Screening of Twelve Pea (Pisum sativum L.) Cultivars and Their Isolates Focusing on the Protein Characterization, Functionality, and Sensory Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Pea Flour
2.3. Production of Pea Protein Isolate
2.4. Chemical Composition
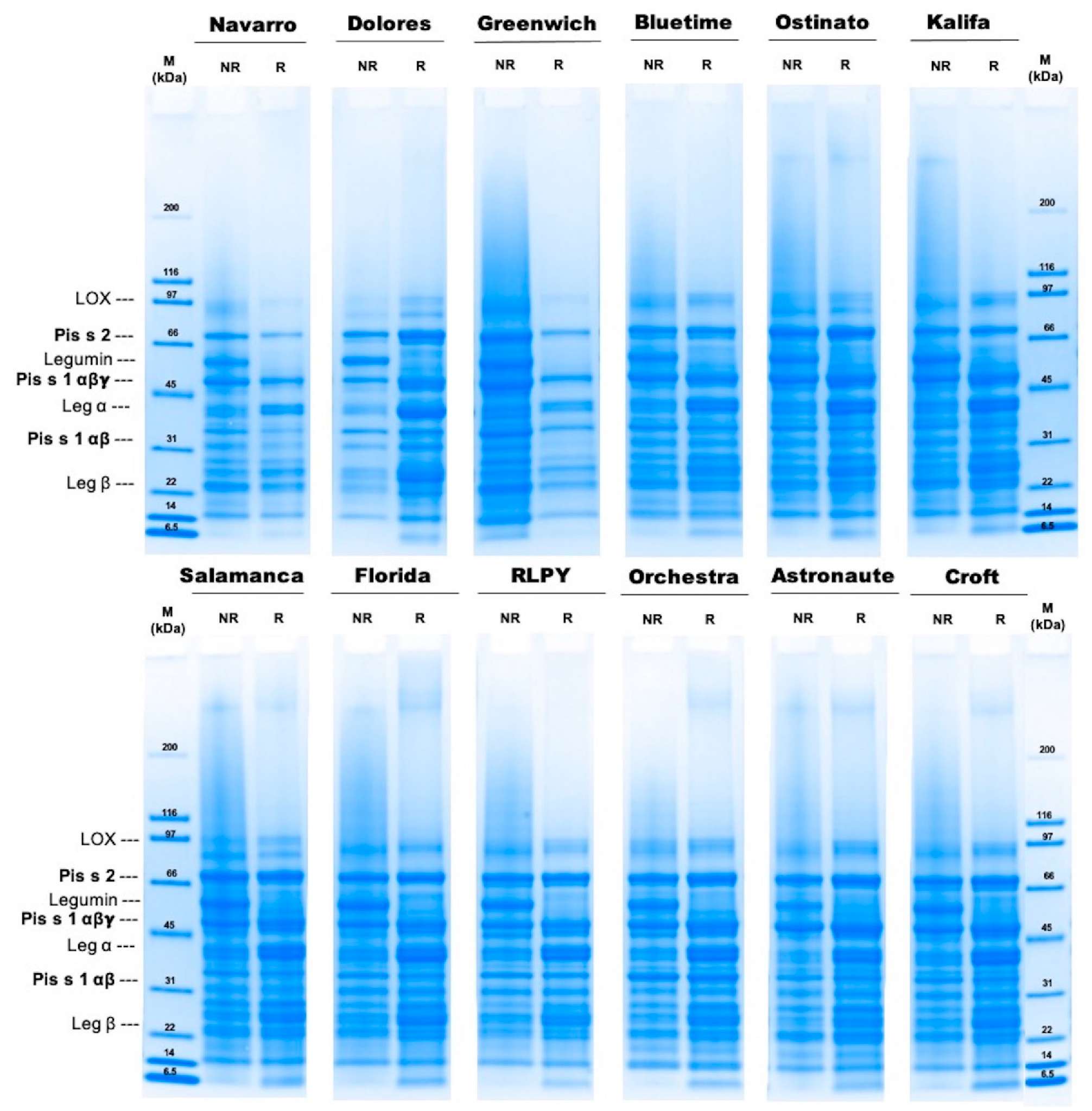
2.5. Molecular Weight Distribution Using Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6. Color
2.7. Particle Size
2.8. Functional Properties
2.8.1. Protein Solubility
2.8.2. Foaming Capacity
2.8.3. Emulsifying Capacity
2.9. Sensory Analysis
2.9.1. Sample Preparation
2.9.2. Sample Evaluation
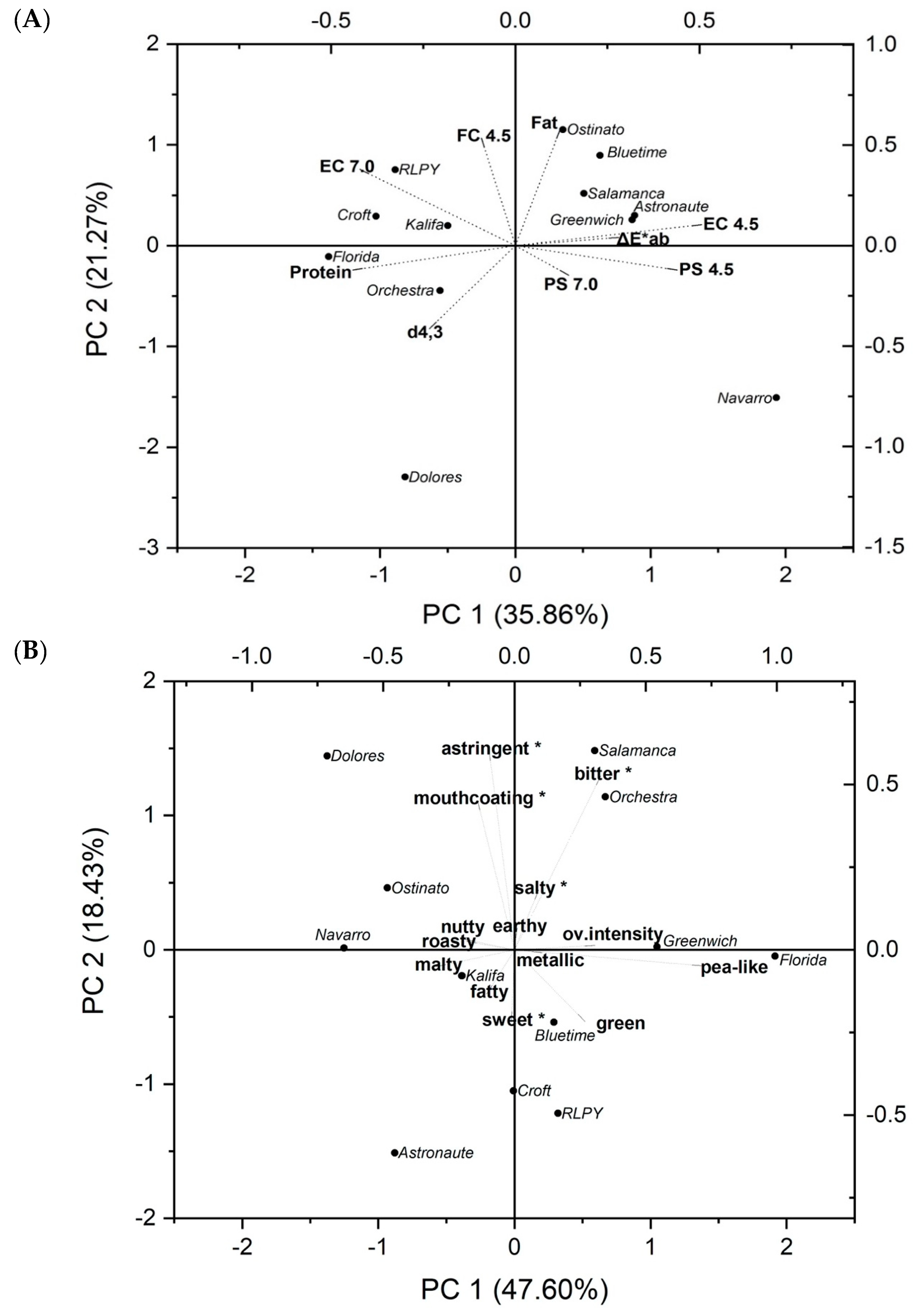
2.10. Principal Component Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition and Protein Yield
3.1.1. Pea Flours
3.1.2. Pea Protein Isolates
3.2. Molecular Weight Distribution
3.3. Color
3.4. Particle Size
3.5. Functional Properties
3.5.1. Protein Solubility
3.5.2. Emulsifying Capacity
3.5.3. Foaming Capacity
3.6. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Crops Production. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 11 January 2021).
- Kreplak, J.; Madoui, M.-A.; Cápal, P.; Novák, P.; Labadie, K.; Aubert, G.; Bayer, P.E.; Gali, K.K.; Syme, R.A.; Main, D.; et al. A reference genome for pea provides insight into legume genome evolution. Nat. Genet. 2019, 51, 1411–1422. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, A.; Mamidi, S.; McPhee, K. Genetic Diversity and Population Structure Among Pea (Pisum sativum L.) Cultivars as Revealed by Simple Sequence Repeat and Novel Genic Markers. Mol. Biotechnol. 2014, 56, 925–938. [Google Scholar] [CrossRef]
- Tulbek, M.C.; Lam, R.S.H.; Wang, Y.; Asavajaru, P.; Lam, A. Chapter 9—Pea: A Sustainable Vegetable Protein Crop. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 145–164. [Google Scholar] [CrossRef]
- Vidal-Valverde, C.; Frias, J.; Hernández, A.; Martín-Alvarez, P.J.; Sierra, I.; Rodríguez, C.; Blazquez, I.; Vicente, G. Assessment of nutritional compounds and antinutritional factors in pea (Pisum sativum) seeds. J. Sci. Food Agric. 2003, 83, 298–306. [Google Scholar] [CrossRef]
- Nikolopoulou, D.; Grigorakis, K.; Stasini, M.; Alexis, M.N.; Iliadis, K. Differences in chemical composition of field pea (Pisum sativum) cultivars: Effects of cultivation area and year. Food Chem. 2007, 103, 847–852. [Google Scholar] [CrossRef]
- Al-Karaki, G.; Ereifej, K. Relationships between Seed Yield and Chemical Composition of Field Peas Grown under Semi-arid Mediterranean Conditions. J. Agron. Crop Sci. 2001, 182, 279–284. [Google Scholar] [CrossRef]
- Azarnia, S.; Boye, J.I.; Warkentin, T.; Malcolmson, L.; Sabik, H.; Bellido, A.S. Volatile flavour profile changes in selected field pea cultivars as affected by crop year and processing. Food Chem. 2011, 124, 326–335. [Google Scholar] [CrossRef]
- Cui, L.; Kimmel, J.; Zhou, L.; Rao, J.; Chen, B. Identification of extraction pH and cultivar associated aromatic compound changes in spray dried pea protein isolate using untargeted and targeted metabolomic approaches. J. Agric. Food Res. 2020, 2, 100032. [Google Scholar] [CrossRef]
- Kniskern, M.A.; Johnston, C.S. Protein dietary reference intakes may be inadequate for vegetarians if low amounts of animal protein are consumed. Nutrition 2011, 27, 727–730. [Google Scholar] [CrossRef]
- Ahuja, K.; Mamtani, K. Pea Protein Market Share Forcasts 2020–2026; GMI362; Global Market Insights: Selbyville, DE, USA, 2020; p. 180. [Google Scholar]
- Koyoro, H.; Powers, J.R. Functional properties of pea globulin fractions. Cereal Chem. 1987, 64, 97–101. [Google Scholar]
- Gueguen, J.; Barbot, J. Quantitative and qualitative variability of pea (Pisum sativum L.) protein composition. J. Sci. Food Agric. 1988, 42, 209–224. [Google Scholar] [CrossRef]
- Tzitzikas, E.N.; Vincken, J.P.; de Groot, J.; Gruppen, H.; Visser, R.G. Genetic variation in pea seed globulin composition. J. Agric. Food Chem. 2006, 54, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Casey, R.; Domoney, C. Pea Globulins. In Seed Proteins; Shewry, P.R., Casey, R., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 171–208. [Google Scholar] [CrossRef]
- Bourgeois, M.; Jacquin, F.; Savois, V.; Sommerer, N.; Labas, V.; Henry, C.; Burstin, J. Dissecting the proteome of pea mature seeds reveals the phenotypic plasticity of seed protein composition. Proteomics 2009, 9, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Barac, M.; Cabrilo, S.; Pesic, M.; Stanojevic, S.; Zilic, S.; Macej, O.; Ristic, N. Profile and functional properties of seed proteins from six pea (Pisum sativum) genotypes. Int. J. Mol. Sci. 2010, 11, 4973–4990. [Google Scholar] [CrossRef] [PubMed]
- Tanger, C.; Engel, J.; Kulozik, U. Influence of extraction conditions on the conformational alteration of pea protein extracted from pea flour. Food Hydrocoll. 2020, 107, 105949. [Google Scholar] [CrossRef]
- Haque, M.A.; Adhikari, B. Drying and Denaturation of Proteins in Spray Drying Process. In Handbook of Industrial Drying, 4th ed.; CRC Press; Taylor and Francis Group: Boca Raton, FL, USA, 2014; Volume 49, pp. 971–984. [Google Scholar]
- LfL. Bayerische Landesanstalt für Landwirtschaft. Available online: https://www.lfl.bayern.de/ipz/oelfruechte/066649/index.php (accessed on 11 January 2021).
- Reinkensmeier, A.; Bußler, S.; Schlüter, O.; Rohn, S.; Rawel, H.M. Characterization of individual proteins in pea protein isolates and air classified samples. Food Res. Int. 2015, 76, 160–167. [Google Scholar] [CrossRef]
- Sanchez-Monge, R.; Lopez-Torrejon, G.; Pascual, C.Y.; Varela, J.; Martin-Esteban, M.; Salcedo, G. Vicilin and convicilin are potential major allergens from pea. Clin. Exp. Allergy 2004, 34, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, L.; Astier, C.; Dano, D.; Hosotte, M.; Jarlot-Chevaux, S.; Sergeant, P.; Kanny, G. Consommation croissante d’aliments contenant du pois jaune: Un risque d’allergie? Rev. Fr. Allergol. 2014, 54, 20–26. [Google Scholar] [CrossRef]
- Codreanu-Morel, F.; Morisset, M.; Cordebar, V.; Larré, C.; Denery-Papini, S. L’allergie au pois. Rev. Fr. Allergol. 2019, 59, 162–165. [Google Scholar] [CrossRef]
- Tian, S.J.; Kyle, W.S.A.; Small, D.M. Pilot scale isolation of proteins from field peas (Pisum sativum L.) for use as food ingredients. Int. J. Food Sci. Technol. 1999, 34, 33–39. [Google Scholar] [CrossRef]
- AOACa. Method 923.03. Ash of flour. In Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC); Horwitz, W.A., Ed.; AOAC International: Gaithersburg, MD, USA, 2003. [Google Scholar]
- AOACb. Method 968.06. Protein (crude) in animal feed. In Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC); Horwitz, W.A., Ed.; AOAC International: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Gertz, C.; Fiebig, H.-J. Determination of fat content by the Caviezel® method (rapid method). Eur. J. Lipid Sci. Technol. 2000, 102, 154–158. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Morr, C.V.; German, B.; Kinsella, J.E.; Regenstein, J.M.; Vanburen, J.P.; Kilara, A.; Lewis, B.A.; Mangino, M.E. A collaborative study to develop a standarized food protein solubility procedure. J. Food Sci. 1985, 50, 1715–1718. [Google Scholar] [CrossRef]
- AACC Method 46-15.01 Crude Protein—5-Minute Biuret Method for Wheat and Other Grains. Available online: https://methods.aaccnet.org/summaries/46-15-01.aspx (accessed on 1 April 2021).
- Phillips, L.G.; Haque, Z.; Kinsella, J.E. A Method for the Measurement of Foam Formation and Stability. J. Food Sci. 1987, 52, 1074–1077. [Google Scholar] [CrossRef]
- Wang, C.; Johnson, L.A. Functional properties of hydrothermally cooked soy protein products. J. Am. Oil Chem. Soc. 2001, 78, 189–195. [Google Scholar] [CrossRef]
- García Arteaga, V.; Apéstegui Guardia, M.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innov. Food Sci. Emerg. Technol. 2020, 65, 102449. [Google Scholar] [CrossRef]
- García Arteaga, V.; Kraus, S.; Schott, M.; Muranyi, I.; Schweiggert-Weisz, U.; Eisner, P. Screening of Twelve Pea (Pisum sativum L.) Cultivars and its Isolates. Mendeley Data 2021, V2. [Google Scholar] [CrossRef]
- Shen, S.; Hou, H.; Ding, C.; Bing, D.-J.; Lu, Z.-X.; Navabi, A. Protein content correlates with starch morphology, composition and physicochemical properties in field peas. Can. J. Plant Sci. 2016, 96, 404–412. [Google Scholar] [CrossRef]
- Maury, M.; Murphy, K.; Kumar, S.; Shi, L.; Lee, G. Effects of process variables on the powder yield of spray-dried trehalose on a laboratory spray-dryer. Eur. J. Pharm. Biopharm. 2005, 59, 565–573. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Zbicinski, I. Modeling and Scaling Up of Industrial Spray Dryers: A Review. J. Chem. Eng. Jpn. 2017, 50, 757–767. [Google Scholar] [CrossRef]
- Gao, Z.; Shen, P.; Lan, Y.; Cui, L.; Ohm, J.-B.; Chen, B.; Rao, J. Effect of alkaline extraction pH on structure properties, solubility, and beany flavor of yellow pea protein isolate. Food Res. Int. 2020, 131, 109045. [Google Scholar] [CrossRef]
- Sternberg, M.J.E.; Thornton, J.M. On the conformation of proteins: Hydrophobic ordering of strands in β-pleated sheets. J. Mol. Biol. 1977, 115, 1–17. [Google Scholar] [CrossRef]
- Sumner, A.K.; Nielsen, M.A.; Youngs, C.G. Production and Evaluation of Pea Protein Isolate. J. Food Sci. 1981, 46, 364–366. [Google Scholar] [CrossRef]
- Shi, Y.; Mandal, R.; Singh, A.; Pratap Singh, A. Legume lipoxygenase: Strategies for application in food industry. Legume Sci. 2020, 2, e44. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2016, 45, D158–D169. [Google Scholar] [CrossRef]
- Gatehouse, J.A.; Lycett, G.W.; Croy, R.R.; Boulter, D. The post-translational proteolysis of the subunits of vicilin from pea (Pisum sativum L.). Biochem. J. 1982, 207, 629–632. [Google Scholar] [CrossRef] [PubMed]
- McClain, S.; Stevenson, S.E.; Brownie, C.; Herouet-Guicheney, C.; Herman, R.A.; Ladics, G.S.; Privalle, L.; Ward, J.M.; Doerrer, N.; Thelen, J.J. Variation in Seed Allergen Content From Three Varieties of Soybean Cultivated in Nine Different Locations in Iowa, Illinois, and Indiana. Front. Plant Sci. 2018, 9, 1025. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.C.Y.; Karaca, A.C.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2016, 34, 126–147. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, L.; Zhang, X.; Liu, H.; Zhu, Y. Effects of ultrafine grinding time on the functional and flavor properties of soybean protein isolate. Colloids Surf. B Biointerfaces 2020, 196, 111345. [Google Scholar] [CrossRef]
- Kerr, W.L.; Ward, C.D.W.; McWatters, K.H.; Resurreccion, A.V.A. Effect of Milling and Particle Size on Functionality and Physicochemical Properties of Cowpea Flour. Cereal Chem. 2000, 77, 213–219. [Google Scholar] [CrossRef]
- Vicente, J.; Pinto, J.; Menezes, J.; Gaspar, F. Fundamental analysis of particle formation in spray drying. Powder Technol. 2013, 247, 1–7. [Google Scholar] [CrossRef]
- Taherian, A.R.; Mondor, M.; Labranche, J.; Drolet, H.; Ippersiel, D.; Lamarche, F. Comparative study of functional properties of commercial and membrane processed yellow pea protein isolates. Food Res. Int. 2011, 44, 2505–2514. [Google Scholar] [CrossRef]
- Adebiyi, A.P.; Aluko, R.E. Functional properties of protein fractions obtained from commercial yellow field pea (Pisum sativum L.) seed protein isolate. Food Chem. 2011, 128, 902–908. [Google Scholar] [CrossRef]
- Chao, D.F.; Aluko, R.E. Modification of the structural, emulsifying, and foaming properties of an isolated pea protein by thermal pretreatment. CyTA J. Food 2018, 16, 357–366. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Cases, E.; Chambin, O.; Saurel, R. Interfacial and Emulsifying Characteristics of Acid-treated Pea Protein. Food Biophys. 2009, 4, 273–280. [Google Scholar] [CrossRef]
- Dickinson, E. Protein Adsorption at Liquid Interfaces and the Relationship to Foam Stability. In Foams: Physics, Chemistry and Structure; Springer: London, UK, 1989; pp. 39–53. [Google Scholar] [CrossRef]
- Denkov, N.; Marinova, K.; Denkov, N.; Marinova, K. Chapter 10—Antifoam effects of solid particles, oil drops and oil-solid compounds in aqueous foams. In Colloidal Particles at Liquid Interfaces; Binks, B.P., Horozov, T.S., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 383–444. [Google Scholar] [CrossRef]
- Murray, K.E.; Whitfield, F.B. The occurrence of 3-alkyl-2-methoxypyrazines in raw vegetables. J. Sci. Food Agric. 1975, 26, 973–986. [Google Scholar] [CrossRef]
- Murat, C.; Bard, M.-H.; Dhalleine, C.; Cayot, N. Characterisation of odour active compounds along extraction process from pea flour to pea protein extract. Food Res. Int. 2013, 53, 31–41. [Google Scholar] [CrossRef]
- Heng, L. Flavour Aspects of Pea and Its Protein Preparations in Relation to Novel Protein Foods; Wageningen University: Wageningen, The Netherlands, 2005. [Google Scholar]
- García Arteaga, V.; Leffler, S.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Sensory profile, functional properties and molecular weight distribution of fermented pea protein isolate. Curr. Res. Food Sci. 2021, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.G. 11—Viticultural and vineyard management practices and their effects on grape and wine quality. In Managing Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 365–444. [Google Scholar] [CrossRef]
- Schlegel, K.; Leidigkeit, A.; Eisner, P.; Schweiggert-Weisz, U. Technofunctional and Sensory Properties of Fermented Lupin Protein Isolates. Foods 2019, 8, 678. [Google Scholar] [CrossRef]
| Cultivar | Harvest Year | Place of Cultivation | Cotyledon Color | Admitted in |
|---|---|---|---|---|
| Navarro | 2018 | Malchow/Mecklenburg-Vorpommern | yellow | Germany |
| Dolores | 2015 | Oderaue/Mecklenburg-Vorpommern | yellow | Germany |
| Greenwich | 2018 | Hohenlieth/Schleswig-Holstein | green | Great Britain |
| Bluetime | 2018 | Hohenlieth/Schleswig-Holstein | green | Great Britain |
| Ostinato | 2018 | Rodez/France | yellow | France |
| Kalifa | 2017 | Hohenlieth/Schleswig-Holstein | yellow | Breeding line |
| Salamanca | 2018 | Malchow/Mecklenburg-Vorpommern | yellow | Germany, Czech Republic, etc. |
| Florida | 2015 | Dreveskirchen/Mecklenburg-Vorpommern | yellow | Germany |
| RLPY141091 | 2018 | Rodez/France | yellow | Germany |
| Orchestra | 2018 | Rodez/France | yellow | France, Germany |
| Astronaute | 2018 | Groß Kiesow/Mecklenburg-Vorpommern | yellow | France, Germany, etc. |
| Croft | 2018 | Hohenlieth/Schleswig-Holstein | green | Great Britain |
| Cultivar | Dry Matter | Protein * | Ash 550 * | Fat * | Starch * | Protein Yield ** |
|---|---|---|---|---|---|---|
| [%] | [%] | [%] | [%] | [%] | [g kg−1] | |
| Dehulled Flour | ||||||
| Navarro | 89.6 ± 0.0 | 22.1 ± 0.1 | 2.9 ± 0.0 | 2.3 ± 0.0 | 52.6 ± 0.2 | - |
| Dolores | 91.4 ± 0.0 | 26.5 ± 0.2 | 3.0 ± 0.0 | 1.9 ± 0.0 | 44.3 ± 2.5 | - |
| Greenwich | 90.9 ± 0.0 | 21.3 ± 0.2 | 2.7 ± 0.0 | 2.5 ± 0.0 | 48.2 ± 0.3 | - |
| Bluetime | 91.5 ± 0.1 | 22.4 ± 0.4 | 2.9 ± 0.0 | 2.2 ± 0.0 | 40.3 ± 0.5 | - |
| Ostinato | 91.2 ± 0.0 | 25.0 ± 0.2 | 3.6 ± 0.1 | 1.9 ± 0.0 | 47.6 ± 0.5 | - |
| Kalifa | 91.4 ± 0.1 | 24.2 ± 0.1 | 3.0 ± 0.0 | 1.9 ± 0.0 | 46.6 ± 0.1 | - |
| Salamanca | 90.8 ± 0.0 | 22.4 ± 0.1 | 2.8 ± 0.0 | 2.0 ± 0.0 | 49.2 ± 3.3 | - |
| Florida | 91.2 ± 0.1 | 24.8 ± 0.1 | 2.9 ± 0.0 | 2.7 ± 0.2 | 45.0 ± 4.6 | - |
| RLPY 141091 | 91.3 ± 0.1 | 27.2 ± 0.0 | 2.8 ± 0.0 | 1.9 ± 0.0 | 32.5 ± 0.8 | - |
| Orchestra | 92.2 ± 0.1 | 26.3 ± 0.2 | 3.5 ± 0.2 | 1.9 ± 0.0 | 35.8 ± 0.3 | - |
| Astronaute | 91.2 ± 0.0 | 22.0 ± 0.0 | 2.5 ± 0.1 | 2.0 ± 0.1 | 45.3 ± 1.1 | - |
| Croft | 91.8 ± 0.1 | 22.5 ± 0.1 | 2.6 ± 0.0 | 2.1 ± 0.0 | 48.0 ± 2.2 | - |
| Protein Isolate | ||||||
| Navarro | 93.0 ± 0.0 | 83.5 ± 0.4 | 5.3 ± 0.3 | 5.9 ± 0.0 | - | 33.8 |
| Dolores | 93.5 ± 0.1 | 89.5 ± 0.2 | 5.4 ± 0.1 | 4.7 ± 0.1 | - | 54.4 |
| Greenwich | 93.8 ± 0.0 | 83.6 ± 0.4 | 6.0 ± 0.6 | 9.0 ± 0.2 | - | 34.8 |
| Bluetime | 94.4 ± 0.0 | 84.1 ± 0.0 | 6.4 ± 0.4 | 8.4 ± 0.3 | - | 42.2 |
| Ostinato | 94.1 ± 0.0 | 86.0 ± 0.5 | 7.6 ± 0.4 | 7.1 ± 0.4 | - | 38.6 |
| Kalifa | 93.0 ± 0.0 | 86.9 ± 0.9 | 5.9 ± 0.1 | 7.0 ± 0.5 | - | 46.2 |
| Salamanca | 93.7 ± 0.6 | 85.0 ± 0.3 | 6.1 ± 1.0 | 8.7 ± 0.6 | - | 42.2 |
| Florida | 92.5 ± 0.0 | 87.4 ± 1.1 | 5.6 ± 0.1 | 7.4 ± 0.7 | - | 59.2 |
| RLPY 141091 | 93.4 ± 0.0 | 90.3 ± 0.0 | 8.5 ± 0.7 | 7.3 ± 0.8 | - | 53.6 |
| Orchestra | 92.8 ± 0.3 | 87.1 ± 0.1 | 6.7 ± 1.1 | 6.2 ± 0.9 | - | 62.2 |
| Astronaute | 96.0 ± 0.2 | 86.4 ± 0.1 | 5.4 ± 0.1 | 7.8 ± 0.1 | - | 42.1 |
| Croft | 92.5 ± 0.1 | 86.7 ± 0.6 | 6.2 ± 0.1 | 7.8 ± 0.1 | - | 47.3 |
| Cultivar | Protein Band Intensity [Int] | |||||
|---|---|---|---|---|---|---|
| Convicilin Pis s2 | Vicilin αβγ Pis s1 | Vicilin αβ Pis s1 | ||||
| NR | R | NR | R | NR | R | |
| Navarro | 185 ± 16 | 146 ± 79 | 308 ± 34 | 280 ± 73 | 81 ± 13 | 95 ± 50 |
| Dolores | 214 ± 5 | 367 ± 35 | 166 ± 22 | 282 ± 43 | 118 ± 5 | 97 ± 11 |
| Greenwich | 219 ± 47 | 185 ± 57 | 229 ± 55 | 256 ± 36 | 126 ± 16 | 113 ± 2 |
| Bluetime | 241 ± 17 | 263 ± 49 | 236 ± 66 | 311 ± 39 | 111 ± 7 | 127 ± 9 |
| Ostinato | 253 ± 31 | 343 ± 32 | 252 ± 66 | 361 ± 11 | 105 ± 19 | 116 ± 37 |
| Kalifa | 141 ± 6 | 205 ± 36 | 285 ± 47 | 392 ± 46 | 122 ± 8 | 153 ± 7 |
| Salamanca | 280 ± 2 | 363 ± 5 | 233 ± 51 | 350 ± 20 | 89 ± 3 | 88 ± 16 |
| Florida | 218 ± 16 | 283 ± 51 | 196 ± 88 | 330 ± 60 | 94 ± 1 | 72 ± 6 |
| RLPY 141091 | 294 ± 13 | 379 ± 63 | 149 ± 25 | 285 ± 78 | 117 ± 8 | 106 ± 0 |
| Orchestra | 302 ± 50 | 421 ± 32 | 212 ± 78 | 398 ± 19 | 228 ± 38 | 180 ± 5 |
| Astronaute | 251 ± 34 | 365 ± 44 | 293 ± 72 | 411 ± 20 | 118 ± 28 | 99 ± 8 |
| Croft | 261 ± 55 | 372 ± 79 | 235 ± 79 | 353 ± 29 | 93 ± 7 | 79 ± 20 |
| Cultivar | Pea Isolate CIE Color | |||
|---|---|---|---|---|
| L* | a* | b* | ΔE*ab | |
| Navarro | 89.3 ± 0.4 ab | 2.8 ± 0.1 ab | 23.7 ± 0.2 a | 23.4 ± 0.2 a |
| Dolores | 88.5 ± 0.1 ab | 1.9 ± 0.0 ab | 19.1 ± 0.2 a | 19.2 ± 0.1 a |
| Greenwich | 88.2 ± 0.1 ab | 0.6 ± 0.1 ab | 19.2 ± 0.4 a | 19.3 ± 0.3 a |
| Bluetime | 86.8 ± 0.3 a | 0.9 ± 0.1 ab | 20.5 ± 0.7 a | 21.0 ± 0.7 a |
| Ostinato | 89.4 ± 0.3 ab | 3.2 ± 0.2 ab | 20.5 ± 0.4 a | 20.4 ± 0.5 a |
| Kalifa | 89.5 ± 0.3 ab | 2.8 ± 0.0 ab | 20.5 ± 0.2 a | 20.3 ± 0.2 a |
| Salamanca | 88.3 ± 0.2 ab | 3.3 ± 0.1 ab | 21.3 ± 0.3 a | 21.5 ± 0.3 a |
| Florida | 88.6 ± 0.3 ab | 2.7 ± 0.2 ab | 20.8 ± 0.2 a | 20.8 ± 0.3 a |
| RLPY 141091 | 90.1 ± 0.2 ab | 3.1 ± 0.1 ab | 22.0 ± 0.2 a | 22.0 ± 0.3 a |
| Orchestra | 90.6 ± 0.6 b | 2.6 ± 0.3 ab | 20.9 ± 0.4 a | 20.3 ± 0.5 a |
| Astronaute | 88.2 ± 0.3 ab | 3.5 ± 0.1 a | 22.8 ± 0.3 a | 22.9 ± 0.3 a |
| Croft | 87.3 ± 0.3 ab | -0.5 ± 0.0 b | 19.9 ± 0.2 a | 20.3 ± 0.3 a |
| Particle Size | Protein Solubility ** | Emulsifying Capacity | Foaming Capacity | |||
|---|---|---|---|---|---|---|
| Cultivar | d4,3 | pH 4.5 | pH7.0 | pH 4.5 | pH 7.0 | pH 4.5 |
| [μm] | [%] | [%] | [mL g−1] | [mL g−1] | [%] | |
| Navarro | 13.19 ± 0.56 | 10.3 ± 0.2 | 51.5 ± 0.9 | 405 ± 1 | 600 ± 7 | 805 ± 0 |
| Dolores | 15.81 ± 0.06 | 7.4 ± 0.0 | 60.8 ± 2.8 | 340 ± 7 | 706 ± 14 | 808 ± 4 |
| Greenwich | 12.82 ± 0.19 | 8.8 ± 1.3 | 55.4 ± 3.4 | 396 ± 2 | 734 ± 7 | 839 ± 36 |
| Bluetime | 9.20 ± 0.49 | 7.7 ± 0.2 | 53.8 ± 2.4 | 365 ± 1 | 710 ± 8 | 915 ± 0 |
| Ostinato | 7.86 ± 0.02 | 8.3 ± 1.9 | 60.4 ± 1.9 | 385 ± 14 | 787 ± 32 | 959 ± 10 |
| Kalifa | 13.55 ± 1.53 | 7.3 ± 0.0 | 40.0 ± 2.1 | 354 ± 1 | 747 ± 3 | 911 ± 40 |
| Salamanca | 10.15 ± 0.40 | 5.9 ± 0.6 | 48.6 ± 3.6 | 378 ± 11 | 744 ± 2 | 835 ± 0 |
| Florida | 18.84 ± 1.31 | 0.9 ± 1.3 | 41.3 ± 7.1 | 340 ± 7 | 781 ± 23 | 884 ± 14 |
| RLPY 141091 | 7.53 ± 0.01 | 2.3 ± 0.6 | 52.6 ± 2.8 | 359 ± 5 | 835 ± 7 | 874 ± 13 |
| Orchestra | 11.31 ± 0.21 | 1.5 ± 0.0 | 61.8 ± 6.0 | 366 ± 1 | 790 ± 6 | 835 ± 9 |
| Astronaute | 7.94 ± 0.29 | 6.3 ± 0.3 | 52.4 ± 0.9 | 381 ± 7 | 681 ± 23 | 858 ± 23 |
| Croft | 14.66 ± 1.35 | 0.0 ± 0.0 | 43.6 ± 5.1 | 355 ± 0 | 790 ± 24 | 861 ± 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Arteaga, V.; Kraus, S.; Schott, M.; Muranyi, I.; Schweiggert-Weisz, U.; Eisner, P. Screening of Twelve Pea (Pisum sativum L.) Cultivars and Their Isolates Focusing on the Protein Characterization, Functionality, and Sensory Profiles. Foods 2021, 10, 758. https://doi.org/10.3390/foods10040758
García Arteaga V, Kraus S, Schott M, Muranyi I, Schweiggert-Weisz U, Eisner P. Screening of Twelve Pea (Pisum sativum L.) Cultivars and Their Isolates Focusing on the Protein Characterization, Functionality, and Sensory Profiles. Foods. 2021; 10(4):758. https://doi.org/10.3390/foods10040758
Chicago/Turabian StyleGarcía Arteaga, Verónica, Sonja Kraus, Michael Schott, Isabel Muranyi, Ute Schweiggert-Weisz, and Peter Eisner. 2021. "Screening of Twelve Pea (Pisum sativum L.) Cultivars and Their Isolates Focusing on the Protein Characterization, Functionality, and Sensory Profiles" Foods 10, no. 4: 758. https://doi.org/10.3390/foods10040758
APA StyleGarcía Arteaga, V., Kraus, S., Schott, M., Muranyi, I., Schweiggert-Weisz, U., & Eisner, P. (2021). Screening of Twelve Pea (Pisum sativum L.) Cultivars and Their Isolates Focusing on the Protein Characterization, Functionality, and Sensory Profiles. Foods, 10(4), 758. https://doi.org/10.3390/foods10040758







