Mayten Tree Seed Oil: Nutritional Value Evaluation According to Antioxidant Capacity and Bioactive Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Materials and Seed Oil Extraction
2.2. Physicochemical Analysis
2.3. Fatty Acid Profile
2.4. Lipidic Indices
2.5. Extraction of Antioxidant Compounds
2.6. Quantification of Phenols and Flavonoids, and Determination of Antioxidant Capacity
2.7. Extraction and Quantification of Carotenoids and β-Carotene
2.8. Statistical Analysis
3. Results and Discussion
3.1. Seed Oil Extraction
3.2. Physicochemical Characteristics
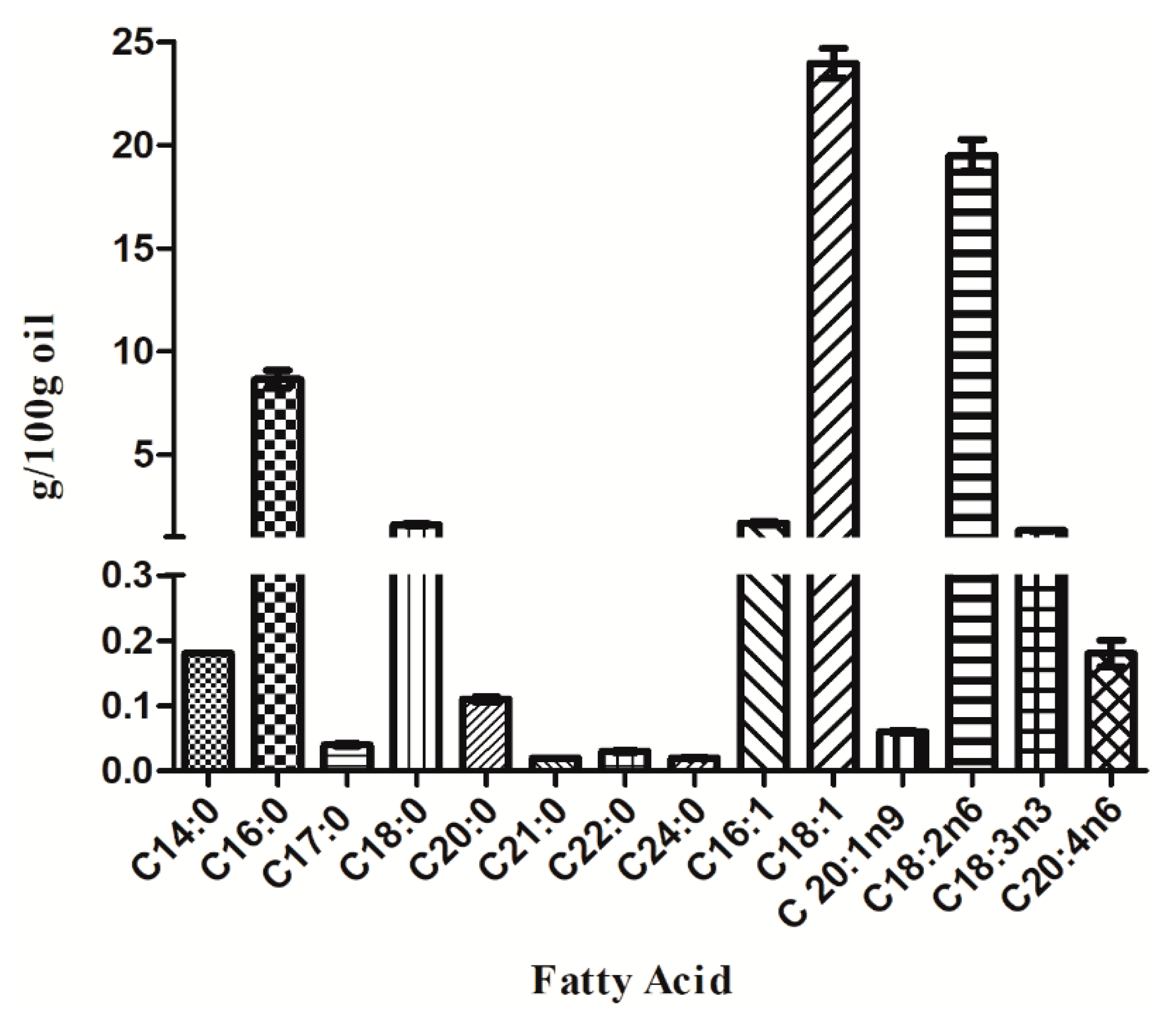
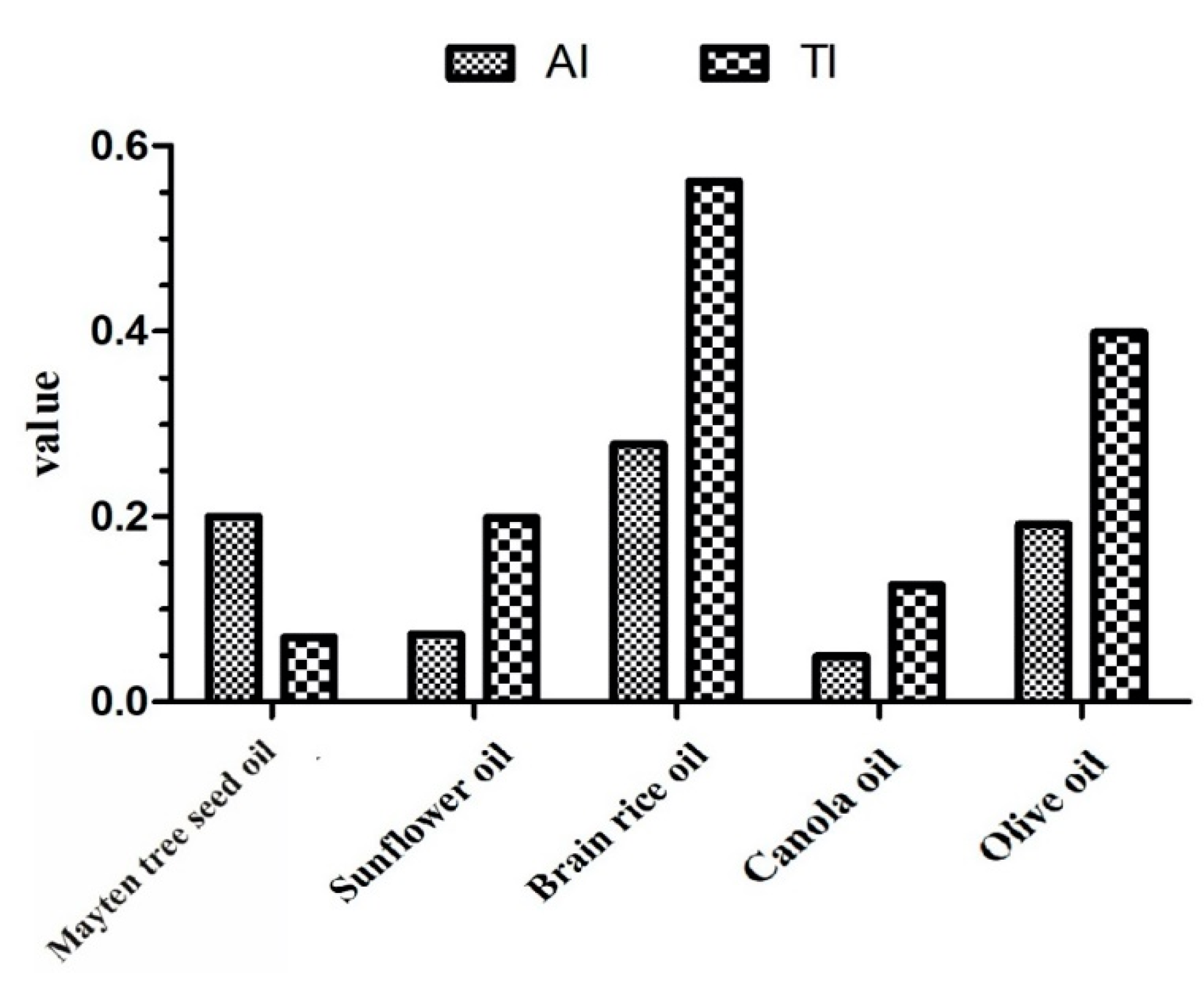
3.3. Fatty Acid Profile and Nutritional Quality
3.4. Polyphenol and Flavonoid Compounds
3.5. Total Carotenoids and β-Carotene
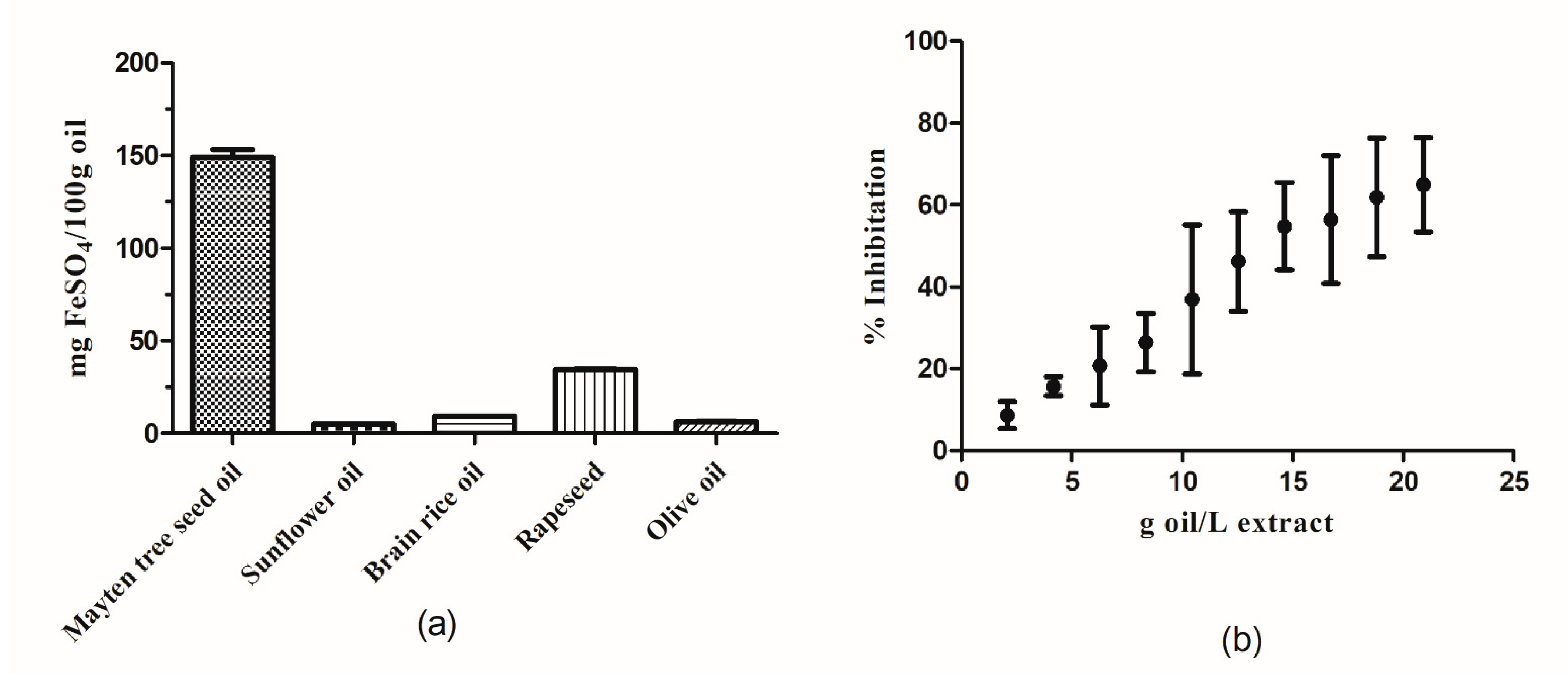
3.6. Antioxidant Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexandros, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision ESA Working Papers; FAO Agricultural Development Economics Division: Rome, Italy, 2012; Volume 54. [Google Scholar] [CrossRef]
- Fine, F.; Lucas, J.L.; Chardigny, J.M.; Redlingshöfer, B.; Renard, M. Food losses and waste in the French oilcrops sector. OCL 2015, 22, A302. [Google Scholar] [CrossRef] [Green Version]
- Varnham, A. Seed Oil: Biological Properties, Health Benefits and Commercial Applications; Nova Science Publishers: New York, NY, USA, 2014; ISBN 9781634630955. [Google Scholar]
- McDonald, M.B.; Copeland, L.O. Seed Production; Springer: Boston, MA, USA, 1997; ISBN 978-1-4613-6825-0. [Google Scholar]
- Huang, A.H.C. Oleosins and oil bodies in seeds and other organs. Plant Physiol. 1996, 110, 1055–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, S.; Gandhi, N.; Tyagi, S.K.; Kaur, A.; Mahajan, B.V.C. Extraction and characterization of guava seed oil: A novel industrial byproduct. LWT 2020, 132, 109882. [Google Scholar] [CrossRef]
- Naderi, M.; Torbati, M.; Azadmard-Damirchi, S.; Asnaashari, S.; Savage, G.P. Common ash (Fraxinus excelsior L.) seeds as a new vegetable oil source. LWT 2020, 131, 109811. [Google Scholar] [CrossRef]
- Marcora, P.I.; Tecco, P.A.; Zeballos, S.R.; Hensen, I. Influence of altitude on local adaptation in upland tree species from central Argentina. Plant Biol. 2017, 19, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.R.; Matthei, S.; Quezada, M. Flora Arbórea De Chile; Biblioteca de Recursos Renovables y no Renovables de Chile; Editorial de la Universidad de Concepción: Concepción, Chile, 1983. [Google Scholar]
- Wendler, J.; Donoso, C. Antecedentes morfológicos y genecológicos de Maytenus boaria. Bosque 1985, 6, 93–99. [Google Scholar]
- Mackenna, B.V. Historia crítica y social de la ciudad de Santiago desde su fundación hasta nuestros días (1541–1868). Impr. Mercur. 1869, 2, 1541–1868. [Google Scholar]
- Boletín de las leyes y de las órdenes y decretos del gobierno. Imprenta Mercur. 1854, 1853, 132–147.
- Memorias, Anales de la Universidad de Chile. Available online: https://anales.uchile.cl/index.php/ANUC/article/view/1815 (accessed on 1 November 2020).
- Tinto, J. Utilización de los recursos forestales argentinos, Instituto Forestal Nacional. Buenos Aires. Folleto Técnico For. 1979, 41, 97. [Google Scholar]
- Facciola, S. Cornucopia II. A Source Book of Edible Plants; Kompong Publications: Vista, CA, USA, 1998. [Google Scholar]
- Montenegro, G.; Timmermann, B. Chile, Nuestra Flora Útil: Guía de Plantas de Uso Apícola, en Medicina Folklórica, Artesanal y Ornamental; Ediciones Pontificia Universidad Católica de Chile: Santiago, Chile, 2000; ISBN 9562884945. [Google Scholar]
- Spivey, A.C.; Weston, M.; Woodhead, S. Celastraceae sesquiterpenoids: Biological activity and synthesis. Chem. Soc. Rev. 2002, 31, 43–59. [Google Scholar] [CrossRef]
- Gullo, F.P.; Sardi, J.C.O.; Santos, V.A.F.F.M.; Sangalli-Leite, F.; Pitangui, N.S.; Rossi, S.A.; de Paula Silva, A.C.A.; Soares, L.A.; Silva, J.F.; Oliveira, H.C.; et al. Antifungal Activity of Maytenin and Pristimerin. Evid.-Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Céspedes, C.; Alarcón, J. Biopesticidas de origen botánico, fitoquímicos y extractos de Celastraceae, Rhamnaceae y Scrophulariaceae. Lat. Am. Caribb. Bull. Med. Aromat. Plants 2011, 10, 175–181. [Google Scholar]
- Hidalgo, D. Actividad Nematicida Sobre Meloidogynehapla de Extractos Acuosos de Especies Arbóreas y Arbustivas de la Zona sur de Chile; Universidad Austral de Chile: Valdivia, Chile, 2008. [Google Scholar]
- Zavala, H.A.; Hormazabal, U.E.; Montenegro, R.G.; Rosalez, V.M.; Quiroz, C.A.; Paz, R.C.; Rebolledo, R.R. Effects of extracts from Maytenus on Aegorhinus superciliosus (coleoptera: Curculionidae) and Hippodamia convergens (coleoptera: Coccinellidae). Rev. Colomb. Entomol. 2017, 43, 233–244. [Google Scholar] [CrossRef]
- Cabello, A.; Camelio, M. Germinación de semillas de maitén (Maytenus boaria) y producción de plantas en vivero. Universidad de Chile. Rev. Ciencias For. 1996, 11, 3–17. [Google Scholar]
- Bastías-Montes, J.M.; Monterrosa, K.; Muñoz-Fariña, O.; García, O.; Acuña-Nelson, S.M.; Vidal-San Martín, C.; Quevedo-Leon, R.; Kubo, I.; Avila-Acevedo, J.G.; Domiguez-Lopez, M.; et al. Chemoprotective and antiobesity effects of tocols from seed oil of Maqui-berry: Their antioxidative and digestive enzyme inhibition potential. Food Chem. Toxicol. 2020, 136, 111036. [Google Scholar] [CrossRef] [PubMed]
- Popa, S.; Boran, S. CIELAB and thermal properties of sesame food oil under antocyanin and UV influence. Rev. Chim. 2017, 68, 1499–1503. [Google Scholar] [CrossRef]
- Islam, M.N.; Sabur, A.; Ahmmed, R.; Hoque, M.E. Oil extraction from pine seed (Polyalthia longifolia) by solvent extraction method and its property analysis. Procedia Eng. 2015, 105, 613–618. [Google Scholar] [CrossRef] [Green Version]
- AOCS Official Methods and Recommended Practices of the AOCS; American Oil Chemist’s Society: Urbana, IL, USA, 2009; ISBN 978-1-893997-74-5.
- Petropoulos, S.A.; Fernandes, Â.; Arampatzis, D.A.; Tsiropoulos, N.G.; Petrović, J.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Seed oil and seed oil byproducts of common purslane (Portulaca oleracea L.): A new insight to plant-based sources rich in omega-3 fatty acids. LWT 2020, 123. [Google Scholar] [CrossRef]
- Otero, P.; Gutierrez-Docio, A.; Navarro del Hierro, J.; Reglero, G.; Martin, D. Extracts from the edible insects Acheta domesticus and Tenebrio molitor with improved fatty acid profile due to ultrasound assisted or pressurized liquid extraction. Food Chem. 2020, 314, 126200. [Google Scholar] [CrossRef]
- Basdagianni, Z.; Papaloukas, L.; Kyriakou, G.; Karaiskou, C.; Parissi, Z.; Sinapis, E.; Kasapidou, E. A comparative study of the fatty acid and terpene profiles of ovine and caprine milk from Greek mountain sheep breeds and a local goat breed raised under a semi-extensive production system. Food Chem. 2019, 278, 625–629. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT Food Sci. Technol. 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Velásquez, P.; Montenegro, G.; Giordano, A.; Retamal, M.; Valenzuela, L.M.; Montenegro, G.; Giordano, A.; Retamal, M.; Valenzuela, L.M. Bioactivities of phenolic blend extracts from Chilean honey and bee pollen. CyTA J. Food 2019, 17, 754–762. [Google Scholar] [CrossRef] [Green Version]
- Giordano, A.; Fuentes-Barros, G.; Castro-Saavedra, S.; González-Cooper, A.; Suárez-Rozas, C.; Salas-Norambuena, J.; Acevedo-Fuentes, W.; Leyton, F.; Tirapegui, C.; Echeverría, J.; et al. Variation of Secondary Metabolites in the Aerial Biomass of Cryptocarya alba. Nat. Prod. Commun. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Diniyah, N.; Alam, M.B.; Lee, S.-H. Antioxidant potential of non-oil seed legumes of Indonesian’s ethnobotanical extracts. Arab. J. Chem. 2020. [Google Scholar] [CrossRef]
- Varzakas, T.; Kiokias, S. HPLC Analysis and Determination of Carotenoid Pigments in Commercially Available Plant Extracts. Curr. Res. Nutr. Food Sci. 2016, 4, 1–14. [Google Scholar] [CrossRef]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 Spectrophotometric Methods for Carotenoid Determination in Frequently Consumed Fruits and Vegetables. J. Food Sci. 2010, 75, C55–C61. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.B. Lipids Basics: Fats and Oils in Foods and Health. In Culinary Nutrition; Elsevier: Amsterdam, The Netherland, 2013; pp. 231–277. [Google Scholar] [CrossRef]
- de Almeida, D.T.; Curvelo, F.M.; Costa, M.M.; Viana, T.V.; de Lima, P.C. Oxidative stability of crude palm oil after deep frying akara (Fried bean paste). Food Sci. Technol. 2018, 38, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Mba, O.I.; Dumont, M.J.; Ngadi, M. Palm oil: Processing, characterization and utilization in the food industry—A review. Food Biosci. 2015, 10, 26–41. [Google Scholar] [CrossRef]
- Tiefenbacher, K.F.; Tiefenbacher, K.F. Technology of Main Ingredients—Sweeteners and Lipids. Wafer Waffle 2017, 123–225. [Google Scholar] [CrossRef]
- Berasategi, I.; Barriuso, B.; Ansorena, D.; Astiasarán, I. Stability of avocado oil during heating: Comparative study to olive oil. Food Chem. 2012, 132, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.T.; Ansolin, M.; Batista, E.A.C.; Meirelles, A.J.A.; Tubino, M. Identification of extra virgin olive oils modified by the addition of soybean oil, using ion chromatography. J. Braz. Chem. Soc. 2019, 30, 1055–1062. [Google Scholar] [CrossRef]
- Méndez-Zúñiga, S.M.; Corrales-García, J.E.; Gutiérrez-Grijalva, E.P.; García-Mateos, R.; Pérez-Rubio, V.; Heredia, J.B. Fatty Acid Profile, Total Carotenoids, and Free Radical-Scavenging from the Lipophilic Fractions of 12 Native Mexican Avocado Accessions. Plant Foods Hum. Nutr. 2019, 501–507. [Google Scholar] [CrossRef]
- Corrales-García, J.E.; del Rosario García-Mateos, M.; Martínez-López, E.; Barrientos-Priego, A.F.; Ybarra-Moncada, M.C.; Ibarra-Estrada, E.; Méndez-Zúñiga, S.M.; Becerra-Morales, D. Anthocyanin and Oil Contents, Fatty Acids Profiles and Antioxidant Activity of Mexican Landrace Avocado Fruits. Plant Foods Hum. Nutr. 2019, 74, 210–215. [Google Scholar] [CrossRef]
- Tao, L. Oxidation of Polyunsaturated Fatty Acids and its Impact on Food Quality and Human Health Protective potential of dietary soy View project Enrichment of omega-3 fatty acids by microalgae View project. Adv. Food Technol. Nutr. Sci. Open J. 2015, 1, 135–142. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Chiu, S.; Bergeron, N.; Krauss, R.M. Saturated Fats Versus Polyunsaturated Fats Versus Carbohydrates for Cardiovascular Disease Prevention and Treatment. Annu. Rev. Nutr. 2015, 35, 517–543. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-G.; Won, S.-R.; Rhee, H.-I. Oleic Acid and Inhibition of Glucosyltransferase. Olives Olive Oil Health Dis. Prev. 2010, 1375–1383. [Google Scholar] [CrossRef]
- Zamani, S.; Naderi, M.R.; Soleymani, A.; Nasiri, B.M. Sunflower (Helianthus annuus L.) biochemical properties and seed components affected by potassium fertilization under drought conditions. Ecotoxicol. Environ. Saf. 2020, 190, 110017. [Google Scholar] [CrossRef]
- Zanqui, A.B.; da Silva, C.M.; Ressutte, J.B.; de Morais, D.R.; Santos, J.M.; Eberlin, M.N.; Cardozo-Filho, L.; da Silva, E.A.; Gomes, S.T.M.; Matsushita, M. Extraction and assessment of oil and bioactive compounds from cashew nut (Anacardium occidentale) using pressurized n-propane and ethanol as cosolvent. J. Supercrit. Fluids 2020, 157, 104686. [Google Scholar] [CrossRef]
- Sanders, T.A.B. Introduction: The Role of Fats in Human Diet. The Role of Fats in Human Diet; Elsevier Inc.: Amsterdam, The Netherland, 2016. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Confortin, T.C.; Todero, I.; Luft, L.; Ugalde, G.A.; Mazutti, M.A.; Oliveira, Z.B.; Bottega, E.L.; Knies, A.E.; Zabot, G.L.; Tres, M.V. Oil yields, protein contents, and cost of manufacturing of oil obtained from different hybrids and sowing dates of canola. J. Environ. Chem. Eng. 2019, 7, 102972. [Google Scholar] [CrossRef]
- Ghaeni, M.; Ghahfarokhi, K.N. Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices in Leiognathusbindus and Upeneussulphureus. J. Mar. Sci. Res. Dev. 2013, 3, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Ghazani, S.M.; García-Llatas, G.; Marangoni, A.G. Micronutrient content of cold-pressed, hot-pressed, solvent extracted and RBD canola oil: Implications for nutrition and quality. Eur. J. Lipid Sci. Technol. 2014, 116, 380–387. [Google Scholar] [CrossRef]
- Pérez, M.M.; Yebra, A.; Melgosa, M.; Bououd, N.; Asselman, A.; Boucetta, A. Caracterización colorimétrica y clasificación del aceite de oliva virgen de la cuenca mediterránea hispano-marroquí. Grasas Aceites 2003, 54, 392–396. [Google Scholar]
- Xuan, T.D.; Ngoc, Q.T.; Khanh, T.D. An Overview of Chemical Profiles, Antioxidant and Edible Oils Marketed in Japan. Foods 2018, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic Potential through Its Antioxidant Property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maalik, A.; Khan, F.A.; Mumtaz, A.; Mehmood, A.; Azhar, S.; Atif, M.; Karim, S.; Altaf, Y.; Tariq, I. Pharmacological applications of quercetin and its derivatives: A short review. Trop. J. Pharm. Res. 2014, 13, 1561–1566. [Google Scholar] [CrossRef]
- Bardaa, S.; Halima, N.B.; Aloui, F.; Mansour, R.B.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Othman, N.; Manan, Z.A.; Alwi, S.W.; Sarmidi, M. A review of extraction technology of carotenoids. J. Appl. Sci. 2010, 10, 1187–1191. [Google Scholar] [CrossRef] [Green Version]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Sandmann, G. Antioxidant protection from UV-and light-stress related to carotenoid structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef] [Green Version]
- Hasheminya, S.-M.; Dehghannya, J. Composition, phenolic content, antioxidant and antimicrobial activity of Pistacia atlantica subsp. kurdica hulls’ essential oil. Food Biosci. 2020, 34, 100510. [Google Scholar] [CrossRef]
- Mueller, L.; Boehm, V. Antioxidant Activity of β-Carotene Compounds in Different In Vitro Assays. Molecules 2011, 16, 1055–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Caraballo, M.; Agarwal, A. Total Antioxidant Capacity Measurement by Colorimetric Assay. In Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction; Elsevier: Amsterdam, The Netherland, 2019; pp. 207–215. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szłyk, E. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta 2008, 76, 899–905. [Google Scholar] [CrossRef] [PubMed]
| Analysis | Value | |
|---|---|---|
| Color | ||
| L | 53.94 ± 7.66 * | |
| a | 24.92 ± 8.20 * | |
| b | 41.02 ± 5.15 * | |
| C° | 48.16 ± 8.34 * | |
| H° | 59.40 ± 6.23 * | |
| Density | 1.06 ± 0.07 (g/mL) | |
| Peroxide value | 5.10 ± 0.18 (meq O2/kg oil) | |
| Free Acidity | 3.89 ± 0.19 (% oleic acid) | |
| TBARS | 5.74 ± 0.21(nmol/g lipids) | |
| Rancimat | 52.15 ± 2.15 (h) | |
| Iodine value | 57.63 ± 2.00 * |
| Value | |
|---|---|
| SFA | 10.66 (g/100 g oil) |
| MUFA | 25.70 (g/100 g oil) |
| PUFA | 20.98 (g/100 g oil) |
| n-6 | 19.66 (g/100 g oil) |
| n-3 | 1.32 (g/100 g oil) |
| PUFA/SFA | 1.97 |
| n-6/n-3 | 12.62 |
| AI | 0.20 |
| TI | 0.07 |
| Parameter | Value |
|---|---|
| Total Phenolic content | 16.1 ± 4.3 (mg GAE/100 g oil) |
| Total Flavonoids content | 15.5 ± 2.3 (mg QE/100 g oil) |
| Total Carotenoids content | 153.2 ± 6.5 (mg β-carotene E/100 g oil) |
| β-carotene content | 106.8 ± 40.2 (mg β-carotene/100 g oil) |
| Coumaric acid | 5.47 ± 0.03 (µg/100 g oil) |
| Quercetin | 3.21 ± 0.05 (µg/100 g oil) |
| Myricetin | 7.74 ± 0.04(µg/100 g oil) |
| Ferulic acid | 49.64 ± 0.03 (µg/100 g oil) |
| Properties | Value |
|---|---|
| Antioxidant Capacity-ABTS | 1.25 ± 0.45 (mg TE/100 g of oil) |
| IC50 | 1.06 ± 0.03 (g oil) |
| FRAP | 149 ± 7.45 (mg FeSO4/100 g of oil) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginocchio, R.; Muñoz-Carvajal, E.; Velásquez, P.; Giordano, A.; Montenegro, G.; Colque-Perez, G.; Sáez-Navarrete, C. Mayten Tree Seed Oil: Nutritional Value Evaluation According to Antioxidant Capacity and Bioactive Properties. Foods 2021, 10, 729. https://doi.org/10.3390/foods10040729
Ginocchio R, Muñoz-Carvajal E, Velásquez P, Giordano A, Montenegro G, Colque-Perez G, Sáez-Navarrete C. Mayten Tree Seed Oil: Nutritional Value Evaluation According to Antioxidant Capacity and Bioactive Properties. Foods. 2021; 10(4):729. https://doi.org/10.3390/foods10040729
Chicago/Turabian StyleGinocchio, Rosanna, Eduardo Muñoz-Carvajal, Patricia Velásquez, Ady Giordano, Gloria Montenegro, Germán Colque-Perez, and César Sáez-Navarrete. 2021. "Mayten Tree Seed Oil: Nutritional Value Evaluation According to Antioxidant Capacity and Bioactive Properties" Foods 10, no. 4: 729. https://doi.org/10.3390/foods10040729
APA StyleGinocchio, R., Muñoz-Carvajal, E., Velásquez, P., Giordano, A., Montenegro, G., Colque-Perez, G., & Sáez-Navarrete, C. (2021). Mayten Tree Seed Oil: Nutritional Value Evaluation According to Antioxidant Capacity and Bioactive Properties. Foods, 10(4), 729. https://doi.org/10.3390/foods10040729






