Influence of Different Prebiotics on Viability of Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus Encapsulated in Alginate Microcapsules
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Probiotic Microorganisms
2.2. Prebiotic Activity Testing/Prebiotic Consumption
2.3. Encapsulation Process
2.4. Cell Viability
2.4.1. Cell Viability in Microcapsules
2.4.2. Efficiency of the Microencapsulation Process (E%) and Viability Rate (R%)
2.4.3. Viability of Encapsulated Bacteria after Enapsulation and during Storage
2.4.4. Cell Viability in Simulated Gastro-Intestinal Juices
Survival of Microencapsulated Cells in Simulated Gastric Juice
Survival of Microencapsulated Cells in Intestinal Juice
- (a)
- fresh microcapsules;
- (b)
- fresh microcapsules that have been subjected to gastric acidity for 1 h. After that, the gastric juice was aspirated and intestinal juice was added;
- (c)
- microcapsules that have been subjected to storage time actions at 4 °C;
- (d)
- microcapsules that have been subjected to both the action of storage time at 4 °C and gastric juice.
2.5. Characterization of Capsules
- -
- microcapsules subjected to acidity for one hour in gastric juice;
- -
- microcapsules subjected to intestinal juice for one hour;
- -
- microcapsules initially subjected to one hour in gastric juice and then for one hour in intestinal juice.
2.6. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of the Efficiency of Prebiotic Activity
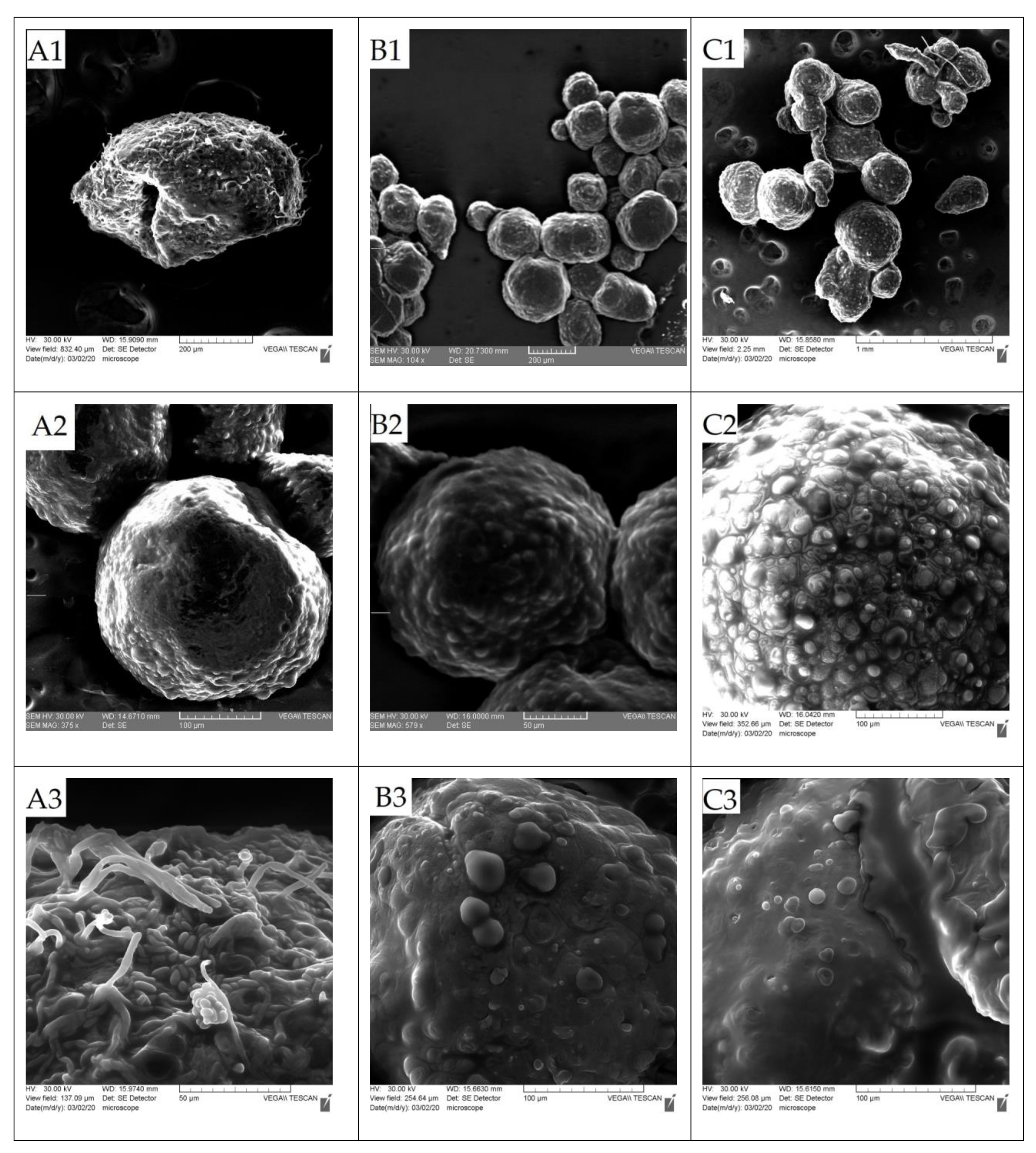
3.2. Characterization of Microcapsules
3.2.1. Evaluation of the Average Diameter and Size Distribution of the Microparticles
3.2.2. Number of Viable Cells. The Efficiency of Microencapsulation
3.3. Viability of Encapsulated Probiotics during Storage
3.4. Cell Viability in Simulated Gastrointestinal Conditions
- -
- a wide variety of enzymes used that differ in their activity and characterization.
- -
- the enzymes used come either from different sources of animal origin such as rabbits, pigs, or even of human origin.
- -
- differences in pH, mineral type, ionic strength, presence or absence of phospholipids, gastric lipase, pancreatin were found.
- -
- the products are subjected to the action of digestive juices for different periods, and different durations of enzymatic activity can significantly change the results [26].
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pirbaglou, M.; Katz, J.; de Souza, R.J.; Stearns, J.C.; Motamed, M.; Ritvo, P. Probiotic supplementation can positively affect anxiety and depressive symptoms: A systematic review of randomized controlled trials. Nutr. Res. 2016, 36, 889–898. [Google Scholar] [CrossRef]
- Avershina, E.; Cabrera Rubio, R.; Lundgård, K.; Perez Martinez, G.; Collado, M.C.; Storrø, O.; Øien, T.; Dotterud, C.K.; Johnsen, R.; Rudi, K. Effect of probiotics in prevention of atopic dermatitis is dependent on the intrinsic microbiota at early infancy. J. Allergy Clin. Immunol. 2017, 139, 1399–1402.e8. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Miles, C.; Tierney, A.C. Effect of probiotics on respiratory, gastrointestinal and nutritional outcomes in patients with cystic fibrosis: A systematic review. J. Cyst. Fibros. 2017, 16, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Capurso, L. FAO/WHO Guidelines on Probiotics. J. Clin. Gastroenterol. 2012, 46. [Google Scholar] [CrossRef] [PubMed]
- Ningtyas, D.W.; Bhandari, B.; Bansal, N.; Prakash, S. The viability of probiotic Lactobacillus rhamnosus (non-encapsulated and encapsulated) in functional reduced-fat cream cheese and its textural properties during storage. Food Control 2019, 100, 8–16. [Google Scholar] [CrossRef]
- Ester, B.; Noelia, B.; Laura, C.J.; Francesca, P.; Cristina, B.; Rosalba, L.; Marco, D.R. Probiotic survival and in vitro digestion of L. salivarius spp. salivarius encapsulated by high homogenization pressures and incorporated into a fruit matrix. LWT 2019, 111, 883–888. [Google Scholar] [CrossRef]
- Chandramouli, V.; Kailasapathy, K.; Peiris, P.; Jones, M. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J. Microbiol. Methods 2004, 56, 27–35. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Frutos, M.J. Effect of different types of encapsulation on the survival of Lactobacillus plantarum during storage with inulin and in vitro digestion. LWT Food Sci. Technol. 2015, 64, 824–828. [Google Scholar] [CrossRef]
- Rather, S.A.; Akhter, R.; Masoodi, F.A.; Gani, A.; Wani, S.M. Effect of double alginate microencapsulation on in vitro digestibility and thermal tolerance of Lactobacillus plantarum NCDC201 and L. casei NCDC297. LWT Food Sci. Technol. 2017, 83, 50–58. [Google Scholar] [CrossRef]
- De Prisco, A.; Mauriello, G. Probiotication of foods: A focus on microencapsulation tool. Trends Food Sci. Technol. 2016, 48, 27–39. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Cąbuk, B.; Harsa, S.T. Improved viability of Lactobacillus acidophilus NRRL-B 4495 during freeze-drying in whey protein-pullulan microcapsules. J. Microencapsul. 2015, 32, 300–307. [Google Scholar] [CrossRef][Green Version]
- De Araújo Etchepare, M.; Raddatz, G.C.; de Moraes Flores, É.M.; Zepka, L.Q.; Jacob-Lopes, E.; Barin, J.S.; Ferreira Grosso, C.R.; de Menezes, C.R. Effect of resistant starch and chitosan on survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT Food Sci. Technol. 2016, 65, 511–517. [Google Scholar] [CrossRef]
- Singh, P.; Medronho, B.; Alves, L.; da Silva, G.J.; Miguel, M.G.; Lindman, B. Development of carboxymethyl cellulose-chitosan hybrid micro- and macroparticles for encapsulation of probiotic bacteria. Carbohydr. Polym. 2017, 175, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H.C. Survival of probiotics encapsulated in chitosan-coated alginate beads in yoghurt from UHT- and conventionally treated milk during storage. LWT Food Sci. Technol. 2006, 39, 177–183. [Google Scholar] [CrossRef]
- Mortazavian, A.M.; Ehsani, M.R.; Sohrabvandi, S.; Reinheimer, J.A. MRS-bile agar: Its suitability for the enumeration of mixed probiotic cultures in cultured dairy products. Milchwissenschaft 2007, 62, 270–272. [Google Scholar]
- Chen, K.N.; Chen, M.J.; Liu, J.E.R.; Lin, C.W.; Chiu, H.Y.I. Optimization of incorporated prebiotics as coating materials for probiotic microencapsulation. J. Food Sci. 2005, 70, M260–M266. [Google Scholar] [CrossRef]
- Rizzardini, G.; Eskesen, D.; Calder, P.C.; Capetti, A.; Jespersen, L.; Clerici, M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2012, 107, 876–884. [Google Scholar] [CrossRef]
- Capurso, L. Thirty Years of Lactobacillus rhamnosus GG: A Review. J. Clin. Gastroenterol. 2019, 53 (Suppl. S1), S1–S41. [Google Scholar] [CrossRef] [PubMed]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and Its Probiotic and Food Potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Luca, L.; Oroian, M. The effect of microencapsulation and potato strarch on the survival of Lactobacillus strains. Food Environ. Saf. J. 2020, 19, 139–147. [Google Scholar]
- Darjani, P.; Hosseini Nezhad, M.; Kadkhodaee, R.; Milani, E. Influence of prebiotic and coating materials on morphology and survival of a probiotic strain of Lactobacillus casei exposed to simulated gastrointestinal conditions. LWT Food Sci. Technol. 2016, 73, 162–167. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Peredo, A.G.; Beristain, C.I.; Pascual, L.A.; Azuara, E.; Jimenez, M. The effect of prebiotics on the viability of encapsulated probiotic bacteria. LWT Food Sci. Technol. 2016, 73, 191–196. [Google Scholar] [CrossRef]
- Martin, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Effect of unmodified starch on viability of alginate-encapsulated Lactobacillus fermentum CECT5716. LWT Food Sci. Technol. 2013, 53, 480–486. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- De Farias, T.G.S.; Ladislau, H.F.L.; Stamford, T.C.M.; Medeiros, J.A.C.; Soares, B.L.M.; Stamford Arnaud, T.M.; Stamford, T.L.M. Viabilities of Lactobacillus rhamnosus ASCC 290 and Lactobacillus casei ATCC 334 (in free form or encapsulated with calcium alginate-chitosan) in yellow mombin ice cream. LWT 2019, 100, 391–396. [Google Scholar] [CrossRef]
- Ashwar, B.A.; Gani, A.; Gani, A.; Shah, A.; Masoodi, F.A. Production of RS4 from rice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem. 2018, 239, 287–294. [Google Scholar] [CrossRef]
- Dupuis, J.H.; Liu, Q. Potato Starch: A Review of Physicochemical, Functional and Nutritional Properties. Am. J. Potato Res. 2019, 96, 127–138. [Google Scholar] [CrossRef]
- Bertoft, E.; Blennow, A. Chapter 4—Structure of Potato Starch BT—Advances in Potato Chemistry and Technology. In Advances in Potato Chemistry and Technology; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Yang, L.; Xia, Y.; Junejo, S.A.; Zhou, Y. Composition, structure and physicochemical properties of three coloured potato starches. Int. J. Food Sci. Technol. 2018, 53, 2325–2334. [Google Scholar] [CrossRef]
- Delgado, P.; Bañón, S. Effects of replacing starch by inulin on the physicochemical, texture and sensory characteristics of gummy jellies. CyTA J. Food 2018, 16. [Google Scholar] [CrossRef]
- Takagi, R.; Tsujikawa, Y.; Nomoto, R.; Osawa, R. Comparison of the Growth of Lactobacillus delbrueckii, L. paracasei and L. plantarum on Inulin in Co-culture Systems. Biosci. Microbiota Food Health 2014, 33. [Google Scholar] [CrossRef]
- Ni, D.; Xu, W.; Zhu, Y.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Inulin and its enzymatic production by inulosucrase: Characteristics, structural features, molecular modifications and applications. Biotechnol. Adv. 2019, 37, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G.; Follador, R. Metabolism of oligosaccharides and starch in lactobacilli: A review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef]
- Humblot, C.; Turpin, W.; Chevalier, F.; Picq, C.; Rochette, I.; Guyot, J.P. Determination of expression and activity of genes involved in starch metabolism in Lactobacillus plantarum A6 during fermentation of a cereal-based gruel. Int. J. Food Microbiol. 2014, 185, 103–111. [Google Scholar] [CrossRef]
- Pimentel-González, D.J.; Campos-Montiel, R.G.; Lobato-Calleros, C.; Pedroza-Islas, R.; Vernon-Carter, E.J. Encapsulation of Lactobacillus rhamnosus in double emulsions formulated with sweet whey as emulsifier and survival in simulated gastrointestinal conditions. Food Res. Int. 2009, 42, 292–297. [Google Scholar] [CrossRef]
- De Souza Oliveira, R.P.; Perego, P.; de Oliveira, M.N.; Converti, A. Effect of inulin on the growth and metabolism of a probiotic strain of Lactobacillus rhamnosus in co-culture with Streptococcus thermophilus. LWT Food Sci. Technol. 2012, 47, 358–363. [Google Scholar] [CrossRef]
- Sousa, A.G.; Nielsen, H.L.; Armagan, I.; Larsen, J.; Sørensen, S.O. The impact of rhamnogalacturonan-I side chain monosaccharides on the rheological properties of citrus pectin. Food Hydrocoll. 2015, 47, 130–139. [Google Scholar] [CrossRef]
- Fareez, I.M.; Lim, S.M.; Mishra, R.K.; Ramasamy, K. Chitosan coated alginate-xanthan gum bead enhanced pH and thermotolerance of Lactobacillus plantarum LAB12. Int. J. Biol. Macromol. 2015, 72, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Zanjani, M.A.; Tarzi, B.G.; Sharifan, A.; Mohammadi, N.; Bakhoda, H.; Madanipour, M.M. Microencapsulation of Lactobacillus casei with calcium alginate-resistant starch and evaluation of survival and sensory properties in cream-filled cake. Afr. J. Microbiol. Res. 2012, 6, 5511–5517. [Google Scholar] [CrossRef]
- Angélica Andrade Lopes, L.; de Siqueira Ferraz Carvalho, R.; Stela Santos Magalhães, N.; Suely Madruga, M.; Julia Alves Aguiar Athayde, A.; Araújo Portela, I.; Eduardo Barão, C.; Colombo Pimentel, T.; Magnani, M.; Christina Montenegro Stamford, T. Microencapsulation of Lactobacillus acidophilus La-05 and incorporation in vegan milks: Physicochemical characteristics and survival during storage, exposure to stress conditions, and simulated gastrointestinal digestion. Food Res. Int. 2020, 135, 109295. [Google Scholar] [CrossRef]
- Khosravi Zanjani, M.A.; Tarzi, B.G.; Sharifan, A.; Mohammadi, N. Microencapsulation of probiotics by calcium alginate-gelatinized starch with chitosan coating and evaluation of survival in simulated human gastro-intestinal condition. Iran. J. Pharm. Res. 2014, 13, 843. [Google Scholar] [CrossRef]
- Mokarram, R.R.; Mortazavi, S.A.; Najafi, M.B.H.; Shahidi, F. The influence of multi stage alginate coating on survivability of potential probiotic bacteria in simulated gastric and intestinal juice. Food Res. Int. 2009, 42, 1040–1045. [Google Scholar] [CrossRef]
- Hansen, L.T.; Allan-Wojtas, P.M.; Jin, Y.L.; Paulson, A.T. Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions. Food Microbiol. 2002, 19, 35–45. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Lenton, D.; Cook, M.T.; Khutoryanskiy, V.V.; Charalampopoulos, D. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr. Polym. 2012, 90, 1281–1287. [Google Scholar] [CrossRef]
- Muthukumarasamy, P.; Allan-Wojtas, P.; Holley, R.A. Stability of Lactobacillus reuteri in different types of microcapsules. J. Food Sci. 2006, 71, M20–M24. [Google Scholar] [CrossRef]
- Smidsrød, O.; Draget, K.I. Alginate Gelation Technologies. Food Colloids; Woodhead Publishing Limited: Cambridge, UK, 2004; pp. 279–293. [Google Scholar]
- Rayment, P.; Wright, P.; Hoad, C.; Ciampi, E.; Haydock, D.; Gowland, P.; Butler, M.F. Investigation of alginate beads for gastro-intestinal functionality, Part 1: In vitro characterisation. Food Hydrocoll. 2009, 23, 816–822. [Google Scholar] [CrossRef]
- Sousa, S.; Gomes, A.M.; Pintado, M.M.; Silva, J.P.; Costa, P.; Amaral, M.H.; Duarte, A.C.; Rodrigues, D.; Rocha-Santos, T.A.P.; Freitas, A.C. Characterization of freezing effect upon stability of, probiotic loaded, calcium-alginate microparticles. Food Bioprod. Process. 2015, 93, 90–97. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [PubMed]
- Norton, I.T.; Frith, W.J.; Ablett, S. Fluid gels, mixed fluid gels and satiety. Food Hydrocoll. 2006, 20, 229–239. [Google Scholar] [CrossRef]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; del Villarán, M.C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Jantarathin, S.; Borompichaichartkul, C.; Sanguandeekul, R. Microencapsulation of probiotic and prebiotic in alginate-chitosan capsules and its effect on viability under heat process in shrimp feeding. Mater. Today Proc. 2017, 4, 6166–6172. [Google Scholar] [CrossRef]
- Raddatz, G.C.; Poletto, G.; de Deus, C.; Codevilla, C.F.; Cichoski, A.J.; Jacob-Lopes, E.; Muller, E.I.; Flores, E.M.M.; Esmerino, E.A.; de Menezes, C.R. Use of prebiotic sources to increase probiotic viability in pectin microparticles obtained by emulsification/internal gelation followed by freeze-drying. Food Res. Int. 2020, 130, 108902. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Zhao, J.; Liu, X.; Tian, F.; Zhang, H.P.; Zhang, H.; Chen, W. Microencapsulation of Bifidobacterium bifidum F-35 in reinforced alginate microspheres prepared by emulsification/internal gelation. Int. J. Food Sci. Technol. 2011, 46, 1672–1678. [Google Scholar] [CrossRef]
- Heidebach, T.; Först, P.; Kulozik, U. Influence of casein-based microencapsulation on freeze-drying and storage of probiotic cells. J. Food Eng. 2010, 98, 309–316. [Google Scholar] [CrossRef]
- Huang, S.; Vignolles, M.L.; Chen, X.D.; Le Loir, Y.; Jan, G.; Schuck, P.; Jeantet, R. Spray drying of probiotics and other food-grade bacteria: A review. Trends Food Sci. Technol. 2017, 63, 1–17. [Google Scholar] [CrossRef]
 MRS G-culture medium made according to the original recipe, glucose culture medium,
MRS G-culture medium made according to the original recipe, glucose culture medium,  MRS WG-culture medium without carbon source, glucose-free culture medium,
MRS WG-culture medium without carbon source, glucose-free culture medium,  MRS INU-culture medium with inulin as a carbon source,
MRS INU-culture medium with inulin as a carbon source,  MRS OLI-culture medium with oligofructose as a carbon source, oligofructose culture medium,
MRS OLI-culture medium with oligofructose as a carbon source, oligofructose culture medium,  MRS STH-culture medium with potato starch as a carbon source, starch culture medium).
MRS STH-culture medium with potato starch as a carbon source, starch culture medium).
 MRS G-culture medium made according to the original recipe, glucose culture medium,
MRS G-culture medium made according to the original recipe, glucose culture medium,  MRS WG-culture medium without carbon source, glucose-free culture medium,
MRS WG-culture medium without carbon source, glucose-free culture medium,  MRS INU-culture medium with inulin as a carbon source,
MRS INU-culture medium with inulin as a carbon source,  MRS OLI-culture medium with oligofructose as a carbon source, oligofructose culture medium,
MRS OLI-culture medium with oligofructose as a carbon source, oligofructose culture medium,  MRS STH-culture medium with potato starch as a carbon source, starch culture medium).
MRS STH-culture medium with potato starch as a carbon source, starch culture medium).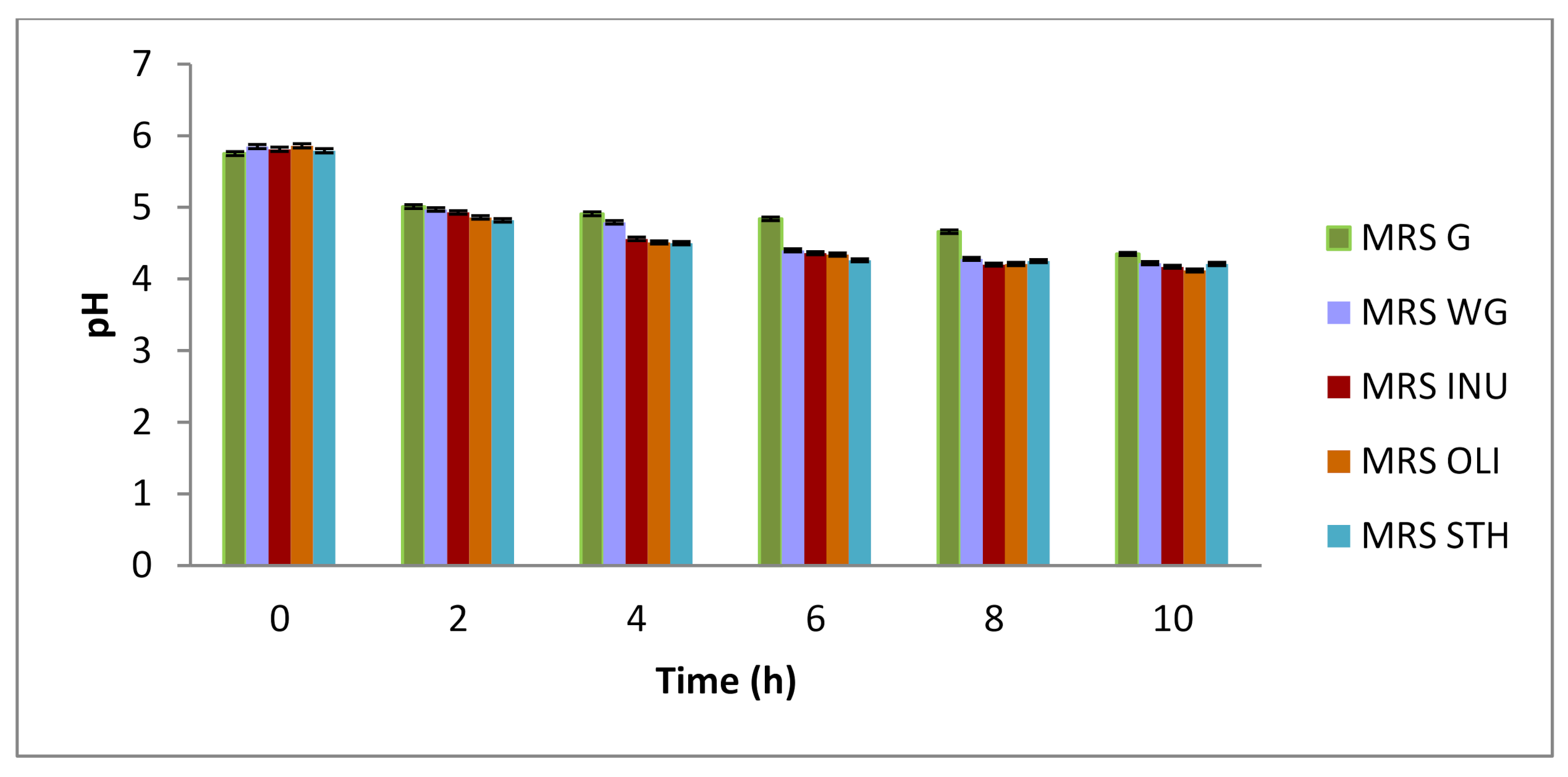



 —T0,
—T0,  —T7,
—T7,  —T14,
—T14,  —T21,
—T21,  —T30).
—T30).
 —T0,
—T0,  —T7,
—T7,  —T14,
—T14,  —T21,
—T21,  —T30).
—T30).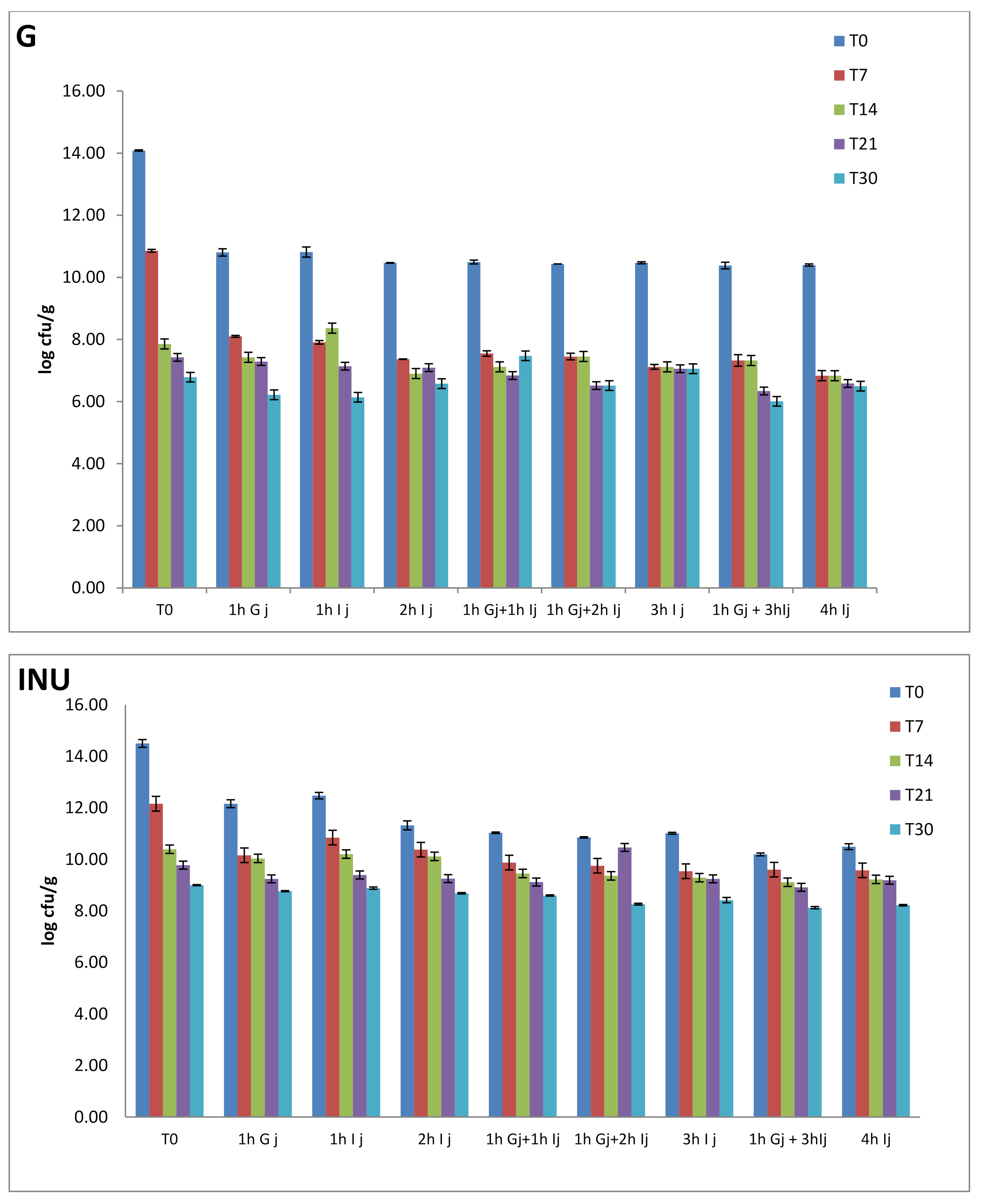

| Time (h) | MRS G | MRS WG | MRS INU | MRS OLI | MRS STH |
|---|---|---|---|---|---|
| 0 | 8.51 ± 0.01 d A | 8.53 ± 0.03 c A | 8.48 ± 0.02 a A | 8.49 ± 0.01 a A | 8.46 ± 0.02 a A |
| 2 | 8.96 ± 0.01 e D | 8.56 ± 0.01 c A | 8.75 ± 0.02 b B | 8.81 ± 0.01 b C | 8.57 ± 0.02 b A |
| 4 | 9.01 ± 0.01 e B | 8.62 ± 0.02 c A | 9.06 ± 0.01 c B | 9.17 ± 0.01 c C | 8.58 ± 0.03 b A |
| 6 | 8.32 ± 0.04 c B | 7.70 ± 0.02 b A | 9.15 ± 0.03 c C | 9.49 ± 0.01 d D | 9.48 ± 0.04 d D |
| 8 | 7.84 ± 0.02 b A | 7.61 ± 0.06 b A | 9.51 ± 0.03 d B | 9.51 ± 0.04 d B | 9.32 ± 0.04 e B |
| 10 | 7.30 ± 0.02 a B | 3.60 ± 0.01 a A | 9.64 ± 0.02 e D | 9.52 ± 0.01 d C | 9.54 ± 0.01 d C |
| log10 cfu/mL | log10 cfu/g | % | |
|---|---|---|---|
| Cell Viability in Emulsion | Cell Viability in Microcapsules | Encapsulation Efficiency | |
| INU | 16.45 ± 0.09 b | 14.50 ± 0.04 c | 88.16 ± 0.13 c |
| OLI | 16.17 ± 0.05 a | 14.26 ± 0.03 b | 88.19 ± 0.09 c |
| STH | 16.12 ± 0.08 a | 14.03 ± 0.03 a | 87.02 ± 0.12 a |
| G | 16.09 ± 0.04 a | 14.09 ± 0.02 a | 87.54 ± 0.11 b |
| Time | Glucose | Oligofructose | Starch | Inulin |
|---|---|---|---|---|
| T0–T7 | 77.06 ± 0.14 d A | 81.96 ± 1.28 c B | 84.11 ± 0.31 c C | 83.86 ± 0.82 d B C |
| T0–T14 | 55.76 ± 0.24 c A | 62.69 ± 0.18 b B | 66.43 ± 0.12 b D | 71.67 ± 0.42 c C |
| T0–T21 | 52.71 ± 0.25 b A | 59.62 ± 0.19 a B | 66.19 ± 0.12 b D | 67.42 ± 0.28 b C |
| T0–T30 | 48.17 ± 0.97 a A | 58.23 ± 0.11 a B | 63.82 ± 0.28 a D | 62.07 ± 0.20 a C |
| Storage Period | G | OLI | STH | INU |
|---|---|---|---|---|
| T0 | 14.08 ± 0.02 e A | 14.25 ± 0.02 d B | 14.03 ± 0.03 d A | 14.50 ± 0.04 e C |
| T7 | 10.85 ± 0.04 d A | 11.68 ± 0.20 c B | 11.80 ± 0.07 c B | 12.16 ± 0.15 d B |
| T14 | 7.85 ± 0.05 c A | 8.94 ± 0.04 b B | 9.32 ± 0.04 b C | 10.39 ± 0.09 c D |
| T21 | 7.42 ± 0.05 b A | 8.50 ± 0.04 a B | 9.24 ± 0.04 b C | 9.77 ± 0.07 b D |
| T30 | 6.78 ± 0.15 a A | 8.30 ± 0.00 a B | 8.98 ± 0.06 a C | 9.00 ± 0.02 a C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, L.; Oroian, M. Influence of Different Prebiotics on Viability of Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus Encapsulated in Alginate Microcapsules. Foods 2021, 10, 710. https://doi.org/10.3390/foods10040710
Luca L, Oroian M. Influence of Different Prebiotics on Viability of Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus Encapsulated in Alginate Microcapsules. Foods. 2021; 10(4):710. https://doi.org/10.3390/foods10040710
Chicago/Turabian StyleLuca, Liliana, and Mircea Oroian. 2021. "Influence of Different Prebiotics on Viability of Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus Encapsulated in Alginate Microcapsules" Foods 10, no. 4: 710. https://doi.org/10.3390/foods10040710
APA StyleLuca, L., & Oroian, M. (2021). Influence of Different Prebiotics on Viability of Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus Encapsulated in Alginate Microcapsules. Foods, 10(4), 710. https://doi.org/10.3390/foods10040710





