.; Coppola, C.; Lopez, A.; Bellagamba, F.; Bernardi, C.; Moretti, V.M. Comparison of Chemical Composition and Safety Issues in Fish Roe Products: Application of Chemometrics To Chemical Data. Foods 2020, 9. [Google Scholar] [CrossRef]
- Himelbloom, B.H.; Crapo, C.A. Microbial Evaluation of Alaska Salmon Caviar. J. Food Prot. 1998, 61, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Medina, I. Solid-Phase Microextraction Method for The Determination of Volatile Compounds Associated to Oxidation of Fish Muscle. J. Chromatogr. A 2008, 1192, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Aro, T.; Tahvonen, R.; Koskinen, L.; Kallio, H. Volatile Compounds of Baltic Herring Analysed by Dynamic Headspace Sampling-Gas Chromatography-Mass Spectrometry. Eur. Food Res. Technol. 2003, 216, 483–488. [Google Scholar] [CrossRef]
- Duflos, G.; Coin, V.M.; Cornu, M.; Antinelli, J.F.; Malle, P. Determination of Volatile Compounds To Characterize Fish Spoilage Using Headspace/Mass Spectrometry And Solid-Phase Microextraction/Gas Chromatography/Mass Spectrometry. J. Sci. Food Agric. 2006, 86, 600–611. [Google Scholar] [CrossRef]
- Duflos, G.; Leduc, F.; N’guessan, A.; Krzewinski, F.; Kol, O.; Malle, P. Freshness Characterisation of Whiting (Merlangius Merlangus) Using an SPME/GC/MS Method and a Statistical Multivariate Approach. J. Sci. Food Agric. 2010, 90, 2568–2575. [Google Scholar] [CrossRef]
- Alasalvar, C.; Taylor, K.D.A.; Shahidi, F. Comparison of Volatiles of Cultured and Wild Sea Bream (Sparus Aurata) During Storage in Ice by Dynamic Headspace Analysis/Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2005, 53, 2616–2622. [Google Scholar] [CrossRef]
- Jonsdottir, R.; Olafsdottir, G.; Martinsdottir, E.; Stefansson, G. Flavor Characterization of Ripened Cod Roe by Gas Chromatography, Sensory Analysis, and Electronic Nose. J. Agric. Food Chem. 2004, 52, 6250–6256. [Google Scholar] [CrossRef]
- Caprino, F.; Moretti, V.M.; Bellagamba, F.; Turchini, G.M.; Busetto, M.L.; Giani, I.; Paleari, M.A.; Pazzaglia, M. Fatty Acid Composition and Volatile Compounds of Caviar from Farmed White Sturgeon (Acipenser Transmontanus). Analytica Chimica Acta 2008, 617, 139–147. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9783540699330. [Google Scholar]
- Ardö, Y. Flavour Formation by Amino Acid Catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef]
- Pripi-Nicolau, L.; De Revel, G.; Bertrand, A.; Maujean, A. Formation of Flavor Components by The Reaction of Amino Acid and Carbonyl Compounds in Mild Conditions. J. Agric. Food Chem. 2000, 48, 3761–3766. [Google Scholar] [CrossRef]
- Weenen, H.; Van Der Ven, J.G.M. The Formation of Strecker Aldehydes. ACS Symp. Ser. 2001, 794, 183–195. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Volatile Organic Compounds of Microbial and Non-Microbial Origin Produced on Model Fish Substrate Un-Inoculated and Inoculated with Gilt-Head Sea Bream Spoilage Bacteria. LWT Food Sci. Technol. 2017, 78, 54–62. [Google Scholar] [CrossRef]
- Josephson, D.B.; Lindsay, R.C. Enzymic Generation of Volatile Aroma Compounds from Fresh Fish. In Biogeneration of Aromas; Parliment, T.H., Croteau, R., Eds.; ACS: Washington, DC, USA, 1986; pp. 201–219. [Google Scholar]
- Lopez, A.; Vasconi, M.; Bellagamba, F.; Mentasti, T.; Moretti, V.M. Sturgeon Meat and Caviar Quality from Different Cultured Species. Fishes 2020, 5. [Google Scholar] [CrossRef]
- Ólafsdóttir, G. Volatile Compounds as Quality Indicators in Chilled Fish: Evaluation of Microbial Metabolites by An Electronic Nose. Ph.D. Thesis, University of Iceland, Reykjavík, Island, 2005. [Google Scholar]
- Jónsdóttir, R.; Ólafsdóttir, G.; Chanie, E.; Haugen, J.E. Volatile Compounds Suitable for Rapid Detection as Quality Indicators of Cold Smoked Salmon (Salmo Salar). Food Chem. 2008, 109, 184–195. [Google Scholar] [CrossRef]
- Olafsdottir, G.; Jonsdottir, R.; Lauzon, H.L.; Luten, J.; Kristbergsson, K. Characterization of Volatile Compounds in Chilled Cod (Gadus Morhua) Fillets by Gas Chromatography and Detection of Quality Indicators by An Electronic Nose. J. Agric. Food Chem. 2005, 53, 10140–10147. [Google Scholar] [CrossRef]
- Jørgensen, L.V.; Huss, H.H.; Dalgaard, P. Significance of Volatile Compounds Produced By Spoilage Bacteria In Vacuum-Packed Cold-Smoked Salmon (Salmo Salar) Analyzed By GC-MS And Multivariate Regression. J. Agric. Food Chem. 2001, 49, 2376–2381. [Google Scholar] [CrossRef]
- Miller, A.; Scanlan, R.A.; Lee, J.S.; Libbey, L.M.; Morgan, M.E. Volatile Compounds Produced in Sterile Fish Muscle (Sebastes Melanops) By Pseudomonas Perolens. Appl. Microbiol. 1973, 25, 257–261. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish Spoilage Mechanisms and Preservation Techniques: Review Department Of Process Engineering And Applied Science, Dalhousie University Halifax, Nova Scotia, Canada. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Milo, C.; Grosch, W. Changes in The Odorants of Boiled Trout (Salmo Fario) As Affected by The Storage of The Raw Material. J. Agric. Food Chem. 1993, 41, 2076–2081. [Google Scholar] [CrossRef]
- Mcgill, A.S.; Hardy, R.; Burt, J.R.; Gunstone, F.D. Hept-Cis-4-Enal And Its Contribution to The Off-Flavour in Cold Stored Cod. J. Sci. Food Agric. 1974, 25, 1477–1489. [Google Scholar] [CrossRef]
- Hardy, R.; Mcgill, A.S.; Gunstone, F.D. Lipid and Autoxidative Changes in Cold Stored Cod (Gadus Morhua). J. Sci. Food Agric. 1979, 30, 999–1006. [Google Scholar] [CrossRef]
- Nordvi, B.; Langsrud, O.; Egelandsdal, B.; Slinde, E.; Vogt, G.; Gutierrez, M.; Olsen, E. Characterization of Volatile Compounds in A Fermented and Dried Fish Product During Cold Storage. J. Food Sci. 2007, 72. [Google Scholar] [CrossRef] [PubMed]
- Josephson, D.B.; Lindsay, R.C.; Stuiber, D.A. Variations in The Occurrences of Enzymically Derived Volatile Aroma Compounds in Salt- And Freshwater Fish. J. Agric. Food Chem. 1984, 32, 1344–1347. [Google Scholar] [CrossRef]
- Hsieh, R.J.; Kinsella, J.E. Lipoxygenase Generation of Specific Volatile Flavor Carbonyl Compounds in Fish Tissues. J. Agric. Food Chem. 1989, 37, 279–286. [Google Scholar] [CrossRef]
- Joffraud, J.J.; Leroi, F.; Roy, C.; Berdagué, J.L. Characterisation of Volatile Compounds Produced by Bacteria Isolated from The Spoilage Flora of Cold-Smoked Salmon. Int. J. Food Microbiol. 2001, 66, 175–184. [Google Scholar] [CrossRef]
- Iglesias, J.; Medina, I.; Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Study of The Volatile Compounds Useful for The Characterisation of Fresh and Frozen-Thawed Cultured Gilthead Sea Bream Fish by Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry. Food Chem. 2009, 115, 1473–1478. [Google Scholar] [CrossRef]
| March 2019 Raw Eggs | July 2019 4 Months | November 2019 8 Months | May 2020 14 Months | |||
|---|---|---|---|---|---|---|
| ID | Series | Can | t0 | t1 | t2 | t3 |
| 1 | NC 3.8% | 500 g | n = 2 | n = 2 | n = 2 | n = 2 |
| 2 | ||||||
| 3 | 1800 g | n = 2 | n = 2 | n = 2 | n = 2 | |
| 4 | ||||||
| 1 | OAM 4% | 500 g | n = 2 | n = 2 | n = 1 | n = 2 |
| 2 | ||||||
| 3 | 1800 g | n = 2 | n = 2 | n = 1 | n = 2 | |
| 4 | ||||||
| 1 | BSM 3.8% | 500 g | n = 2 | n = 2 | n = 1 | n = 2 |
| 2 | ||||||
| 3 | 1800 g | n = 2 | n = 2 | n = 1 | n = 2 | |
| 4 | ||||||
| N= | 12 | 12 | 8 | 12 | ||
| pH | aw | Total Viable Count (Log CFU/g) and Countable | |
|---|---|---|---|
| t0 = unripened roes | |||
| NC | 5.86 ± 0.02 | 0.30 ± 0.00 | 1.24 ± 0.17 b (2/4) |
| OAM | 6.12 ± 0.09 | 0.30 ± 0.00 | 1.08 ± 0.08 b (4/4) |
| BSM | 6.15 ± 0.01 | 0.30 ± 0.00 | 1.48 ± 0.20 b (4/4) |
| t1 = 4 months of ripening | |||
| NC | 5.97 ± 0.01 | 0.29 ± 0.00 | 5.89 ± 1.29 a (4/4) |
| OAM | 6.28 ± 0.07 | 0.29 ± 0.00 | 1.20 ± 0.17 b (4/4) |
| BSM | 6.42 ± 0.02 | 0.29 ± 0.00 | 1.50 ± 0.17 b (3/4) |
| t2 = 8 months of ripening | |||
| NC | 5.90 ± 0.02 | 0.29 ± 0.00 | 5.90 ± 1.20 a (4/4) |
| OAM | 6.26 ± 0.01 | 0.29 ± 0.00 | nd |
| BSM | 6.31 ± 0.04 | 0.29 ± 0.00 | nd |
| t3 = 14 months of ripening | |||
| NC | 5.84 ± 0.02 | 0.29 ± 0.00 | 6.89 ± 0.09 a (3/4) |
| OAM | 6.12 ± 0.08 | 0.29 ± 0.0 | nd |
| BSM | 6.30 ± 0.03 | 0.29 ± 0.00 | 1.30 (1/4) |
| t0 = Unripened Roes | t1 = 4 Months of Ripening | t2 = 8 Months of Ripening | t3 = 14 Months of Ripening | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC N = 4 | OAM N = 4 | BSM N = 4 | NC N = 4 | OAM N = 4 | BSM N = 4 | NC N = 4 | OAM N = 2 | BSM N = 2 | NC N = 4 | OAM N = 4 | BSM N = 4 | |
| ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | |
| 3-methyl butanal | 5.21 ± 0.03 d | 5.38 ± 0.04 cd | 3.91 ± 0.28 e | 5.51 ± 0.32 bcd | 5.73 ± 0.10 cd | 5.48 ± 0.08 bcd | 6.36 ± 0.07 a | 6.26 ± 0.04 a | 6.03 ± 0.02 ab | 6.36 ± 0.05 bcd | 6.32 ± 0.06 a | 5.96 ± 0.08 abc |
| 2-methyl butanal | nd | nd | nd | 4.98 ± 0.31 b | 5.22 ± 0.09 b | 4.99 ± 0.10 b | 5.92 ± 0.06 a | nd | 5.51 ± 0.00 ab | 5.95 ± 0.07 a | 5.91 ± 0.06 a | 5.54 ± 0.09 ab |
| 2-methyl, 2-butenal | nd | 5.46 ± 0.09 b | nd | nd | 4.89 ± 0.07 c | nd | nd | 5.79 ± 0.02 a | nd | 5.84 ± 0.16 a | 5.53 ± 0.08 ab | nd |
| 2-pentenal | nd | 5.36 ± 0.05 ab | nd | nd | 4.82 ± 0.04 c | nd | nd | 5.49 ± 0.04 a | 5.22 ± 0.03 ab | 5.18 ± 0.08 bc | 5.40 ± 0.14 ab | 5.24 ± 0.09 ab |
| 3-methyl, 2-butenal | nd | nd | nd | nd | nd | nd | nd | 2.50 ± 1.77 ab | nd | nd | 4.87 ± 0.07 a | nd |
| Hexanal | 5.58 ± 0.26 def | 5.78 ± 0.05 cdef | 4.59 ± 0.29 g | 5.43 ± 0.37 ef | 5.37 ± 0.07 f | 5.91 ± 0.10 cdef | 6.14 ± 0.19 bcd | 5.98 ± 0.06 bcde | 6.76 ± 0.00 a | 6.60 ± 0.10 ab | 6.35 ± 0.17 abc | 6.68 ± 0.08 ab |
| 2-hexenal | nd | nd | nd | nd | nd | nd | 4.99 ± 0.07 bc | 4.55 ± 0.04 c | 5.44 ± 0.08 a | 5.30 ± 0.08 ab | 4.92 ± 0.16 bc | 5.28 ± 0.11 ab |
| 4-heptenal | nd | nd | nd | nd | nd | nd | nd | nd | 5.23 ± 0.02 a | 4.99 ± 0.07 b | nd | 5.20 ± 0.11 ab |
| Heptanal | nd | nd | nd | nd | nd | nd | nd | nd | 5.44 ± 0.02 a | 5.36 ± 0.08 ab | 5.16 ± 0.14 b | 5.39 ± 0.08 ab |
| 3-methyltio, propanal | nd | nd | nd | 4.88 ± 0.13 de | 5.01 ± 0.15 cd | 4.43 ± 0.10 e | 5.71 ± 0.13 ab | 5.87 ± 0.03 a | 5.37 ± 0.04 bc | nd | nd | 5.43 ± 0.08 bc |
| 2-heptenal | nd | nd | nd | nd | nd | nd | nd | nd | nd | 5.09 ± 0.08 a | nd | 5.13 ± 0.09 a |
| Benzaldehyde | 4.96 ± 0.23 cde | 5.53 ± 0.05 abc | nd | 4.39 ± 0.34 e | 5.07 ± 0.06 bcde | 4.67 ± 0.05 de | 5.55 ± 0.06 abc | 5.84 ± 0.04 a | 5.58 ± 0.02 abc | 5.34 ± 0.04 abcd | 5.74 ± 0.06 ab | 5.44 ± 0.08 abc |
| Octanal | 5.04 ± 0.28 ab | 5.18 ± 0.17 a | 3.48 ± 0.30 d | 4.16 ± 0.35 cd | 4.58 ± 0.05 abc | 4.36 ± 0.11 bc | 4.99 ± 0.05 ab | 4.97 ± 0.04 ab | 5.20 ± 0.02 a | 5.36 ± 0.06 a | 5.27 ± 0.08 a | 5.13 ± 0.07 ab |
| 2,4-heptadienal | nd | nd | nd | nd | nd | nd | 2.47 ± 1.43 b | nd | 5.60 ± 0.03 a | 5.30 ± 0.09 a | 3.72 ± 1.25 ab | 5.39 ± 0.14 a |
| Benzeneacetaldehyde | 4.88 ± 0.30 c | 5.36 ± 0.14 bc | 3.95 ± 0.16 d | 5.45 ± 0.34 bc | 5.68 ± 0.16 abc | 5.46 ± 0.06 bc | 6.47 ± 0.10 a | 6.33 ± 0.02 a | 6.14 ± 0.02 ab | 6.24 ± 0.09 ab | 6.23 ± 0.07 ab | 5.96 ± 0.07 ab |
| 2-octenal | nd | nd | nd | nd | nd | nd | 2.54 ± 1.47 b | nd | 5.43 ± 0.04 a | 5.28 ± 0.12 a | 5.10 ± 0.19 a | 5.29 ± 0.13 a |
| Nonanal | 5.83 ± 0.27 ab | * | 4.53 ± 0.29 d | 5.19 ± 0.30 c | * | 5.37 ± 0.05 bc | 5.72 ± 0.05 abc | * | 6.00 ± 0.06 a | 6.10 ± 0.05 a | * | 6.05 ± 0.05 a |
| 2,6-nonadienal | nd | nd | nd | nd | nd | nd | nd | nd | 5.25 ± 0.05 a | nd | nd | nd |
| 2-Nonenal | nd | nd | nd | nd | nd | nd | nd | nd | 4.87 ± 0.04 a | 5.00 ± 0.05 a | 4.93 ± 0.11 a | 4.90 ± 0.06 a |
| Decanal | 4.58 ± 0.24 | 4.83 ± 0.10 | 4.22 ± 0.17 | 4.15 ± 0.32 | 4.65 ± 0.03 | 4.53 ± 0.00 | 4.54 ± 0.08 | 4.91 ± 0.03 | 4.73 ± 0.04 | 5.07 ± 0.09 | 5.10 ± 0.08 | 4.96 ± 0.07 |
| Sum of aldehydes | 6.22 ± 0.22 c | 6.35 ± 0.05 bc | 5.03 ± 0.26 d | 6.16 ± 0.31 c | 6.28 ± 0.09 c | 6.28 ± 0.05 c | 6.99 ± 0.06 ab | 6.88 ± 0.00 ab | 7.09 ± 0.00 a | 7.12 ± 0.05 a | 6.98 ± 0.06 ab | 7.02 ± 0.07 a |
| t0 = Unripened Roes | t1 = 4 Months of Ripening | t2 = 8 Months of Ripening | t3 = 14 Months of Ripening | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC N = 4 | OAM N = 4 | BSM N = 4 | NC N = 4 | OAM N = 4 | BSM N = 4 | NC N = 4 | OAM N = 2 | BSM N = 2 | NC N = 4 | OAM N = 4 | BSM N = 4 | |
| ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | ± SEM | |
| 1-penten-3-ol | nd | nd | nd | 4.75 ± 0.35 d | nd | 5.25 ± 0.03 cd | 5.53 ± 0.12 bc | 5.08 ± 0.02 cd | 6.10 ± 0.03 a | 5.65 ± 0.09 bc | 5.58 ± 0.16 bc | 6.06 ± 0.05 ab |
| 3-methyl,1-butanol | nd | nd | nd | nd | nd | nd | 2.98 ± 1.72 b | nd | nd | 6.26 ± 0.14 a | nd | nd |
| 2-penten-1-ol | nd | nd | nd | nd | nd | nd | nd | nd | 5.57 ± 0.02 a | nd | nd | 5.49 ± 0.05 b |
| 1-octen-3-ol | nd | nd | nd | 4.52 ± 0.38 ab | nd | 4.81 ± 0.15 ab | 5.32 ± 0.02 bc | nd | 5.98 ± 0.04 a | 5.97 ± 0.11 a | 5.62 ± 0.18 a | 6.08 ± 0.10 a |
| 2-ethyl, 1-hexanol | nd | nd | nd | 5.70 ± 0.32 ab | nd | 5.87 ± 0.12 a | 4.85 ± 0.06 c | 5.31 ± 0.02 ab | nd | 5.18 ± 0.03 bc | nd | nd |
| 4-methyl, 1-heptanol | nd | nd | nd | nd | nd | nd | nd | nd | nd | 5.83 ± 0.26 a | 5.73 ± 0.06 a | 5.54 ± 0.10 a |
| 6-methyl, 1-octanol | nd | nd | nd | nd | nd | nd | nd | nd | nd | 5.41 ± 0.11 | 5.54 ± 0.04 | 5.35 ± 0.08 |
| Sum of alcohols | nd | nd | nd | 5.78 ± 0.33 cd | nd | 6.01 ± 0.09 bcd | 5.98 ± 0.09 bcd | 5.51 ± 0.01 d | 6.41 ± 0.03 ab | 6.65 ± 0.13 a | 6.25 ± 0.10 abc | 6.52 ± 0.05 ab |
| 3-pentanone | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 5.06 ± 0.11 |
| 3-hydroxy, 2-butanone | nd | nd | nd | nd | nd | nd | 6.55 ± 0.31 | nd | nd | 6.83 ± 0.19 | nd | nd |
| Acetophenone | nd | nd | nd | nd | nd | nd | nd | nd | nd | 4.97 ± 0.06 b | 5.15 ± 0.04 a | 4.99 ± 0.04 b |
| Sum of ketones | nd | nd | nd | nd | nd | nd | 6.55 ± 0.31 a | nd | nd | 6.84 ± 0.19 a | 5.15 ± 0.04 b | 5.35 ± 0.05 b |
| α-pinene | 5.10 ± 0.29 abc | 5.65 ± 0.10 a | 4.71 ± 0.32 bc | 4.63 ± 0.31 c | 5.24 ± 0.09 abc | 5.29 ± 0.07 abc | 5.38 ± 0.12 abc | 5.57 ± 0.08 | 5.66 ± 0.04 a | 5.35 ± 0.09 abc | 5.59 ± 0.09 ab | 5.58 ± 0.07 ab |
| 3-carene | nd | nd | nd | 3.72 ± 0.32 | 4.43 ± 0.06 | 4.27 ± 0.01 | 4.64 ± 0.01 | 4.73 ± 0.01 | 4.76 ± 0.03 | 5.13 ± 0.05 | 5.13 ± 0.04 | 5.06 ± 0.05 |
| Limonene | 4.82 ± 0.04 b | 5.33 ± 0.07 a | 4.23 ± 0.25 c | nd | 4.93 ± 0.20 abc | nd | nd | nd | 5.20 ± 0.04 a | nd | 5.28 ± 0.06 a | 5.21 ± 0.05 ab |
| Β-ocimene | 4.68 ± 0.06 c | 5.51 ± 0.09 a | 4.11 ± 0.34 d | nd | 5.30 ± 0.12 ab | 4.88 ± 0.10 bc | nd | 5.42 ± 0.12 a | 5.29 ± 0.04 ab | nd | 5.41 ± 0.07 a | 5.32 ± 0.07 ab |
| Caryophyllene | nd | 5.05 ± 0.05 | nd | nd | 4.47 ± 0.09 | 3.79 ± 0.00 | nd | 4.23 ± 0.45 | nd | nd | 4.81 ± 0.08 | nd |
| Sum of terpenes | 5.46 ± 0.12 ab | 6.03 ± 0.08 a | 4.92 ± 0.30 bc | 4.68 ± 0.31 c | 5.75 ± 0.06 a | 5.47 ± 0.07 ab | 5.41 ± 0.13 abc | 5.86 ± 0.10 a | 5.94 ± 0.04 a | 5.57 ± 0.06 ab | 6.01 ± 0.06 a | 5.94 ± 0.06 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, A.; Bellagamba, F.; Tirloni, E.; Vasconi, M.; Stella, S.; Bernardi, C.; Pazzaglia, M.; Moretti, V.M. Evolution of Food Safety Features and Volatile Profile in White Sturgeon Caviar Treated with Different Formulations of Salt and Preservatives during a Long-Term Storage Time. Foods 2021, 10, 850. https://doi.org/10.3390/foods10040850
Lopez A, Bellagamba F, Tirloni E, Vasconi M, Stella S, Bernardi C, Pazzaglia M, Moretti VM. Evolution of Food Safety Features and Volatile Profile in White Sturgeon Caviar Treated with Different Formulations of Salt and Preservatives during a Long-Term Storage Time. Foods. 2021; 10(4):850. https://doi.org/10.3390/foods10040850
Chicago/Turabian StyleLopez, Annalaura, Federica Bellagamba, Erica Tirloni, Mauro Vasconi, Simone Stella, Cristian Bernardi, Mario Pazzaglia, and Vittorio Maria Moretti. 2021. "Evolution of Food Safety Features and Volatile Profile in White Sturgeon Caviar Treated with Different Formulations of Salt and Preservatives during a Long-Term Storage Time" Foods 10, no. 4: 850. https://doi.org/10.3390/foods10040850
APA StyleLopez, A., Bellagamba, F., Tirloni, E., Vasconi, M., Stella, S., Bernardi, C., Pazzaglia, M., & Moretti, V. M. (2021). Evolution of Food Safety Features and Volatile Profile in White Sturgeon Caviar Treated with Different Formulations of Salt and Preservatives during a Long-Term Storage Time. Foods, 10(4), 850. https://doi.org/10.3390/foods10040850









