3.3.1. α-Amylase, Starch Damage and Water Absorption
The α-amylase activity and level of starch damage were characterised, as well as the water absorption of the WMFs (
Table 9). The highest α-amylase activity was found in amaranth WMF (0.15 ± 0.06 CU/g dm), which may have an impact on the technological properties of this flour. Kaur et al. [
8] studied the pasting behaviour of several
A. hypochondriacus and
A. caudatus lines and calculated the α-amylase activity from the difference in peak viscosity measured in the presence and absence of AgNO
3. Inactivation of α-amylase increased the peak viscosity for
A. hypochondriacus, while no difference was observed for
A. caudatus. The
A. hypochondriacus lines showed a wider and higher α-amylase activity which might explain the lower viscosity of these lines [
8]. No significant correlations with the pasting properties were observed within the present study. It is possible that the α-amylase activity was too low to have an impact on the pasting behaviour, similar to what was observed for the
A. caudatus lines in the study by Kaur et al. [
8]. However, the difference in measuring technique does not allow one to compare the activities of both studies.
The α-amylase activity ranged between 0.05 and 0.11 CU/g dm for most quinoa samples. Other studies reported an activity of 0.04 CU/g [
76] to 0.09 CU/g [
77]. QU4 and QU5 showed higher activity of 0.32 and 1.07 CU/g dm, respectively. Aluwi et al. [
78] suggested that the persisting trend of low pasting temperature, peak and final viscosity of quinoa variety Temuco was attributed to a higher α-amylase activity. Indeed, QU4 and QU5 showed a lower peak and final viscosity but this was also the case for sample QU1 with an activity of only 0.11 CU/g dm. Furthermore, no significant correlations between the pasting parameters and α-amylase activity were observed. A re-measurement of the pasting profile, in the presence of AgNO
3 to inactivate α-amylase [
8], could ensure more clarity about the impact of the high α-amylase activity in samples QU4 and QU5. However, Elgeti et al. [
76] found a high α-glucosidase activity (approximately 9 units/g) in quinoa WMF and suggested that this enzyme had a greater impact on starch hydrolysis.
The lowest α-amylase activity was measured in buckwheat WMF and ranged between 0.03 and 0.10 CU/g dm. Phiarais et al. [
79] reported an activity of 0.1 CU/g.
The dry-milling technique used in the WMF production caused physically damaged some of the starch granules [
5]. The level of starch damage varied between 3.10 and 3.95% dm for amaranth (
Table 9). This is considerably lower compared to the findings of Srichuwong et al. (11.7% dm) [
5]. Nevertheless, the differences in starch damage may be caused by the different milling techniques applied [
14]. The level of starch damage related positively to the TKW (
r = 0.794,
p = 0.019) of the amaranth seeds. Thus, the milling of larger seeds resulted in more starch damage. The amount of starch damage was higher in quinoa WMF, which contrasts with the findings of Srichuwong et al. [
5]. Values ranged between 3.51 and 4.44% dm. Other studies reported higher levels of 5.4 and 10.6% dm [
5,
14]. The level of starch damage in buckwheat WMF was considerably lower compared to quinoa WMF, which is confirmed by the study of Hager et al. [
14]. Torbica et al. [
44] attributed the low level of starch damage to the less compact structure of the buckwheat seed. In other words, buckwheat seeds are more easily reduced in size during the milling process. The buckwheat samples contained between 0.94 and 1.43% dm damaged starch, which is lower compared to the findings of Hager et al. (3.0% dm) [
14].
Water absorption is the ability of the WMF to physically hold water while exposed to a centrifugal force [
80]. Collar et al. [
81] concluded that amaranth and quinoa flour had a similar water absorption, while the water absorption of buckwheat flour was lower. Within the present study, amaranth WMF was characterised by the highest water absorption (
Table 9). The high capacity to absorb water might be attributed to the high protein content. It is known that the ability of flour to associate with water, at room temperature, mainly depends on proteins [
78,
82]. The absorption ranged between 1.86 and 2.15 g/g, which is in accordance with previous studies (1.60–2.15 g/g) [
82,
83]. Amaranth samples with a high water absorption capacity were usually characterised by a lower peak viscosity (
r = −0.812,
p = 0.014) and holding strength (
r = −0.788,
p = 0.020).
The water absorption of quinoa WMF varied from 1.52 to 1.74 g/g, which is higher compared to the findings of Aluwi et al. (0.89–1.22 g/g) [
78]. Sample QU5 was characterised by high water absorption of 2.05 g/g. Water absorption correlated positively with the α-amylase activity (
r = 0.929,
p = 0.002), which indicates that the high absorption capacity of QU5 is attributed to its high α-amylase activity. This is unexpected as the enzymatic hydrolysis of starch is known to reduce water absorption [
84].
The water absorption capacity of buckwheat (1.67 ± 0.06 g/g) was comparable to quinoa. Higher values were reported (2.25–2.73 g/g) [
20]. The water absorption of buckwheat WMF related positively with the starch (
r = 0.686,
p = 0.029) and TDF (
r = 0.718,
p = 0.019) content of the flour, while a lower protein content (
r = −0.697,
p = 0.025) was associated with a higher absorption capacity. This indicates that starch and dietary fibre are the main hydrophilic constituents, attributing to the water absorption capacity of buckwheat WMF. Water absorption correlated positively with the swelling power at 95 °C (
r = 0.835,
p = 0.003) and important pasting parameters, such as peak viscosity (
r = 0.882,
p = 0.001), holding strength (
r = 0.916,
p < 0.001) and final viscosity (
r = 0.965,
p < 0.001).
3.3.2. Swelling Power
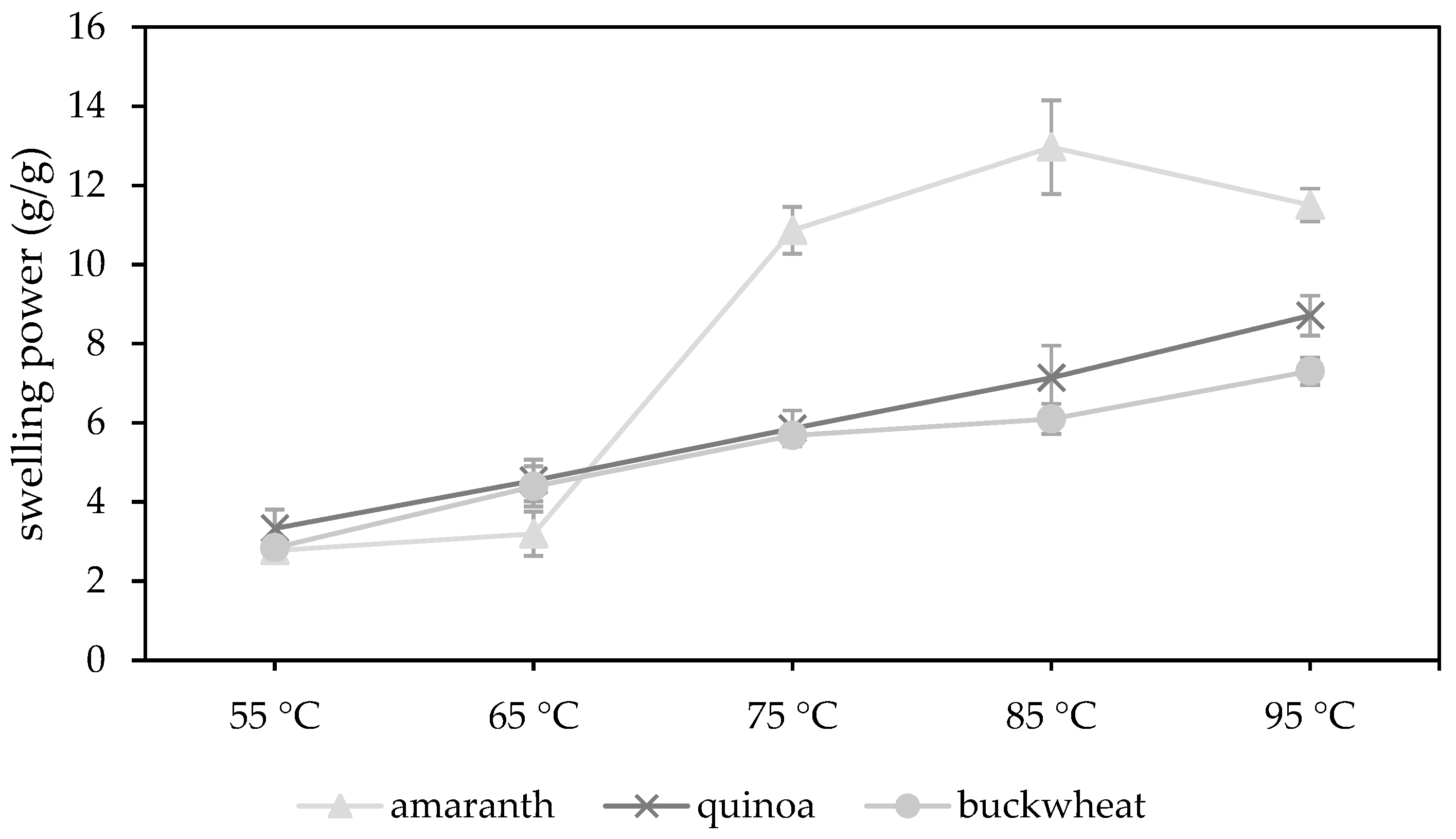
The swelling behaviour of amaranth, quinoa and buckwheat WMF were characterised by measuring the swelling power at 55, 65, 75, 85 and 95 °C (
Figure 1). The data table is presented in the
Supplementary Materials (Table S1). Each pseudocereal had a different swelling behaviour, which indicates differences in the molecular organization of the starch granules [
85]. The swelling power of a starch granule is attributed to its strength and micellar structure and is in fact a property of amylopectin [
86,
87].
The swelling power of amaranth WMFs showed a strong increase from 3.18 ± 0.56 to 10.87 ± 0.59 g/g when temperature increased from 65 to 75 °C. Siwatch et al. [
88] also reported a strong increase in the swelling power from 60 to 70 °C for amaranth starch. Furthermore, the swelling power reached a maximum at 85 °C (12.97 ± 1.19 g/g) and eventually dropped to 11.50 ± 0.42 g/g at 95 °C. The temperature increase causes damage to the starch granules, which enables the granules to absorb more water [
86]. At peak temperature, the rate of starch swelling is equal to the rate of starch disintegration [
69]. As such, the swelling power of amaranth WMF increased as long as the temperature was reaching its peak temperature (84.25 ± 0.91 °C,
Section 3.3.3) but decreased at 95 °C. This decrease is attributed to the increased solubility and leaching out of more solids [
86]. Quinoa (95.07 ± 0.11 °C) and buckwheat (95.08 ± 0.03 °C) WMFs had a higher peak temperature causing a continued increase of the swelling power at 95 °C.
The swelling power of quinoa WMF showed a steady increase from 3.33 ± 0.47 g/g at 55 °C to 8.71 ± 0.50 g/g at 95 °C. Similar results were reported by Li et al. [
21]. The swelling power of buckwheat followed a similar trend from 55 to 75 °C but showed a less strong increase from 75 to 95 °C. The high amylose content of buckwheat starch (21.1–46.6%) might attribute to the lower swelling power at temperatures above 75 °C [
10]. Amylose reinforces the internal structure within the starch granule, which renders the granule more resistant to swelling. The presence of amylose-lipid complexes can also inhibit the swelling and solubilisation of the starch granule as it prevents the amylose from leaching out [
87]. In amaranth starch, the amylose content is much lower (0.1–11.1%) and the inhibitory effects of amylose-lipid complexes are absent or negligible [
5,
6]. This explains why amaranth WMFs showed a much higher swelling power at temperatures above 65 °C compared to quinoa or buckwheat WMF.
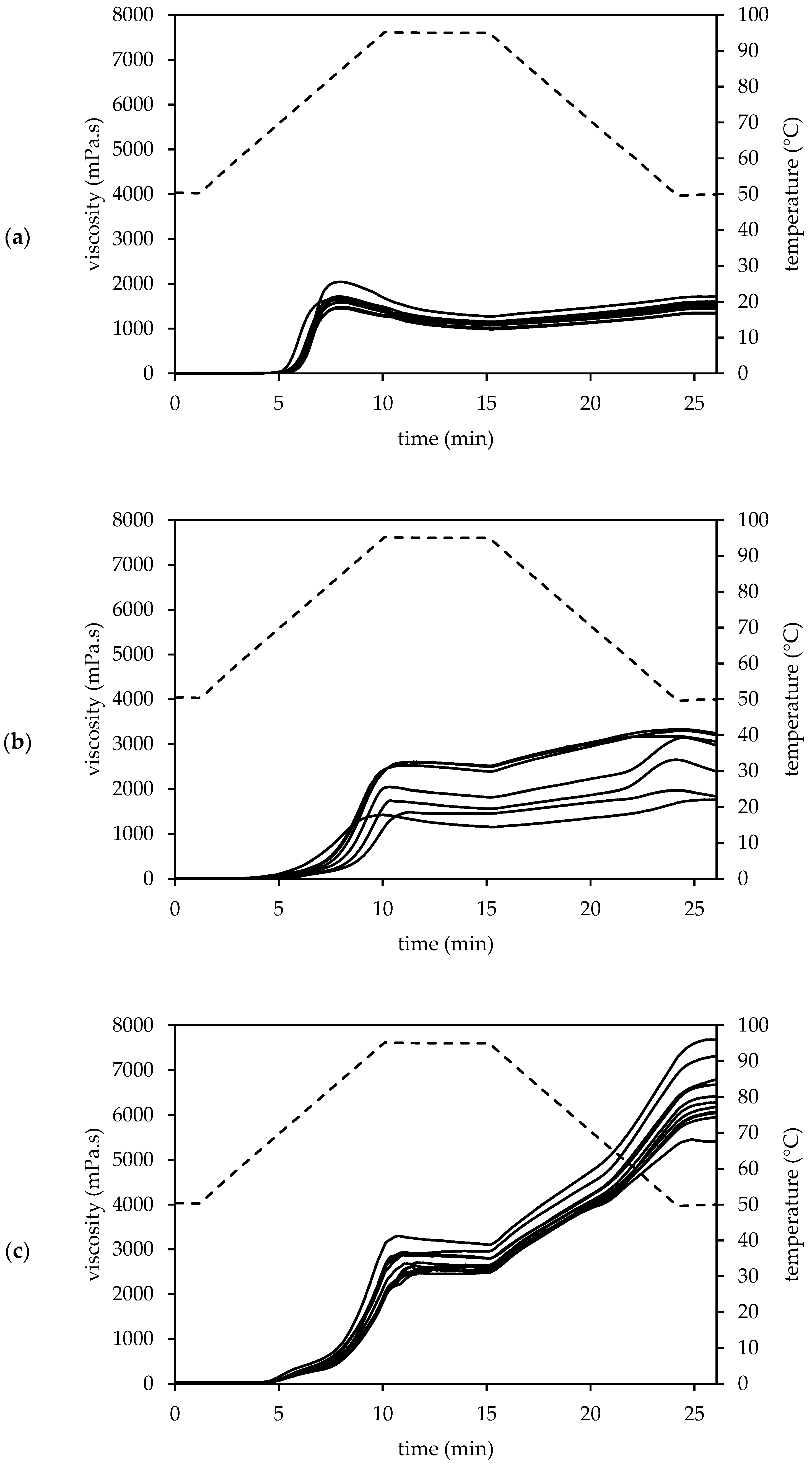
3.3.3. Pasting Properties
The pasting profiles of amaranth, quinoa and buckwheat WMF (
Figure 2) were characterised by the pasting parameters in
Table S2 (Supplementary Materials). Amaranth WMF had the highest pasting temperature which varied between 65.09 and 68.89 °C. This pasting temperature increased along with the TDF content of the amaranth WMF (
r = 0.900,
p = 0.002), which might be attributed to the competition for hydration between dietary fibre and starch [
5]. The amaranth WMFs with a high swelling power at 65 °C were characterised by a lower pasting temperature (
r = −0.895,
p = 0.006) due to their ability to swell more freely [
5]. A similar correlation was found between the swelling power at 55 °C and the pasting temperature of quinoa (
r = −0.895,
p = 0.006) and buckwheat WMFs (
r = −0.723,
p = 0.016). Quinoa and buckwheat WMF had a lower pasting temperature of 62.22 ± 2.95 and 65.08 ± 0.63 °C, respectively.
Despite the high swelling capacity, amaranth WMFs had the lowest peak viscosity (1657 ± 180 mPa.s). The presence of amylose-lipid complexes is crucial for the heat and shear force resistance of swollen starch granules. The effect of amylose-lipid complexes is limited for amaranth WMFs due to the low amylose content. This leads to high shear sensitivity and low peak viscosity [
5]. The higher peak viscosity of AM7 (2045 mPa.s) suggests a higher amylose content as viscosity is directly affected by the amylose content [
48]. The low TDF content of AM5 resulted in a lower peak time (7.491 min) and temperature (82.19 °C). Kaur et al. [
8] evaluated the pasting parameters of forty-eight
A. hypochondriacus lines and eleven
A. caudatus lines and found a peak viscosity between 879 and 1613 mPa.s.
The peak viscosity strongly varied among the quinoa samples and ranged from 1418 to 2606 mPa.s. Other studies also reported a great diversity in the peak viscosity of quinoa. According to Li et al. [
21], the peak viscosity of seven quinoa WMFs ranged between 2904 and 5820 mPa.s. Wu et al. [
30] studied eleven quinoa varieties and two commercial quinoa samples and found a peak viscosity between 708 and 2364 mPa.s. The diversity in peak viscosity might be attributed to a wide variation in the amylose content [
89]. The pasting profile of quinoa WMF showed a less distinct peak, especially if compared to amaranth WMF. Contreras-Jiménez et al. [
67] compared the pasting profile of quinoa flour with that of its isolated starch and concluded that the starch developed a significantly different and higher viscosity peak. Authors attributed this to the higher fat content in the flour which produced a more fluid system with lower viscosity. Within the present study, the peak viscosity negatively correlated with the TDF (
r = −0.920,
p = 0.003) and K (
r = −0.756,
r = 0.049) content of the WMFs, indicating that other non-starch components also affected the pasting properties.
Buckwheat WMF had a significantly higher peak viscosity (2771 ± 242 mPa.s) compared to the other pseudocereals, probably due to the higher starch and amylose content [
6]. Buckwheat samples with a higher starch content were generally characterised by a higher peak viscosity (
r = 0.822,
p = 0.004), while the protein content had a negative effect on this pasting property (
r = −0.798,
p = 0.006). Furthermore, the peak viscosity positively correlated with the Mn (
r = 0.747,
p = 0.013) and Zn (
r = 0.892,
p = 0.001) content. Bhinder et al. [
20] mentioned a flatter peak in the pasting profile of buckwheat and considered this an indication of a low degree of shear-thinning. A similar peak was observed in the present study.
Amaranth WMFs were characterised by the lowest holding strength (1107 ± 87 mPa.s). The pasting profile showed a strong viscosity decrease after reaching maximum, indicating that amaranth WMFs were less resistant to shearing. Quinoa and buckwheat WMFs showed higher shear stability due to the stabilizing effect of amylose-lipid complexes [
5]. Holding strength ranged between 1156 and 2513 mPa.s for quinoa, while Wu et al. [
30] reported a holding strength between 600 and 2016 mPa.s. Holding strength negatively correlated with the TDF content of the quinoa WMF (
r = −0.870,
p = 0.011). Buckwheat showed the highest shear stability as viscosity showed a minor decrease or even slight increase (BU1, BU2, BU3) during the holding phase. Therefore, the holding strength of buckwheat samples was calculated as the average viscosity at the end of the holding phase. The holding strength ranged between 2494 and 3113 mPa.s and correlated with the starch (
r = 0.788,
p = 0.007) and protein (
r = −0.791,
p = 0.009) content.
The viscosity increased upon cooling as a result of the re-association of the starch granules [
8,
52]. The final viscosity of amaranth WMF (1502 ± 124 mPa.s) was eventually lower compared to the peak viscosity, as also reported by Kaur et al. [
8]. The total setback of amaranth WMFs was low (395 ± 42 mPa.s), indicating a low rate of starch retrogradation [
52]. The final viscosity (2677 ± 647 mPa.s) and total setback (767 ± 261 mPa.s) was higher for quinoa WMFs. Wu et al. [
30] reported a final viscosity of 672 to 2436 mPa.s and a total setback between −744 and 876 mPa.s for quinoa. Li et al. [
21] estimated the total setback between 1068 and 2352 mPa.s. Final viscosity negatively correlated with the TDF content (
r = −0.961,
p = 0.001). Dietary fibre is known to retard starch retrogradation. Lipids may also retard retrogradation due to interactions with amylopectin [
21].
Cooling of the paste caused a strong viscosity increase for buckwheat as reflected in the high total setback (3702 ± 493 mPa.s). The final viscosity (6421 ± 673 mPa.s) was eventually two to three times higher compared to quinoa. This short-term development of gel structure is attributed to the retrogradation of amylose so the high viscosity increase is expected for buckwheat WMF. The retrogradation of amylopectin occurs at a much slower rate as it involves the re-association of the branch chains [
5]. A lower final viscosity (620–2959 mPa.s) and total setback (184–1736 mPa.s) was found by Bhinder et al. [
20].