An Approach to Investigate Content-Related Quality of Nutraceuticals Used by Slovenian Consumers: A Case Study with Folate and Vitamin D Supplements
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.1.1. Food Supplements for Supplementation with Folate in Pregnancy
2.1.2. Food Supplements for Supplementation with Vitamin D in the General Population
2.2. Quantification of the Active Ingredient
2.3. Data Analyses and Conformity with Regulatory Requirements
3. Results and Discussion
3.1. Food Supplements for Supplementation with Folate in Pregnancy
3.2. Food Supplements for Supplementation with Vitamin D in the General Population
3.3. Study Limitations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Skeie, G.; Braaten, T.; Hjartåker, A.; Lentjes, M.; Amiano, P.; Jakszyn, P.; Pala, V.; Palanca, A.; Niekerk, E.M.; Verhagen, H.; et al. Use of Dietary Supplements in the European Prospective Investigation into Cancer and Nutrition Calibration Study. Eur. J. Clin. Nutr. 2009, 63, S226–S238. [Google Scholar] [CrossRef] [PubMed]
- Kantor, E.D.; Rehm, C.D.; Du, M.; White, E.; Giovannucci, E.L. Trends in Dietary Supplement Use among US Adults from 1999–2012. JAMA 2016, 316, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- EC. Directive 2002/46/EC on the Approximation of the Laws of the Member States Relating to Food Supplements. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32002L0046 (accessed on 15 February 2021).
- Pravst, I. Dietary supplement labelling and health claims. In Dietary Supplements; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–24. [Google Scholar]
- Coppens, P.; da Silva, M.F.; Pettman, S. European regulations on nutraceuticals, dietary supplements and functional foods: A framework based on safety. Toxicology 2006, 221, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Bieniek, M.; Manning, L. Food supplements’ non-conformity in Europe—Poland: A case study. Trends Food Sci. Technol. 2019, 93, 262–270. [Google Scholar] [CrossRef]
- Wheatley, V.M.; Spink, J. Defining the Public Health Threat of Dietary Supplement Fraud. Compr. Rev. Food Sci. Food Saf. 2013, 12, 599–613. [Google Scholar] [CrossRef]
- Costa, J.G.; Vidovic, B.; Saraiva, N.; do Céu Costa, M.; Del Favero, G.; Marko, D.; Oliveira, N.G.; Fernandes, A.S. Contaminants: A dark side of food supplements? Free Radic. Res. 2019, 53, 1113–1135. [Google Scholar] [CrossRef]
- Rocha, T.; Amaral, J.S.; Oliveira, M.B.P.P. Adulteration of Dietary Supplements by the Illegal Addition of Synthetic Drugs: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 43–62. [Google Scholar] [CrossRef]
- Czepielewska, E.; Makarewicz-Wujec, M.; Różewski, F.; Wojtasik, E.; Kozłowska-Wojciechowska, M. Drug adulteration of food supplements: A threat to public health in the European Union? Regul. Toxicol. Pharmacol. 2018, 97, 98–102. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, H.N.; Park, O.R.; Kim, N.S.; Park, S.K.; Kang, H. Screening of illegal sexual enhancement supplements and counterfeit drugs sold in the online and offline markets between 2014 and 2017. Forensic Sci. Int. 2019, 298, 10–19. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Niedzielski, P.; Kozak, L.; Rzymski, P.; Wachelka, M.; Rzymska, I.; Karczewski, J.; Rzymski, P. Monitoring of essential and toxic elements in multi-ingredient food supplements produced in European Union. J. Consum. Prot. Food Saf. 2018, 13, 41–48. [Google Scholar] [CrossRef]
- Pravst, I.; Zmitek, K. The coenzyme Q10 content of food supplements. J. Verbrauch. Lebensm. J. Consum. Prot. Food Saf. 2011, 6, 457–463. [Google Scholar] [CrossRef]
- Prado-Cabrero, A.; Beatty, S.; Howard, A.; Stack, J.; Bettin, P.; Nolan, J.M. Assessment of lutein, zeaxanthin and meso-zeaxanthin concentrations in dietary supplements by chiral high-performance liquid chromatography. Eur. Food Res. Technol. 2016, 242, 599–608. [Google Scholar] [CrossRef]
- Phelan, D.; Prado-Cabrero, A.; Nolan, J.M. Stability of Commercially Available Macular Carotenoid Supplements in Oil and Powder Formulations. Nutrients 2017, 9, 1133. [Google Scholar] [CrossRef]
- Gaspar, D.P.; Lechtenberg, M.; Hensel, A. Quality Assessment of Bilberry Fruits (Vaccinium myrtillus) and Bilberry-Containing Dietary Supplements. J. Agric. Food Chem. 2021, 69, 2213–2225. [Google Scholar] [CrossRef]
- Mannino, G.; Di Stefano, V.; Lauria, A.; Pitonzo, R.; Gentile, C. Vaccinium macrocarpon (Cranberry)-Based Dietary Supplements: Variation in Mass Uniformity, Proanthocyanidin Dosage and Anthocyanin Profile Demonstrates Quality Control Standard Needed. Nutrients 2020, 12, 992. [Google Scholar] [CrossRef]
- Verkaik-Kloosterman, J.; Seves, S.M.; Ocké, M.C. Vitamin D concentrations in fortified foods and dietary supplements intended for infants: Implications for vitamin D intake. Food Chem. 2017, 221, 629–635. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Roškar, R. Vitamin D in supplements and medicines. In Vitamin D deficiency; Nighat, Y., Mandal, A., Amiri, W., Eds.; Open Access eBooks: Las Vegas, NV, USA, 2018; pp. 1–19. [Google Scholar]
- LeBlanc, E.S.; Perrin, N.; Johnson, J.D.; Ballatore, A.; Hillier, T. Over-the-Counter and Compounded Vitamin D: Is Potency What We Expect? JAMA Intern. Med. 2013, 173, 585–586. [Google Scholar] [CrossRef]
- Andrews, K.W.; Pehrsson, P.R.; Betz, J.M. Variability in vitamin D content among products for multivitamin and mineral supplements. JAMA Intern. Med. 2013, 173, 1752–1753. [Google Scholar] [CrossRef]
- Garg, S.; Sabri, D.; Kanji, J.; Rakkar, P.S.; Lee, Y.; Naidoo, N.; Svirskis, D. Evaluation of vitamin D medicines and dietary supplements and the physicochemical analysis of selected formulations. J. Nutr. Health Aging 2013, 17, 158–161. [Google Scholar] [CrossRef]
- Rajakumari, R.; Oluwafemi, O.S.; Thomas, S.; Kalarikkal, N. Dietary supplements containing vitamins and minerals: Formulation, optimization and evaluation. Powder Technol. 2018, 336, 481–492. [Google Scholar] [CrossRef]
- Kłaczkow, G.; Anuszewska, E.L. Simultaneous determination of vitamins A, D3 and E in multiple pharmaceutical preparations by HPLC method. Acta Pol. Pharm. 2000, 57, 167–170. [Google Scholar] [PubMed]
- Rakusa, A.T.; Grobin, A.; Roskar, R. A comprehensive approach for the simultaneous analysis of all main water-soluble vitamins in multivitamin preparations by a stability-indicating HPLC-DAD method. Food Chem. 2021, 337, 11. [Google Scholar]
- Byrdwell, W.C.; DeVries, J.; Exler, J.; Harnly, J.M.; Holden, J.M.; Holick, M.F.; Hollis, B.W.; Horst, R.L.; Lada, M.; Lemar, L.E.; et al. Analyzing vitamin D in foods and supplements: Methodologic challenges. Am. J. Clin. Nutr. 2008, 88, 554S–557S. [Google Scholar] [CrossRef] [PubMed]
- Jehangir, M.; Ahmed, M.; Imtiaz Shafiq, M.; Samad, A.; Iftikhar Ul, H. UHPLC-PDA Assay for Simultaneous Determination of Vitamin D(3) and Menaquinone-7 in Pharmaceutical Solid Dosage Formulation. J. Anal. Methods Chem. 2017, 2017, 1208753. [Google Scholar] [CrossRef] [PubMed]
- Temova, Ž.; Roškar, R. Stability-Indicating HPLC-UV Method for Vitamin D3 Determination in Solutions, Nutritional Supplements and Pharmaceuticals. J. Chromatogr. Sci. 2016, 54, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L. Current regulatory guidelines and resources to support research of dietary supplements in the United States. Crit. Rev. Food Sci. Nutr. 2020, 60, 298–309. [Google Scholar] [CrossRef]
- EC. Guidance Document for Competent Authorities, Tolerances for the Control of Compliance of Nutrient Values Declared on a Label with EU Legislation. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/labelling_nutrition-vitamins_minerals-guidance_tolerances_1212_en.pdf (accessed on 15 February 2021).
- Argyridis, S. Folic acid in pregnancy. Obstet. Gynaecol. Reprod. Med. 2019, 29, 118–120. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the substantiation of a health claim related to increasing maternal folate status by supplemental folate intake and reduced risk of neural tube defects pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2013, 11, 3328. [Google Scholar]
- Ferrazzi, E.; Tiso, G.; Di Martino, D. Folic acid versus 5- methyl tetrahydrofolate supplementation in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 312–319. [Google Scholar] [CrossRef]
- Žmitek, K.; Hribar, M.; Hristov, H.; Pravst, I. Efficiency of vitamin D supplementation in healthy adults is associated with body mass index and baseline serum 25-hydroxyvitamin d level. Nutrients 2020, 12, 1268. [Google Scholar] [CrossRef]
- Hribar, M.; Hristov, H.; Gregorič, M.; Blaznik, U.; Zaletel, K.; Oblak, A.; Osredkar, J.; Kušar, A.; Žmitek, K.; Rogelj, I.; et al. Nutrihealth Study: Seasonal Variation in Vitamin D Status Among the Slovenian Adult and Elderly Population. Nutrients 2020, 12, 1838. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Skrabakova, Z.; Gonzalez-Gross, M.; Valtuena, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Molgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D and Health: Evolution, Biologic Functions, and Recommended Dietary Intakes for Vitamin D. Clin. Rev. Bone. Miner. Metab. 2009, 7, 2–19. [Google Scholar] [CrossRef]
- GNS. New reference values for vitamin D. Ann. Nutr. Metab. 2012, 60, 241–246. [Google Scholar] [CrossRef]
- EFSA. Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Available online: www.lekarnar.com (accessed on 20 September 2020).
- Accredited Method for Quantification of 5-methyl-tetrahydrofolate: SOP M3816 (LC-MS/MS); SGS Institut Fresenius GmbH: Berlin/Heidelberg, Germany, 2020.
- Accredited Method for Quantification of Cholecalciferol: MP 1570 rev 2/2017 (LC-MS/MS); Chelab—Mérieux NutriSciences Corporation: Resana, Italy, 2017.
- SCF. Tolerable Upper Intake Levels for Vitamins and Minerals. Available online: https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf (accessed on 17 February 2021).
- VMK. Risk Assessment of Folic Acid in Food Supplements: Opinion of the Panel on Nutrition, Dietetic Products, Novel Food and Allergy of the Norwegian Scientific Committee for Food Safety. Available online: https://vkm.no/download/18.2994e95b15cc545071650ec1/1498655906655/86f93d4c96.pdf (accessed on 17 February 2021).
- Ebbing, M.; Bønaa, K.H.; Nygård, O.; Arnesen, E.; Ueland, P.M.; Nordrehaug, J.E.; Rasmussen, K.; Njølstad, I.; Refsum, H.; Nilsen, D.W.; et al. Cancer Incidence and Mortality after Treatment with Folic Acid and Vitamin B12. JAMA 2009, 302, 2119–2126. [Google Scholar] [CrossRef]
- ZIRS. Umik Prehranskega Dopolnila Zaradi Odsotnosti Folne Kisline. Available online: https://tinyurl.com/ysntdc74 (accessed on 8 March 2021).
- FSAI. Recall of a Folic Acid Food Supplement Due to Diminishing Levels of Folic Acid within the Shelf Life. Available online: https://tinyurl.com/rpss4x8h (accessed on 8 March 2021).
- Svarc, P.L.; Garcia-Moreno, P.J.; Mendes, A.C.; Fallahasghari, E.Z.; Jakobsen, J. Encapsulation of L-5-methyltetrahydrofolate by electrospraying for food applications. J. Food Eng. 2020, 277, 109901. [Google Scholar] [CrossRef]
- Domínguez, L.; Fernández-Ruiz, V.; Morales, P.; Sánchez-Mata, M.-C.; Cámara, M. Assessment of Health Claims Related to Folic Acid in Food Supplements for Pregnant Women According to the European Regulation. Nutrients 2021, 13, 937. [Google Scholar] [CrossRef]
- Adams, J.S.; Lee, G. Gains in bone mineral density with resolution of vitamin D intoxication. Ann. Intern. Med. 1997, 127, 203–206. [Google Scholar] [CrossRef]
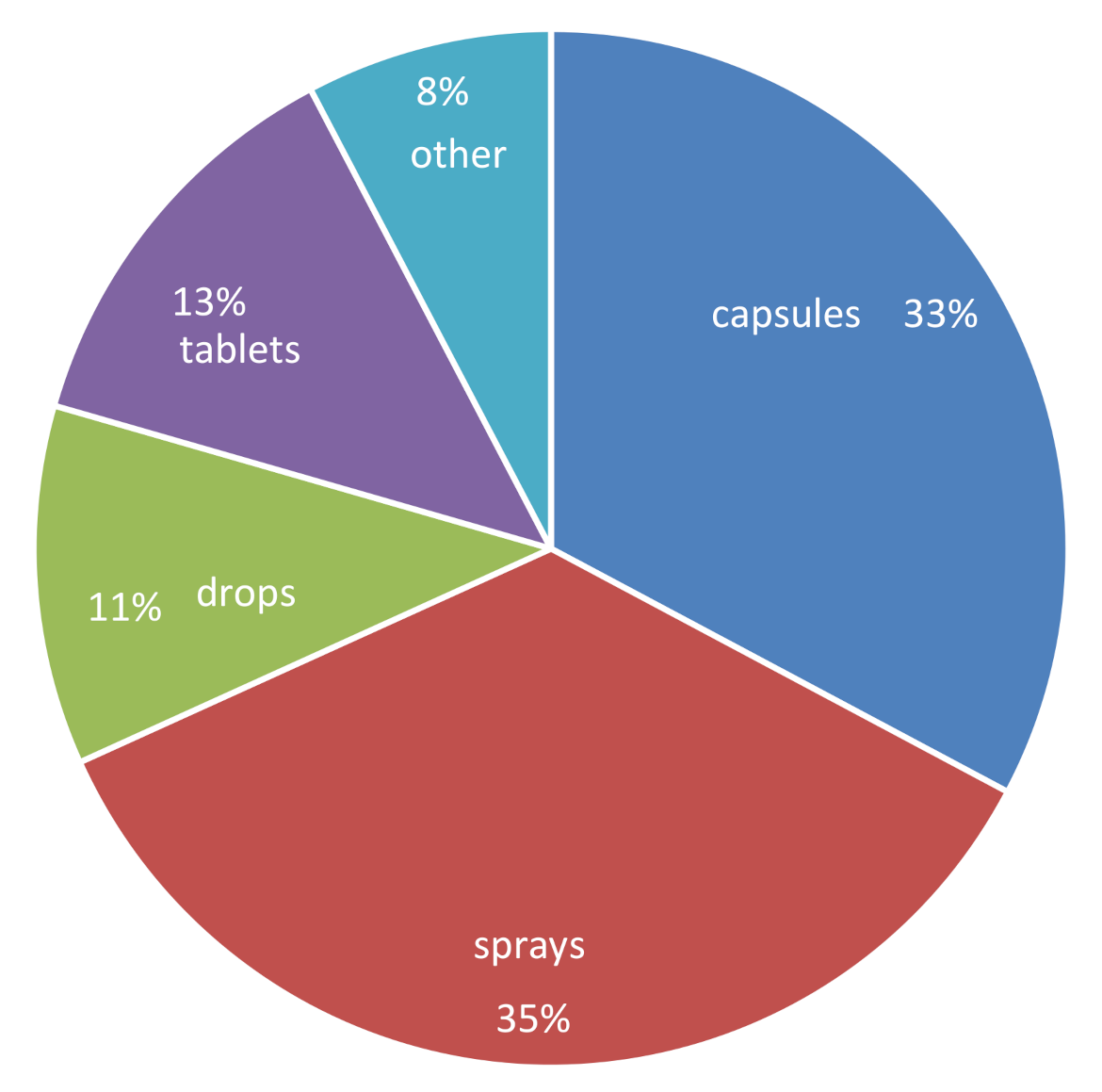
| Sample ID | Labeled Amount of 5-MTHF per Dosage a | Analytically Determined Amount of 5-MTHF per Dosage | % Labeled Content b | Comment c |
|---|---|---|---|---|
| NUT20/F/1 | 400 µg | 587 ± 6 µg | 147% | |
| NUT20/F/2 | 100 µg | 0 µg | 0% | Below limit of quantification. Below 80% limit. |
| NUT20/F/3 | 400 µg | 302 ± 13 µg | 76% | Below 80% limit |
| NUT20/F/4 | 400 µg | 587 ± 25 µg | 147% | |
| NUT20/F/5 | 300 µg | 290 ± 11 µg | 97% | |
| NUT20/F/6 | 400 µg | 1120 ± 35 µg | 280% | Above 150% limit. Above UL level. d |
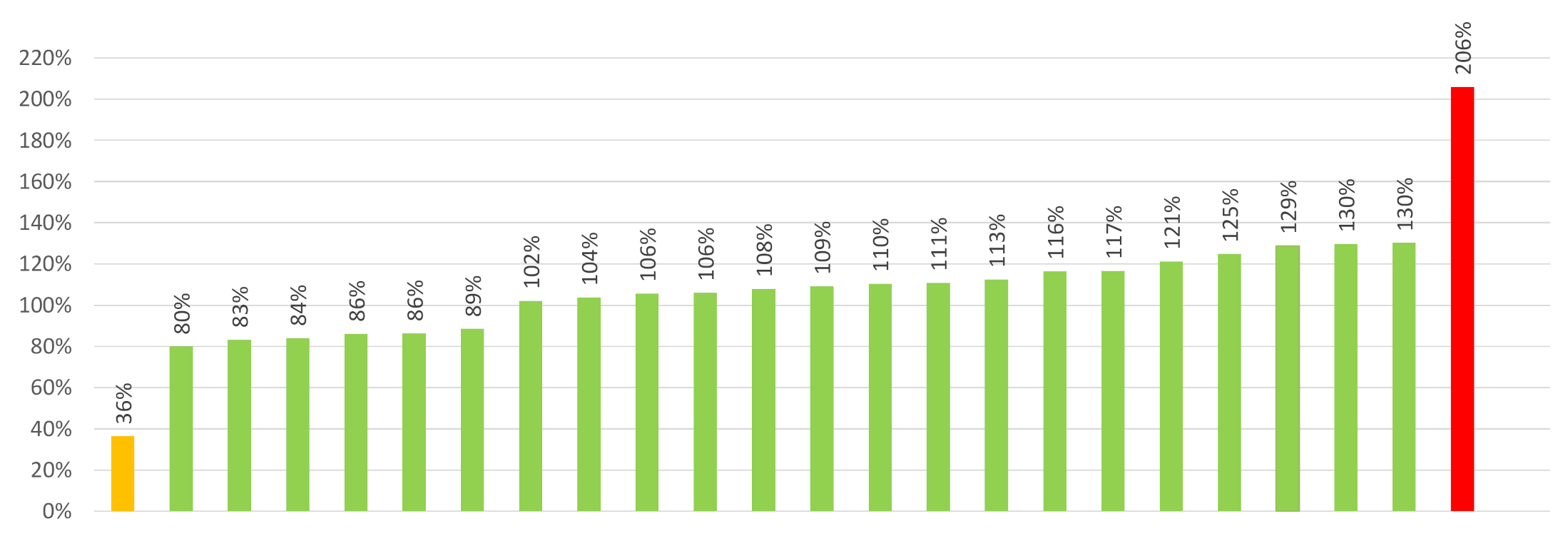
| Vitamin D a Content b | N | % | Actual Range of Cholecalciferol | Comment c |
|---|---|---|---|---|
| Below 80% of declared content | 1 | 4% | 36% | |
| 80–150% of declared content | 22 | 92% | 80–130% | One sample was above the UL limit d |
| Above 80% of declared content | 1 | 4% | 206% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žmitek, K.; Krušič, S.; Pravst, I. An Approach to Investigate Content-Related Quality of Nutraceuticals Used by Slovenian Consumers: A Case Study with Folate and Vitamin D Supplements. Foods 2021, 10, 845. https://doi.org/10.3390/foods10040845
Žmitek K, Krušič S, Pravst I. An Approach to Investigate Content-Related Quality of Nutraceuticals Used by Slovenian Consumers: A Case Study with Folate and Vitamin D Supplements. Foods. 2021; 10(4):845. https://doi.org/10.3390/foods10040845
Chicago/Turabian StyleŽmitek, Katja, Sanja Krušič, and Igor Pravst. 2021. "An Approach to Investigate Content-Related Quality of Nutraceuticals Used by Slovenian Consumers: A Case Study with Folate and Vitamin D Supplements" Foods 10, no. 4: 845. https://doi.org/10.3390/foods10040845
APA StyleŽmitek, K., Krušič, S., & Pravst, I. (2021). An Approach to Investigate Content-Related Quality of Nutraceuticals Used by Slovenian Consumers: A Case Study with Folate and Vitamin D Supplements. Foods, 10(4), 845. https://doi.org/10.3390/foods10040845








