Extraction Method Affects Contents of Flavonoids and Carotenoids in Huanglongbing-Affected “Valencia” Orange Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Chemicals
2.2. Fruit Source and Juice Extraction Process
2.3. Separation of Supernatant and Pellet
2.4. Insoluble Solids Analysis
2.5. Viscosity
2.6. Juice Color Analyses
2.7. Peel Oil Analysis
2.8. Sugar, Acid and pH Analysis
2.9. Flavonoid Analysis
2.10. Carotenoid Extraction and Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. General Juice Features
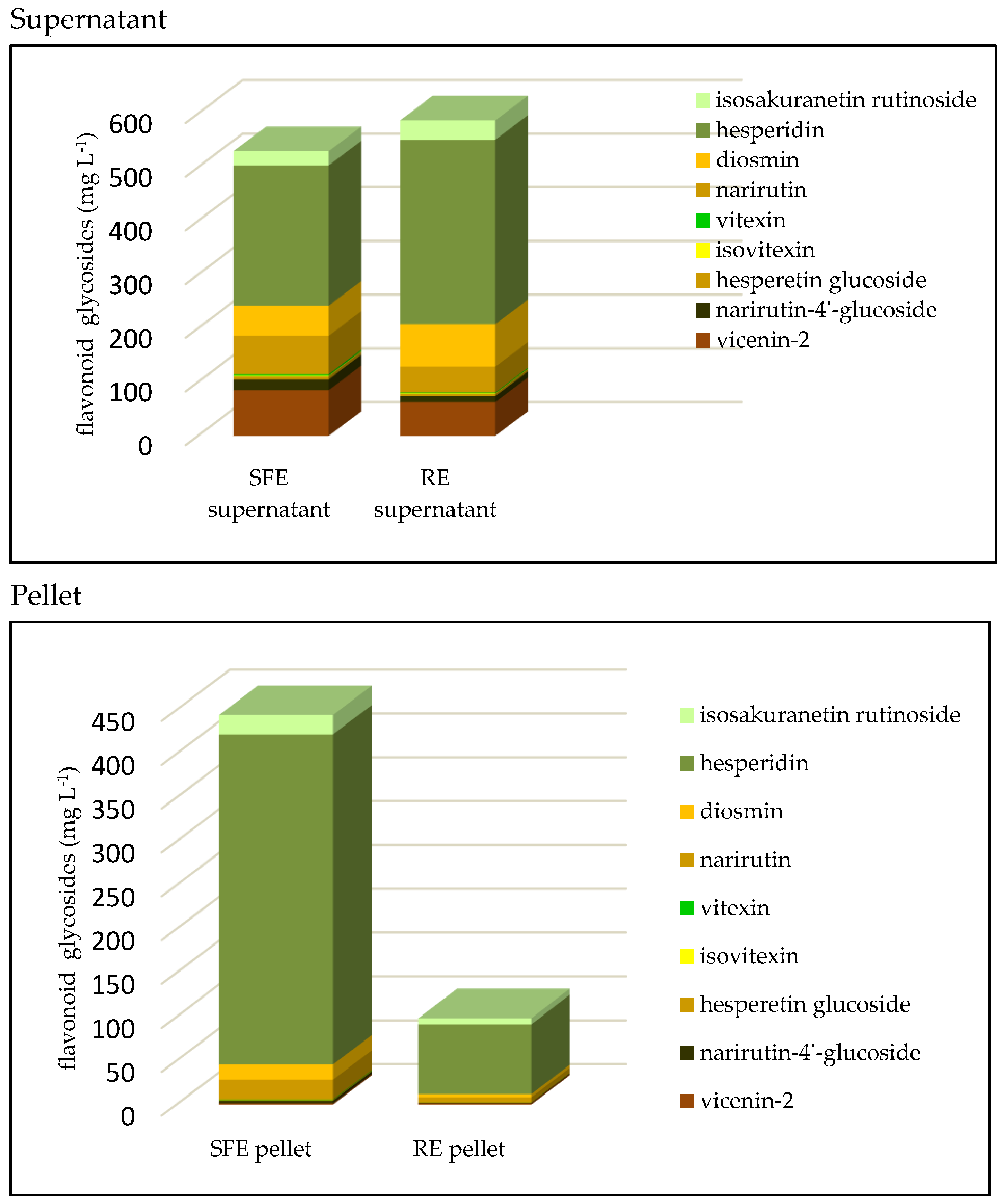
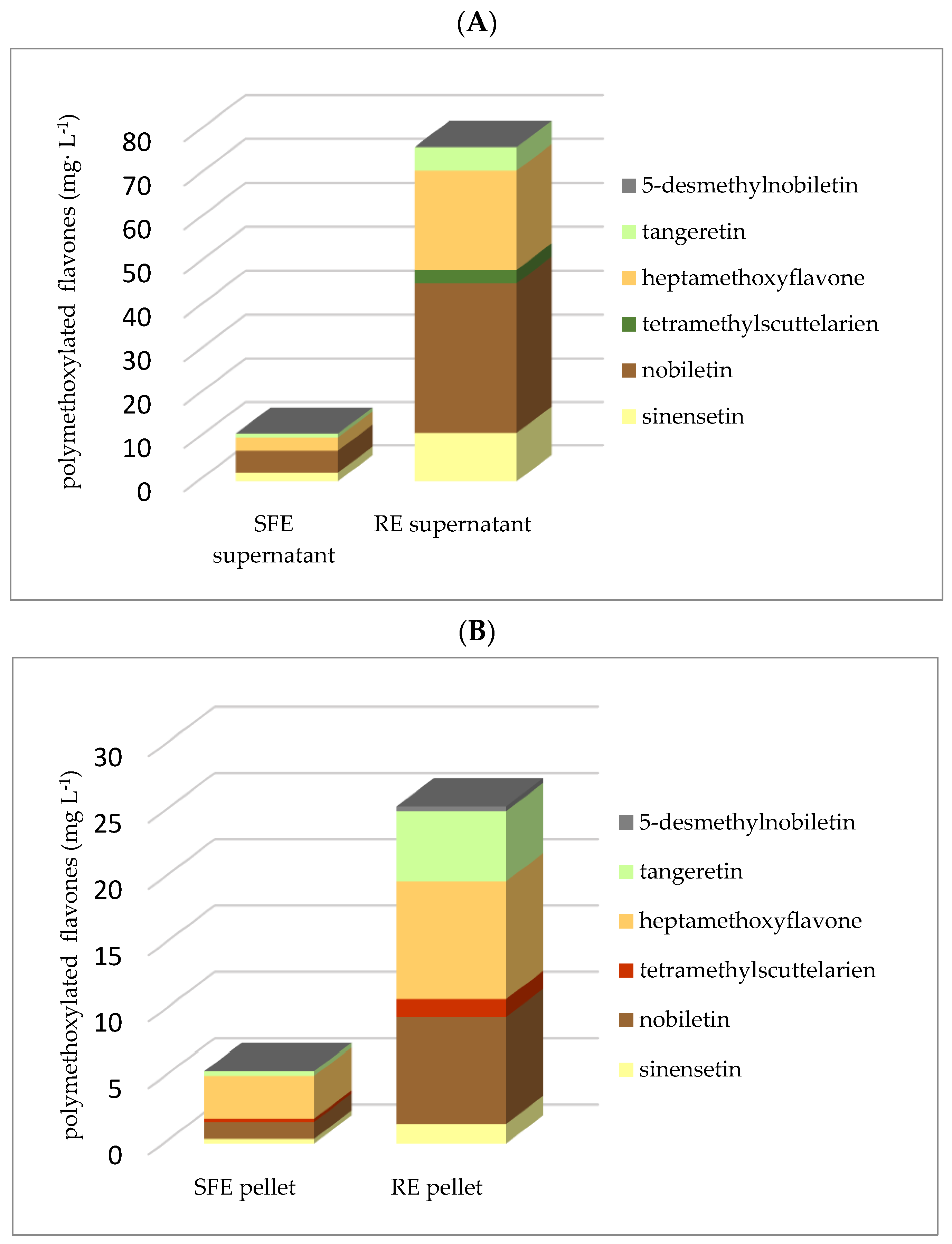
3.2. Flavonoids
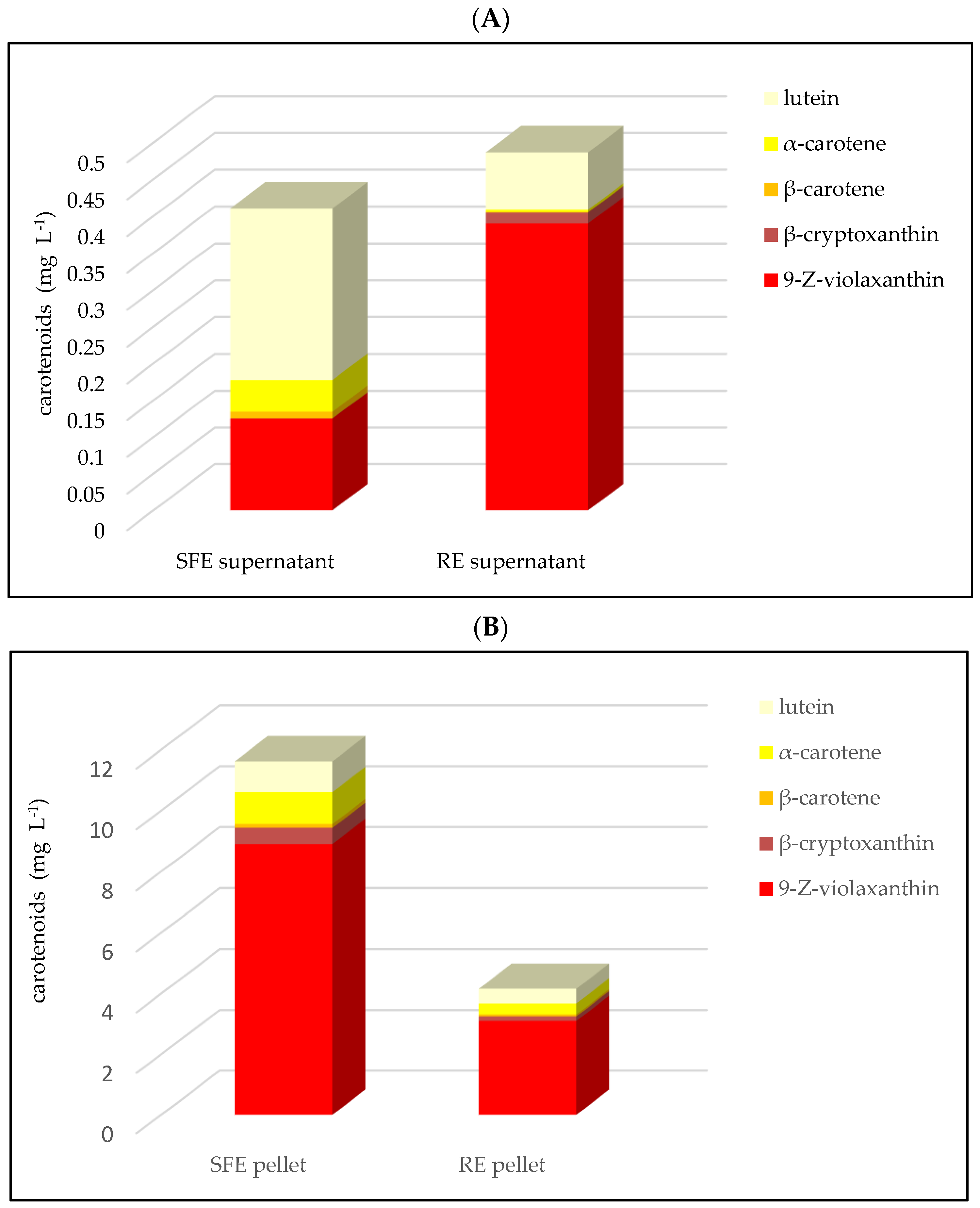
3.3. Carotenoids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar] [CrossRef]
- Wang, N. The Citrus Huanglongbing Crisis and Potential Solutions. Mol. Plant. 2019, 12, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Dagulo, L.; Danyluk, M.D.; Spann, T.M.; Valim, M.F.; Goodrich-Schneider, R.; Sims, C.; Rouseff, R. Chemical characterization of orange juice from trees infected with citrus greening (Huanglongbing). J. Food Sci. 2010, 75, C199–C207. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, E.; Plotto, A.; Manthey, J.; McCollum, G.; Bai, J.; Irey, M.; Cameron, R.; Luzio, G. Effect of liberibacter infection (huanglongbing disease) of citrus on orange fruit physiology and fruit/fruit juice quality: Chemical and physical analyses. J. Agric. Food Chem. 2010, 58, 1247–1262. [Google Scholar] [CrossRef]
- Baldwin, E.; Plotto, A.; Bai, J.; Manthey, J.; Zhao, W.; Raithore, S.; Irey, M. Effect of Abscission Zone Formation on Orange (Citrus sinensis) Fruit/Juice Quality for Trees Affected by Huanglongbing (HLB). J. Agric Food Chem. 2018, 66, 2877–2890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dala-Paula, B.M.; Plotto, A.; Bai, J.; Manthey, J.A.; Baldwin, E.A.; Ferrarezi, R.S.; Gloria, M.B.A. Effect of Huanglongbing or Greening Disease on Orange Juice Quality, a Review. Front Plant Sci. 2019, 9, 1976. [Google Scholar] [CrossRef] [Green Version]
- Kiefl, J.; Kohlenberg, B.; Hartmann, A.; Obst, K.; Paetz, S.; Krammer, G.; Trautzsch, S. Investigation on Key Molecules of Huanglongbing (HLB)-Induced Orange Juice Off-flavor. J. Agric. Food Chem. 2018, 66, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Raithore, S.; Kiefl, J.; Manthey, J.A.; Plotto, A.; Bai, J.; Zhao, W.; Baldwin, E. Mitigation of Off-Flavor in Huanglongbing-Affected Orange Juice Using Natural Citrus Non-Volatile Compounds. J. Agric. Food Chem. 2020, 68, 1038–1050. [Google Scholar] [CrossRef]
- Liao, H.L.; Burns, J.K. Gene expression in Citrus sinensis fruit tissues harvested from huanglongbing-infected trees: Comparison with girdled fruit. J. Exp. Bot. 2012, 63, 3307–3319. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Bai, J.; McCollum, G.; Baldwin, E. High incidence of preharvest colonization of huanglongbing-symptomatic citrus sinensis fruit by Lasiodiplodia theobromae (Diplodia natalensis) and exacerbation of postharvest fruit decay by that fungus. Appl. Environ. Microbiol. 2015, 81, 364–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, J.; Manthey, J.A.; Ford, B.L.; Luzio, G.; Cameron, R.G.; Narciso, J.; Baldwin, E.A. Effect of extraction, pasteurization and cold storage on flavonoids and other secondary metabolites in fresh orange juice. J. Sci. Food Agric. 2013, 93, 2771–2781. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Bai, J.; Plotto, A.; Cameron, R.; Luzio, G.; Narciso, J.; Manthey, J.; Widmer, W.; Ford, B.L. Effect of extraction method on quality of orange juice: Hand-squeezed, commercial-fresh squeezed and processed. J. Sci. Food Agric. 2012, 92, 2029–2042. [Google Scholar] [CrossRef] [PubMed]
- Kimball, D.; Parish, M.; Braddock, R. Oranges and Tangerines. In Processing Fruits: Science and Technology; Barrett, D.M., Somogyi, L.P., Ramaswamy, H.S., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 617–638. [Google Scholar]
- Rampersaud, G.C.; Valim, M.F. 100% citrus juice: Nutritional contribution, dietary benefits, and association with anthropometric measures. Crit. Rev. Food Sci. Nutr. 2017, 57, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.N.; Khomich, L.M.; Perova, I.B. Orange juice nutritional profile. Vopr. Pitan. 2017, 86, 103–113. [Google Scholar] [CrossRef]
- Constans, J.; Bennetau-Pelissero, C.; Martin, J.F.; Rock, E.; Mazur, A.; Bedel, A.; Morand, C.; Bérard, A.M. Marked antioxidant effect of orange juice intake and its phytomicronutrients in a preliminary randomized cross-over trial on mild hypercholesterolemic men. Clin. Nutr. 2015, 34, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.A.; Cooney, R.V.; Henning, S.M.; Custer, L.J. Bioavailability and antioxidant effects of orange juice components in humans. J. Agric. Food Chem. 2005, 53, 5170–5178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. Biofactors 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Sierra, N.; Marqués-Cardete, R.; Gurrea-Martínez, A.; Grau-Del Valle, C.; Morillas, C.; Hernández-Mijares, A.; Bañuls, C. Effect of Fibre-Enriched Orange Juice on Postprandial Glycaemic Response and Satiety in Healthy Individuals: An Acute, Randomised, Placebo-Controlled, Double-Blind, Crossover Study. Nutrients 2019, 11, 3014. [Google Scholar] [CrossRef] [Green Version]
- Kurowska, E.M.; Manthey, J.A. Hypolipidemic effects and absorption of citrus polymethoxylated flavones in hamsters with diet-induced hypercholesterolemia. J. Agric. Food Chem. 2004, 52, 2879–2886. [Google Scholar] [CrossRef]
- Morand, C.; Dubray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef]
- Silveira, J.Q.; Cesar, T.B.; Manthey, J.A.; Baldwin, E.A.; Bai, J.; Raithore, S. Pharmacokinetics of flavanone glycosides after ingestion of single doses of fresh-squeezed orange juice versus commercially processed orange juice in healthy humans. J. Agric. Food Chem. 2014, 62, 12576–12584. [Google Scholar] [CrossRef]
- Gonalves, D.; Ferreira, P.; Baldwin, E.; Cesar, T. Health Benefits of Orange Juice and Citrus Flavonoids. In Phytochemicals in Citrus–Applications in Functional Foods; Ye, X., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 299–323. [Google Scholar]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef] [Green Version]
- Massenti, R.; Lo Bianco, R.; Sandhu, A.K.; Gu, L.; Sims, C. Huanglongbing modifies quality components and flavonoid content of ‘Valencia’ oranges. J. Sci. Food Agric. 2016, 96, 73–78. [Google Scholar] [CrossRef]
- Huang, L.; Grosser, J.; Gmitter, F.G.; Sims, C.A.; Wang, Y. Effects of Scion/Rootstock Combination on Flavor Quality of Orange Juice from Huanglongbing (HLB)-Affected Trees: A Two-Year Study of the Targeted Metabolomics. J. Agric. Food Chem. 2020, 68, 3286–3296. [Google Scholar] [CrossRef]
- Dala Paula, B.M.; Raithore, S.; Manthey, J.A.; Baldwin, E.A.; Bai, J.; Zhao, W.; Glória, M.B.A.; Plotto, A. Active taste compounds in juice from oranges symptomatic for Huanglongbing (HLB) citrus greening disease. LWT 2018, 91, 518–525. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Borges, G.; Van Der Hooft, J.; Clifford, M.N.; Del Rio, D.; Lean, M.E.; Roberts, S.A.; Kellerhals, M.B.; Crozier, A. Orange juice (poly) phenols are highly bioavailable in humans. Am. J. Clin. Nutr. 2014, 100, 1378–1384. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F. Effect of processing techniques at industrial scale on orange juice antioxidant and beneficial health compounds. J. Agric. Food Chem. 2002, 50, 5107–5114. [Google Scholar] [CrossRef]
- Basile, L.G.; Lima, C.G.D.; Cesar, T.B. Daily intake of pasteurized orange juice decreases serum cholesterol, fasting glucose and diastolic blood pressure in adults. Proc. Fla. State Hort. Soc. 2010, 123, 228–233. [Google Scholar]
- Albach, R.F.; Redman, G.H. Composition and inheritance of flavanones in citrus fruit. Phytochem 1969, 8, 127–143. [Google Scholar] [CrossRef]
- Hiroyasu, K.; Yoshiharu, M.; Yoshitomi, I.; Kozo, O.; Katsumi, Y. Structure and hypotensive effect of flavonoid glycosides in Kinkan (fortunella japonica) peelings. Agric. Biol. Chem. 1985, 49, 2613–2618. [Google Scholar] [CrossRef]
- Horowitz, R.M.; Gentili, B. Flavonoid constituents of citrus. Citrus Sci. Technol. 1977, 1, 397–426. [Google Scholar]
- Sun, X.; Yang, H.; Zhao, W.; Bourcier, E.; Baldwin, E.A.; Plotto, A.; Irey, M.; Bai, J. Huanglongbing and foliar spray programs affect the chemical profile of ‘Valencia’orange peel oil. Front Plant Sci. 2021, 12, 611449. [Google Scholar] [CrossRef]
- Manthey, J.A. Differences in secondary metabolites in leaves from orange (Citrus sinensis L.) trees affected with greening disease (Huanglongbing) (HLB). Proc. Fla. State Hort. Soc. 2008, 121, 285–288. [Google Scholar]
- Scott, K.J. Detection and measurement of carotenoids by uv/vis spectrophotometry. Curr. Protoc. Food Anal. Chem. 2001, F2.2.1–F2.2.10. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Anwer, M.K.; Al-Shdefat, R.; Jamil, S.; Alam, P.; Abdel-Kader, M.S.; Shakeel, F. Solubility of bioactive compound hesperidin in six pure solvents at (298.15 to 333.15) K. J. Chem. Eng. Data 2014, 59, 2065–2069. [Google Scholar] [CrossRef]
- Stinco, C.M.; Fernández-Vázquez, R.; Escudero-Gilete, M.L.; Heredia, F.J.; Meléndez-Martínez, A.J.; Vicario, I.M. Effect of orange juice’s processing on the color, particle size, and bioaccessibility of carotenoids. J. Agric. Food Chem. 2012, 60, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Martinez, A.J.; Escudero-Gilete, R.L.; Vicario, R.M.; Heredia, R.J. Study of the influence of carotenoid structure and individual carotenoids in the qualitative and quantitative attributes of orange juice colour. Food Res. Int. 2010, 43. [Google Scholar] [CrossRef]
- Dhuique-Mayer, C.; Caris-Veyrat, C.; Ollitrault, P.; Curk, F.; Amiot, M.J. Varietal and interspecific influence on micronutrient contents in citrus from the Mediterranean area. J. Agric. Food Chem. 2005, 53, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ikomam, Y.; Matsumotom, H.; Sugiura, M.; Hyodo, H.; Yano, M. Accumulation of carotenoids and expression of carotenoid biosynthetic genes during maturation in citrus fruit. Plant Physiol. 2004, 134, 824–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borel, P.; Grolier, P.; Armand, M.; Partier, A.; Lafont, H.; Lairon, D.; Azaïs-Braesco, V. Carotenoids in biological emulsions: Solubility, surface-to-core distribution, and release from lipid droplets. J. Lipid Res. 1996, 37, 250–261. [Google Scholar] [CrossRef]
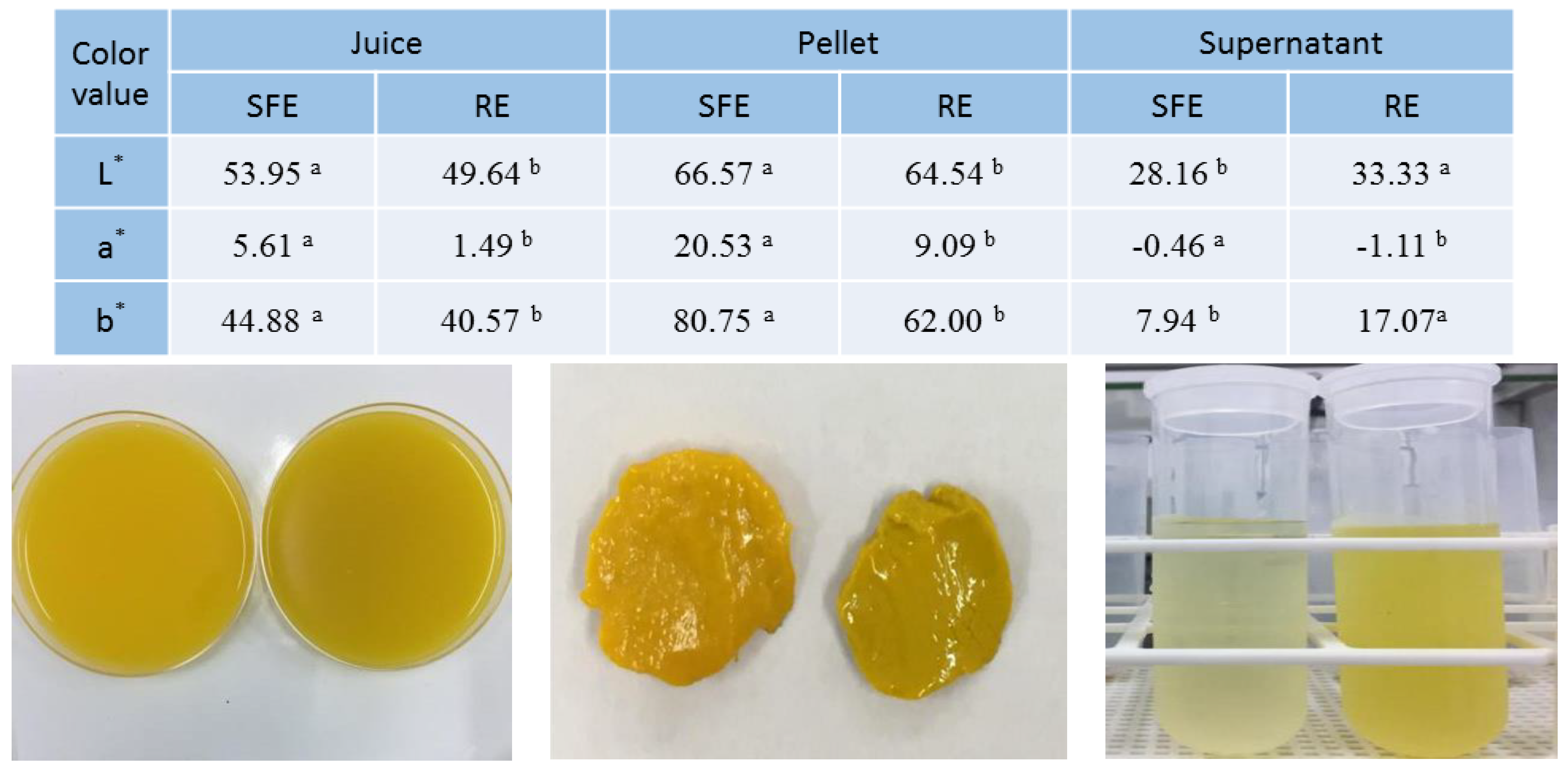
| Attribute | SFE | RE | t-Test a |
|---|---|---|---|
| juice content (%, v/w) | 42.80 ± 0.67 | 44.60 ± 0.71 | 0.05 |
| insoluble solids content (ISC, g L−1) | 4.90 ± 0.19 | 1.75 ± 0.04 | 0.01 |
| soluble solids content (SSC, g L−1) | 94.3 ± 1.2 | 81.3 ± 2.8 | 0.01 |
| titratable acidity (TA, g L−1) | 9.1 ± 0.2 | 9.3 ± 0.3 | NS |
| pH | 3.89 ± 0.10 | 3.80 ± 0.01 | NS |
| SSC/TA ratio | 9.91 ± 0.21 | 7.98 ± 0.17 | 0.01 |
| sucrose (g L−1) | 27.0 ± 1.1 | 26.1 ± 0.9 | 0.05 |
| glucose (g L−1) | 20.8 ± 0.6 | 16.2 ± 1.0 | 0.05 |
| fructose (g kg−1) | 23.5 ± 0.9 | 19.7 ± 0.2 | 0.05 |
| peel oil (g L−1) | 0.22 ± 0.02 | 1.96 ± 0.12 | 0.01 |
| viscosity (mPa s−1) | 58.7 ± 1.8 | 46.0 ± 0.7 | 0.05 |
| Chemical Class | SFE | RE | t-Test a |
|---|---|---|---|
| (Supernatant %: Pellet %) | |||
| flavonoid glycosides | 973.32 ± 71.89 (58:42) | 685.16 ± 51.64 (89:11) | 0.01 |
| polymethoxylated flavones | 16.22 ± 0.84 (66.85:33.15) | 101.46 ± 6.32 (75.04:24.96) | 0.01 |
| carotenoids | 12.03 ± 0.54 (3.41:96.59) | 4.64 ± 0.22 (10.56:89.44) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Li, T.; Baldwin, E.A.; Manthey, J.A.; Plotto, A.; Zhang, Q.; Gao, W.; Bai, J.; Shan, Y. Extraction Method Affects Contents of Flavonoids and Carotenoids in Huanglongbing-Affected “Valencia” Orange Juice. Foods 2021, 10, 783. https://doi.org/10.3390/foods10040783
Li Q, Li T, Baldwin EA, Manthey JA, Plotto A, Zhang Q, Gao W, Bai J, Shan Y. Extraction Method Affects Contents of Flavonoids and Carotenoids in Huanglongbing-Affected “Valencia” Orange Juice. Foods. 2021; 10(4):783. https://doi.org/10.3390/foods10040783
Chicago/Turabian StyleLi, Qili, Tao Li, Elizabeth A. Baldwin, John A. Manthey, Anne Plotto, Qun Zhang, Wei Gao, Jinhe Bai, and Yang Shan. 2021. "Extraction Method Affects Contents of Flavonoids and Carotenoids in Huanglongbing-Affected “Valencia” Orange Juice" Foods 10, no. 4: 783. https://doi.org/10.3390/foods10040783
APA StyleLi, Q., Li, T., Baldwin, E. A., Manthey, J. A., Plotto, A., Zhang, Q., Gao, W., Bai, J., & Shan, Y. (2021). Extraction Method Affects Contents of Flavonoids and Carotenoids in Huanglongbing-Affected “Valencia” Orange Juice. Foods, 10(4), 783. https://doi.org/10.3390/foods10040783






