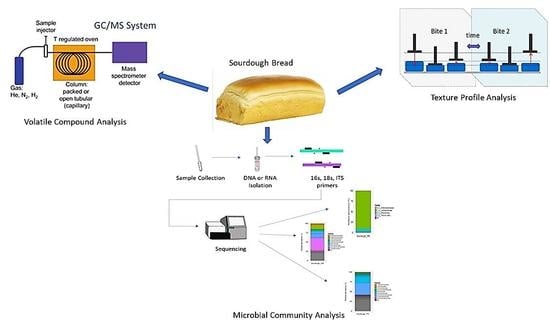
Characterization of Artisanal Spontaneous Sourdough Wheat Bread from Central Greece: Evaluation of Physico-Chemical, Microbiological, and Sensory Properties in Relation to Conventional Yeast Leavened Wheat Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Artisanal Sourdough Preparation
2.2. Bread Preparation
2.3. Microbiological Analysis
2.3.1. Bread Microflora
2.3.2. Sourdough Microbiota-Library Construction and Sequencing
2.3.3. Amplicon Meta-Barcoding Bioinformatics Analysis
2.4. Physicochemical Analysis
2.4.1. pH/Titratable Acidity (TA) Determination
2.4.2. Semi-Quantitative Determination of Flavor Volatiles of Bread
2.4.3. Determination of Water Activity
2.4.4. Determination of Texture Parameters (Texture Profile Analysis, TPA)
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
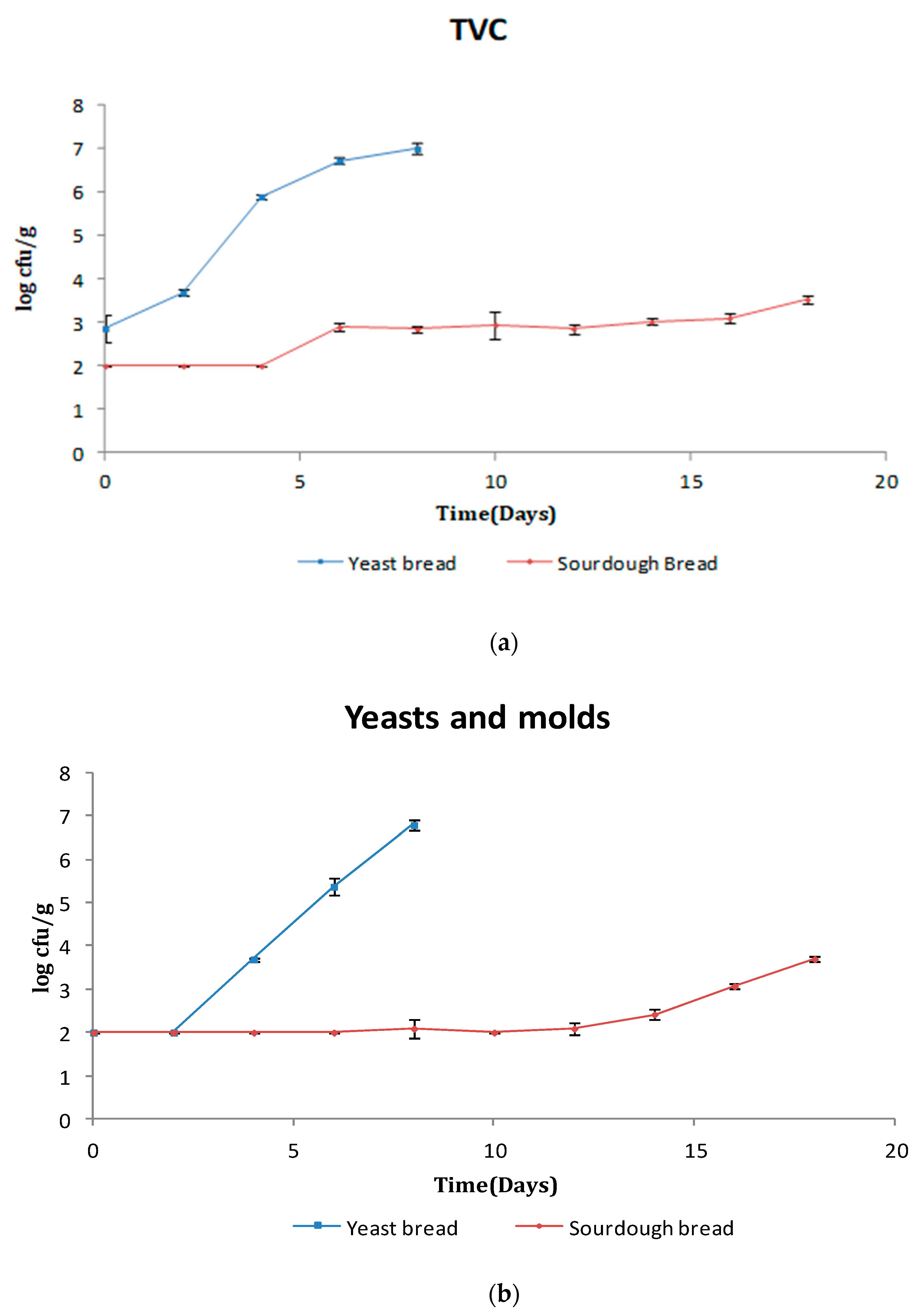
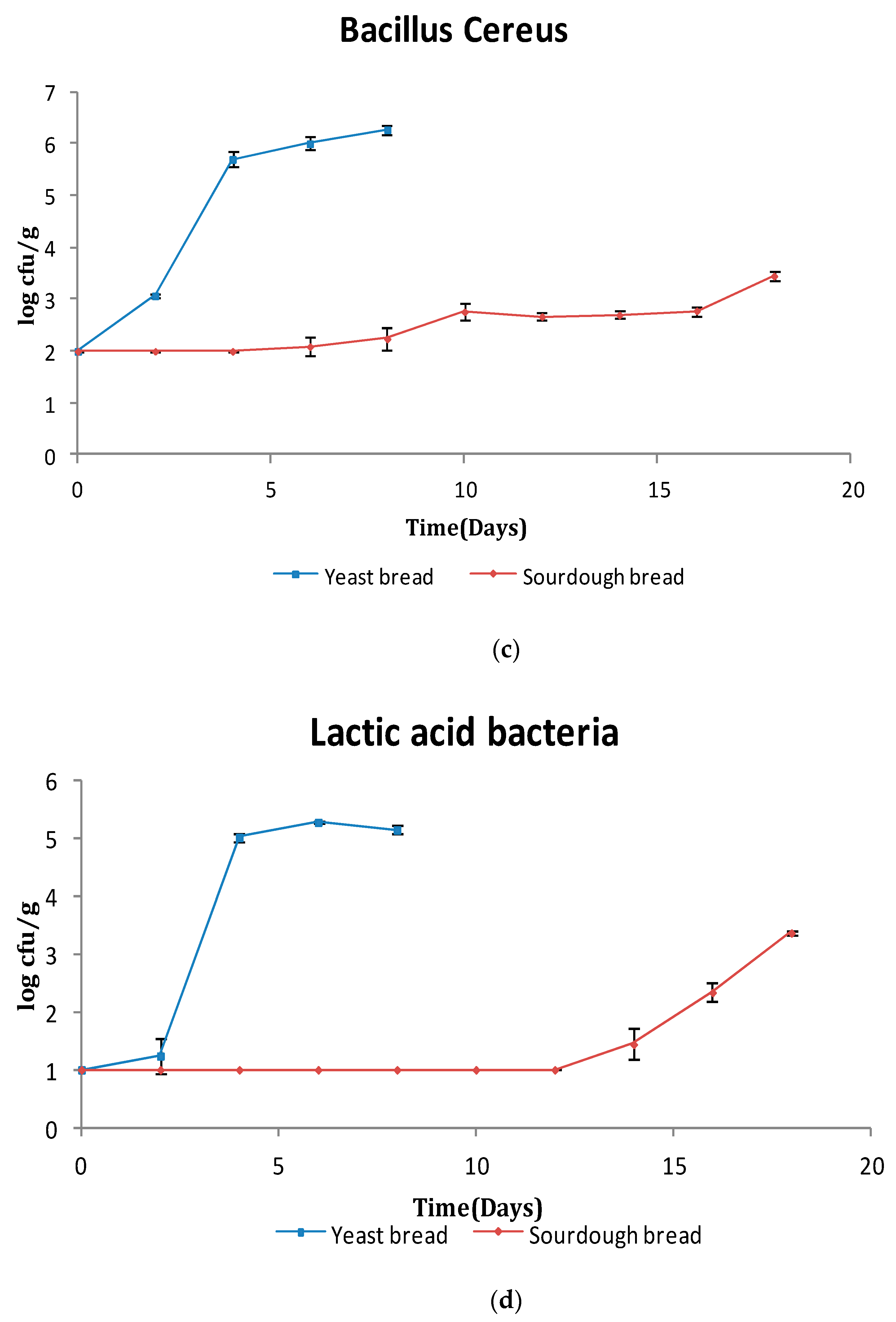
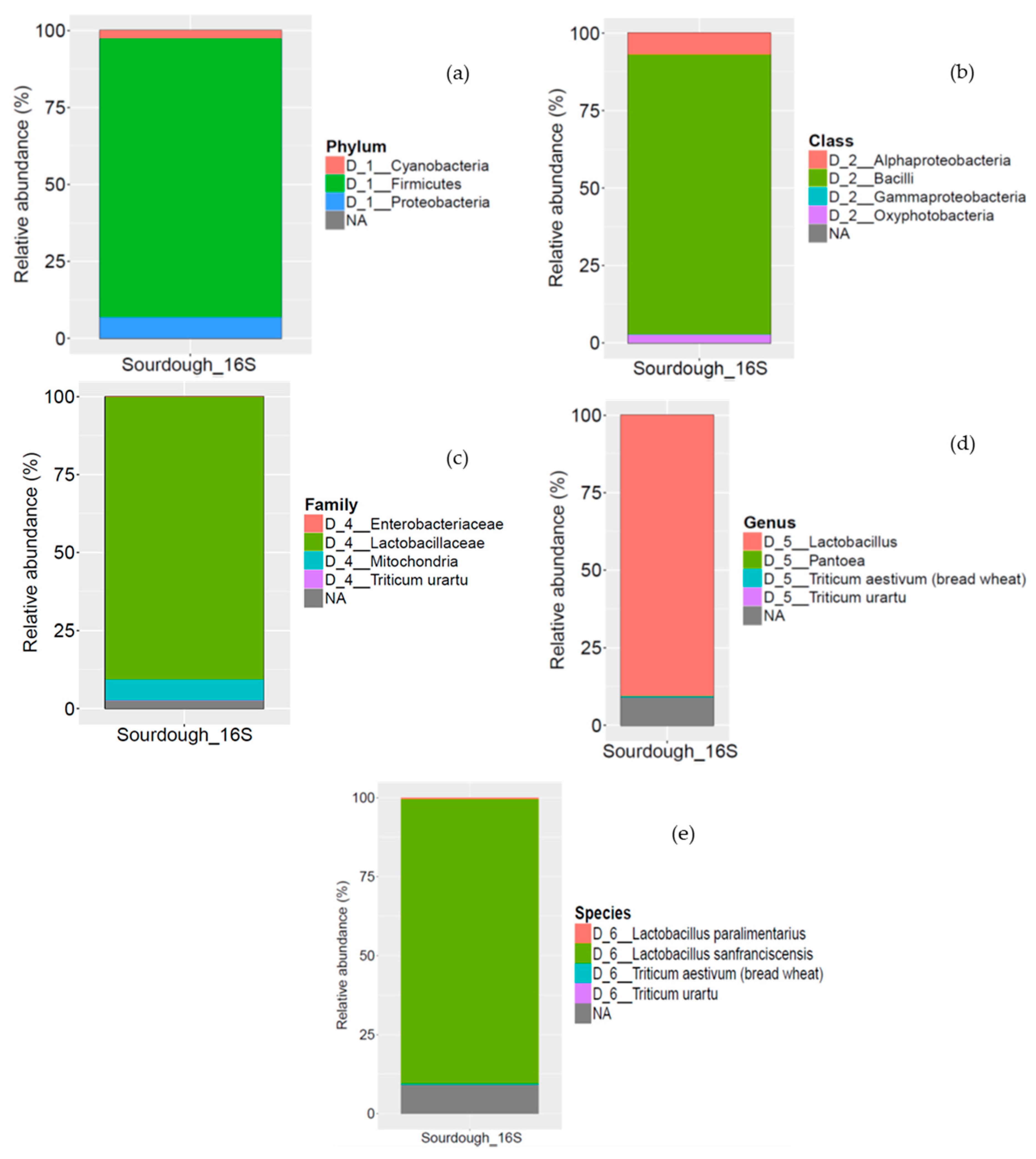
3.1. Microbiological Analysis
3.1.1. Bread Microflora
3.1.2. Microbial Community in Sourdough
3.2. Physico-Chemical Analysis
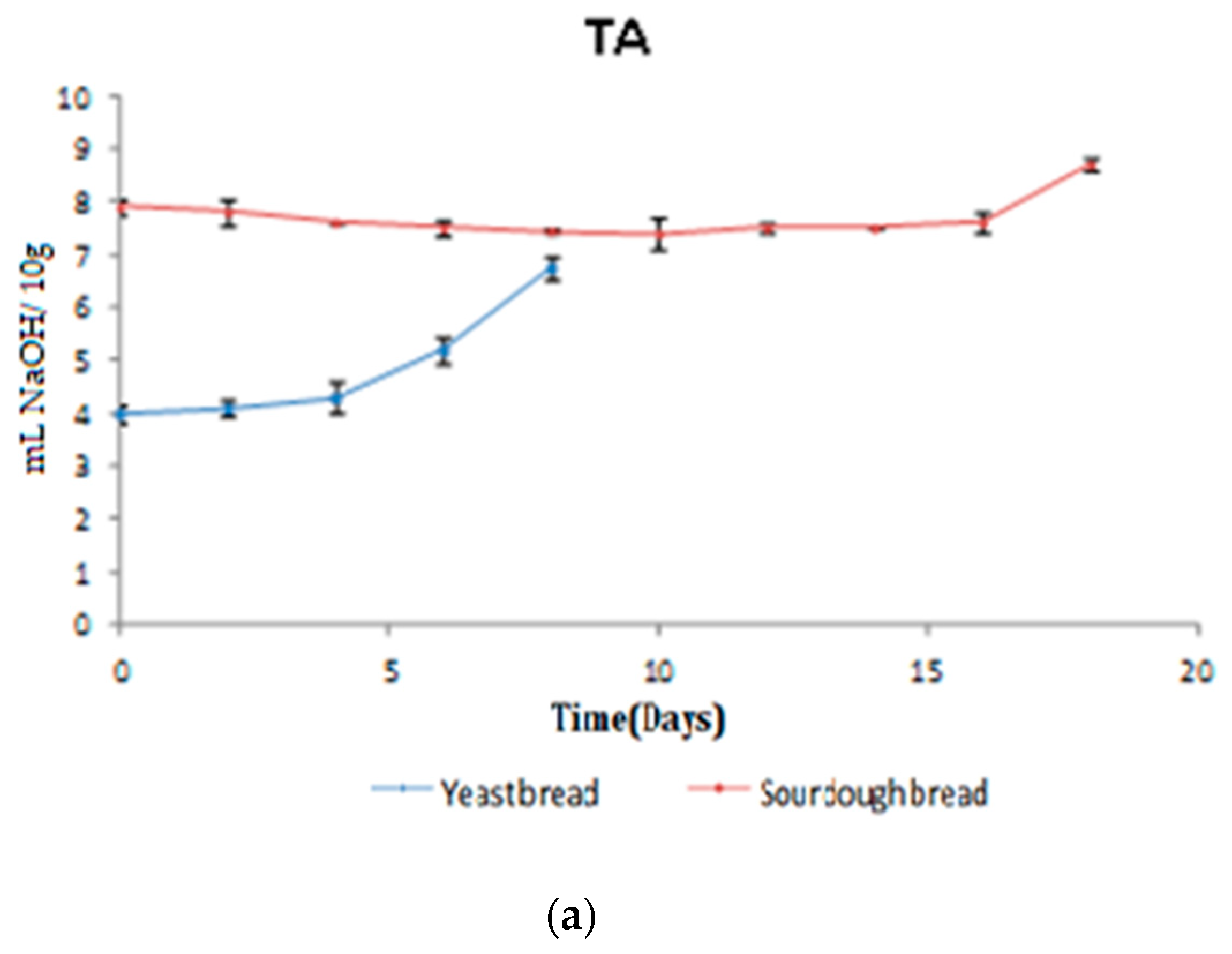
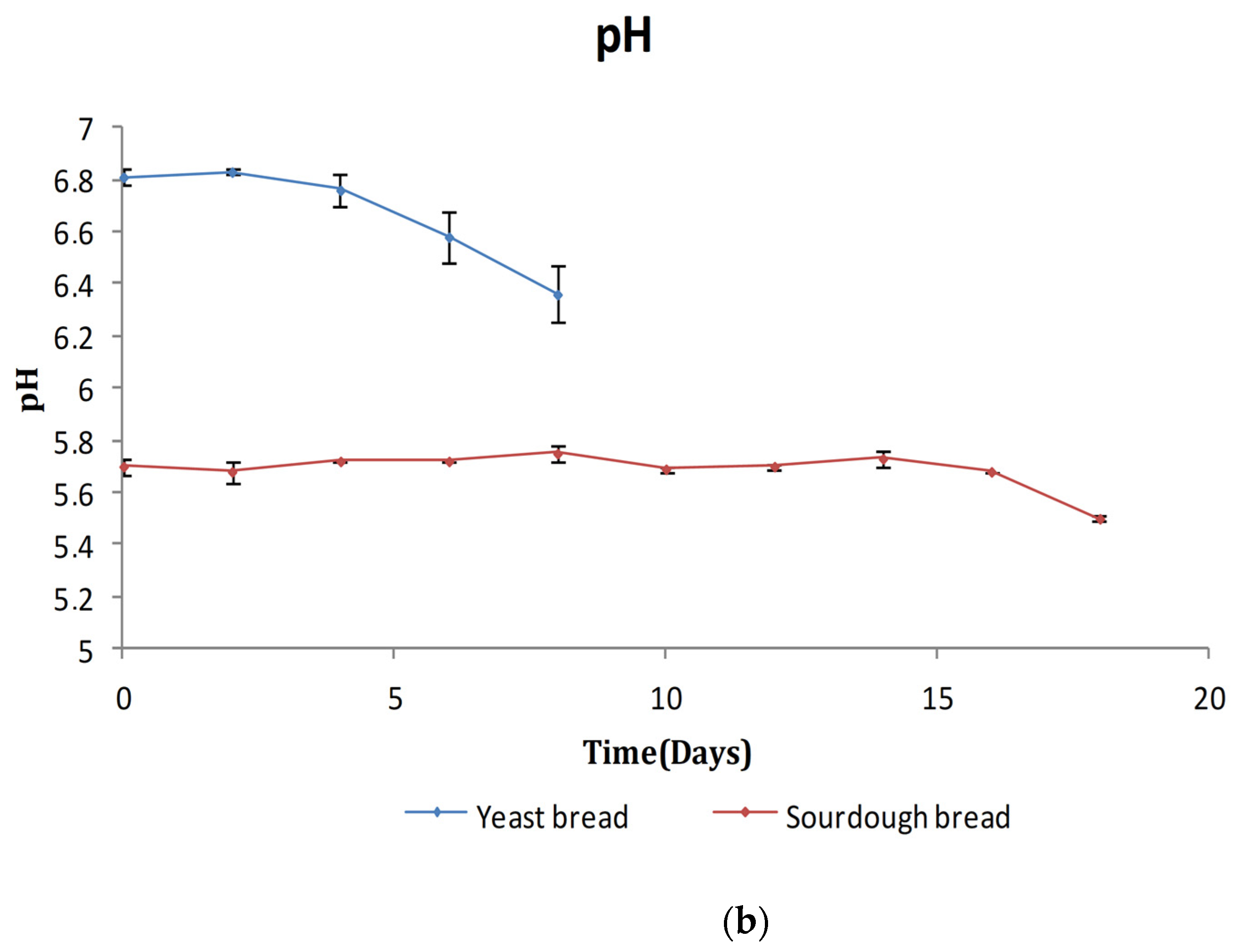
3.2.1. TA/pH/aw
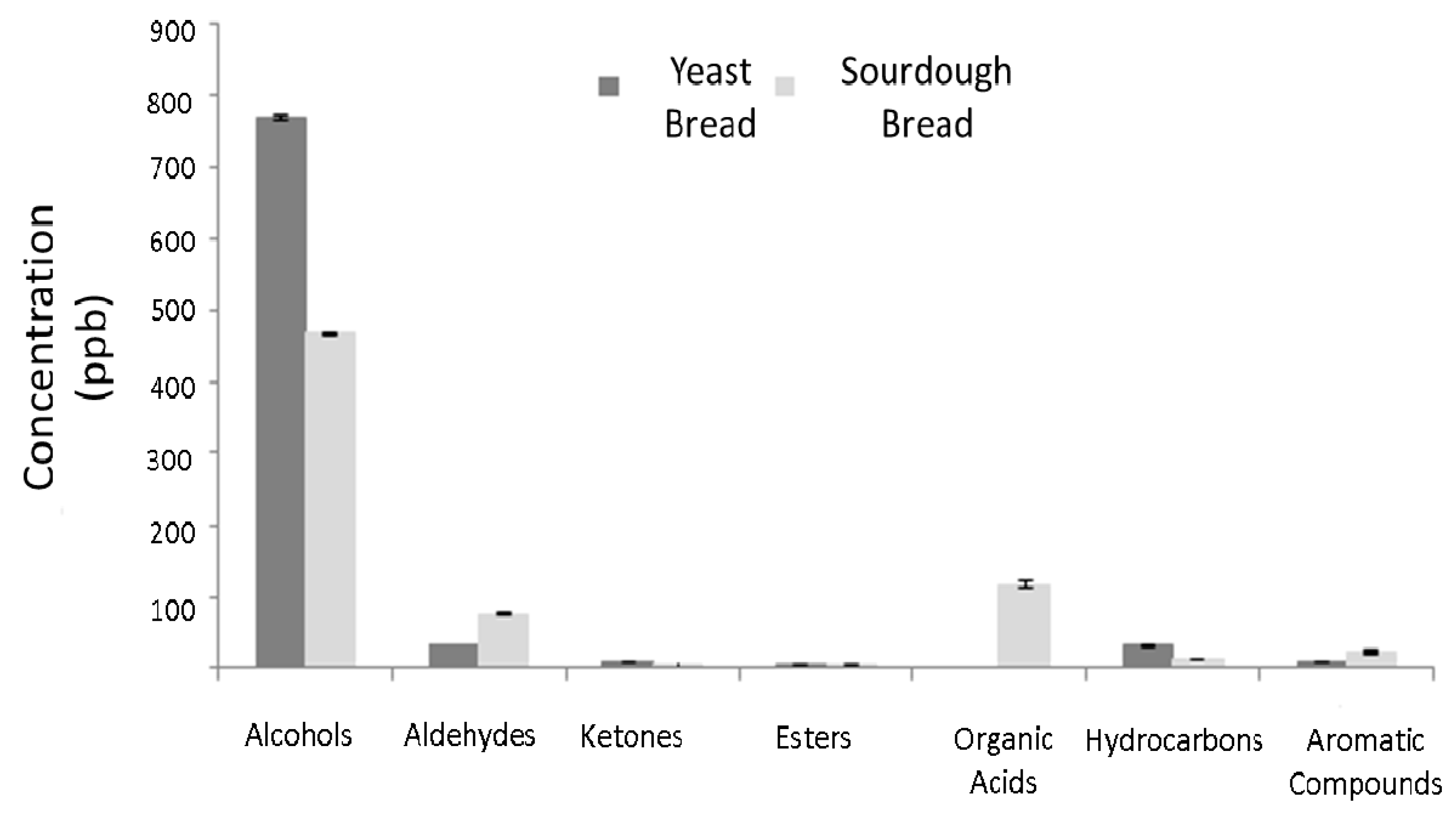
3.2.2. Volatile Compounds
3.2.3. Mechanical Parameters (Texture Profile Analysis—TPA)
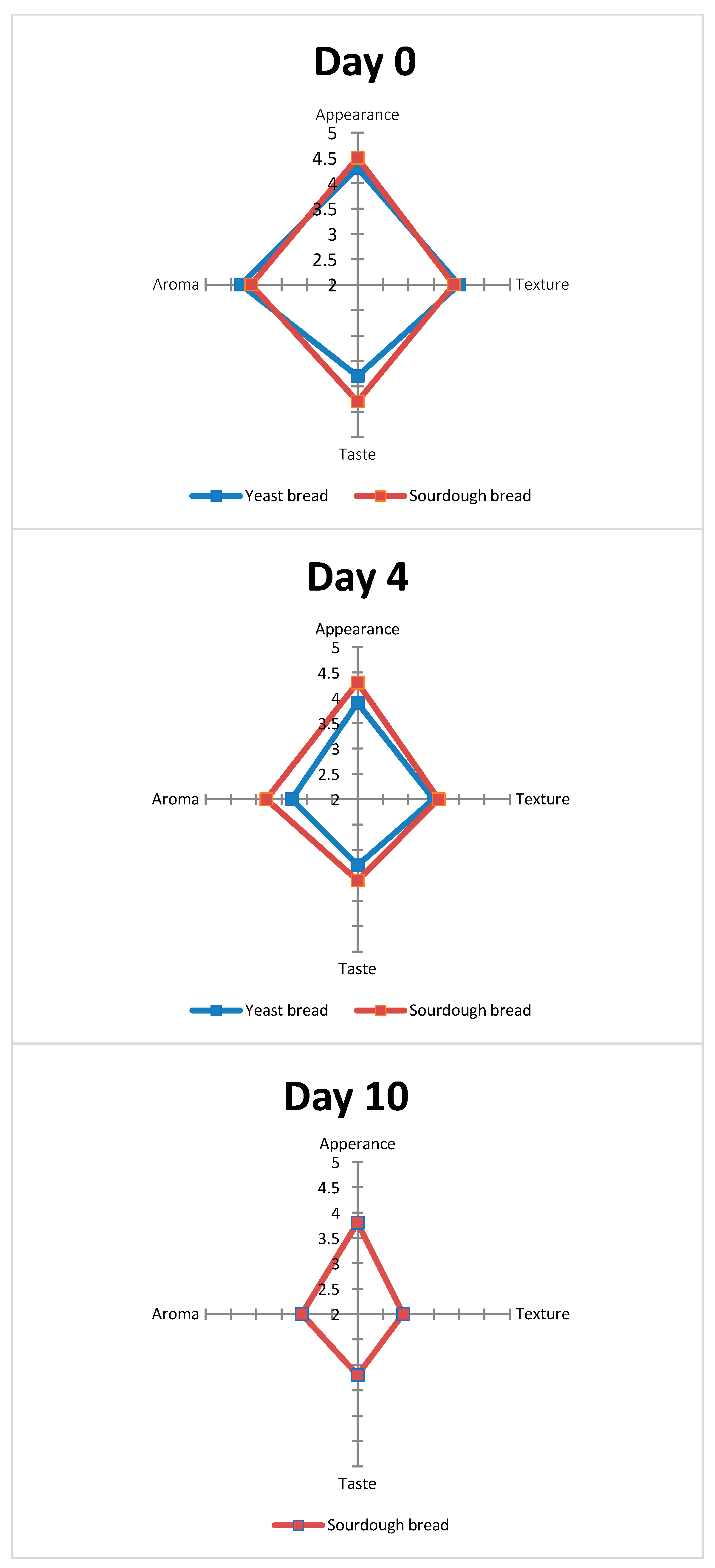
3.3. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagodawithana, T.W.; Trivedi, N. Yeast Selection for Baking. Ιn Yeast Strain Selection; Panchal, C.J., Ed.; Marcel Decker: New York, NY, USA, 1990; pp. 139–184. [Google Scholar]
- Ganzle, M.; Gobbetti, M. Handbook of Sourdough Biotechnology; Springer US: Boston, MA, USA, 2013. [Google Scholar]
- Petel, C.; Onno, B.; Prost, C. Sourdough volatile compounds and their contribution to bread. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Mohsen, S.M.; Aly, M.H.; Attia, A.A.; Osman, D.B. Effect of Sourdough on Shelf Life, Freshness and Sensory Characteristics of Egyptian Balady Bread. J. Appl. Environ. Microbiol. 2016, 4, 39–45. [Google Scholar]
- Hansen, A. Sourdough Bread. In Handbook of Food Science, Technology and Engineering; Hui, Y.H., Sherka, F., Eds.; Taylor and Francis Group, LLC: Boca Raton, FL, USA, 2006; p. 183. [Google Scholar]
- Salovaara, H. Lactic Acid Bacteria in Cereal-Based Products. In Lactic Acid Bacteria. Microbiological and Functional Aspects; Salminen, S., von Wright, A., Ouwehand, A., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 431–451. [Google Scholar]
- Minervini, F.; Di Cagno, R.; Lattanzi, A.; De Angelis, M.; Antonielli, L.; Cardinali, G.; Cappelle, S.; Gobbetti, M. Lactic acid bacterium and yeast microbiotas of 19 sourdoughs used for traditional/typical Italian breads: Interactions between ingredients and microbial species diversity. Appl. Environ. Microbiol. 2012, 78, 1251–1264. [Google Scholar] [CrossRef]
- De Vuyst, L.; Van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.M.; Weckx, S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef]
- Legan, J.D. Mould spoilage of bread: The problem and some solutions. Int. Biodeterior. Biodegrad. 1993, 32, 33–53. [Google Scholar] [CrossRef]
- Novotini, D.; Špoljarić, I.V.; Drakula, S.; Čukelj, N.; Voučko, B.; Ščetar, M.; Galić, K.; Ćurić, D. Influence of Barley Sourdough and Vacuum Cooling on Shelf Life Quality of Partially Baked Bread. Food Technol. Biotechnol. 2017, 55, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Legan, J.D.; Voyseyt, P.A. Yeast spoilage of bakery products and ingredients. J. Appl. Bacteriol. 1991, 70, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.K.; Ryan, L.A.M.; Bello, F.D. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef]
- Katina, K. Sourdough: A Tool for the Improved Flavor, Texture and Shelf Life of Wheat Bread; Vtt Technical Research Center of Finland: Helsinki, Finland, 2005. [Google Scholar]
- Katina, K.; Salmenkallio-Marttila, M.; Partanen, R.; Forssell, P.; Autio, K. Effects of sourdough and enzymes on staling of highfibre wheat bread. LWT Food Sci. Technol. 2006, 39, 479–491. [Google Scholar] [CrossRef]
- European Council. EU Regulation 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods; European Council: Brussels, Belgium, 2006; pp. 9–25. [Google Scholar]
- APHA. Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; APHA: Washington, DC, USA, 2001; p. 676. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Chemidlin Prévost-Bouré, N.; Christen, R.; Dequiedt, S.; Mougel, C.; Lelièvre, M.; Jolivet, C.; Shahbazkia, H.R.; Guillou, L.; Arrouays, D.; Ranjard, L. Validation and application of a PCR primer set to quantify fungal communities in the soil environment by real-time quantitative PCR. PLoS ONE 2011, 6, e24166. [Google Scholar]
- Bokulich, N.A.; Mills, D.A. Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Appl. Environ. Microbiol. 2013, 79, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. Dada2: High-resolution sample inference from illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2010. [Google Scholar]
- McMurdie, P.J.; Holmes, S. Phyloseq: A bioconductor package for handling and analysis of high-throughput phylogenetic sequence data. Pac. Symp. Biocomput. 2012, 17, 235–246. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Latou, E.; Mexis, S.F.; Badeka, A.V.; Kontominas, M.G. Shelf life extension of sliced wheat bread using either an ethanol emitter or an ethanol emitter combined with an oxygen absorber as alternatives to chemical preservatives. J. Cereal Sci. 2010, 52, 457–465. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Chiacaro, E.; Musci, M.; Vittadini, E. Gas chromatographic-mass spectrometric characterization of the Italian protected designation of origin ‘Altamura’ bread volatile profile. Food Chem. 2008, 110, 787–793. [Google Scholar] [CrossRef]
- International Commission on Microbiological Specifications for Foods. Sampling for Microbiological Analysis: Principles and Scientific Applications, 2nd ed.; University of Toronto Press: Toronto, ON, Canada, 1986; Volume 2. [Google Scholar]
- Jonkuvienė, D.; Vaičiulytė-Funk, L.; Šalomskienė, J.; Alenčikienė, G.; Mieželienė, A. Potential of Lactobacillus reuteri from Spontaneous Sourdough as a Starter Additive for Improving Quality Parameters of Bread. Food Technol. Biotechnol. 2016, 54, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, U.; Vodovotz, Y.; Courtney, P.; Pascall, M. Extended shelf life of soy bread using modified atmosphere packaging. J. Food Protect. 2006, 69, 693–698. [Google Scholar] [CrossRef]
- Tatar, O.M.; Rehman, S.; Mueen-Ud-Din, G.; Murtaza, M.A. Studies on the shelf life of bread using acidulants and their salts. Turk. J. Biol. 2010, 34, 133–138. [Google Scholar]
- Samapundo, S.; Deschuyfteleer, N.; Van Laere, D. Effect of NaCl reduction and replacement on the growth of fungi important to the spoilage of bread. J. Food Microbiol. 2010, 27, 749–756. [Google Scholar] [CrossRef]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology, 7th ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- EFSA. Bacillus cereus and other Bacillus spp in foodstuffs. J. EFSA 2005, 175, 1–8. [Google Scholar]
- Bailey, C.P.; von Holy, A. Bacillus spore contamination associated with commercial bread manufacture. Food Microbiol. 1993, 10, 287–294. [Google Scholar] [CrossRef]
- Ravimannan, N. Study on microbial profile of bread during storage. Int. J. Adv. Res. Biol. Sci. 2016, 3, 60–63. [Google Scholar]
- Hutkins, R.W.; Nannen, N.L. pH Homeostasis in Lactic Acid Bacteria. J. Dairy Sci. 1993, 76, 2354–2365. [Google Scholar] [CrossRef]
- Park, J.; Seo, J.S.; Kim, S.; Shin, S.; Park, J.H.; Han, N.S. Microbial Diversity of Commercial Makgeolli and Its Influence on the Organoleptic Characteristics of Korean Rice Sourdough, Jeung-Pyun. J. Microbiol. Biotechnol. 2017, 27, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- NSW Food Authority. Microbiological Quality Guide for Ready-to-Eat Foods. A Guide to Interpreting Microbiological Results; NSW Food Authority: Newington, NSW, Australia, 2009. [Google Scholar]
- De Vuyst, L.; Schrijvers, V.; Paramithiotis, P.; Hoste, B.; Vancanneyt, M.; Swings, J.; Kalantzopoulos, G.; Tsakalidou, E.; Messens, W. The biodiversity of lactic acid bacteria in Greek traditional wheat sourdoughs is reflected in both composition and metabolite formation. Appl. Environ. Microbiol. 2002, 68, 6059–6069. [Google Scholar] [CrossRef] [PubMed]
- Palla, M.; Cristani, C.; Giovannetti, M.; Agnolucci, M. Identification and characterization of lactic acid bacteria and yeasts of PDO Tuscan bread sourdough by culture dependent and independent methods. Int. J. Food Microbiol. 2017, 250, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, E.; Lattanzi, A.; Dousset, X.; Minervini, F.; De Angelis, M.; Lacaze, G.; Onno, B.; Gobbetti, M. Lactic acid bacterium and yeast microbiotas of sixteen French traditional sourdoughs. Int. J. Food Microbiol. 2015, 215, 161–170. [Google Scholar] [CrossRef]
- Banu, I.; Vasilean, I.; Barbu, V.; Iancu, C. The effect of some technological factors on the rye sourdough bread. Chem. Chem. Eng. Biotechnol. Food Ind. 2011, 12, 197–202. [Google Scholar]
- Mert, I.D.; Campanella, O.H.; Sumnu, G.; Sahin, S. Gluten-Free Sourdough Bread Prepared with Chestnut and Rice Flour; Department of Food Engineering, Engineering Faculty, Middle East Technical University, Universiteler Mah: Ankara, Turkey, 2014. [Google Scholar]
- Sanz-Penella, J.M.; Tamayo-Ramos, J.A.; Haros, C.M. Application of Bifidobacteria as starter culture in whole wheat sourdough breadmaking. Food Bioprocess. Technol. 2012, 5, 2370–2380. [Google Scholar] [CrossRef]
- Marcus, C.E.; Mairinger, B.R.; Zannini, E.; Ryan, L.A.M.; Cashman, A.D.; Arendt, E.K. The effect of sourdough and calcium propionate on the microbial shelf-life of salt reduced bread. Appl. Microbiol. Biotechnol. 2012, 96, 493–501. [Google Scholar]
- Chirife, J.; Favetto, G.J. Some physicochemical basis of food preservation by combined methods. Food Res. Int. 1992, 25, 389–396. [Google Scholar] [CrossRef]
- Smith, J.P.; Daifas, D.P.; El-Khoury, W.; Koukoutsis, J.; El-Khoury, A. Shelf life and safety concerns of bakery products—A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 19–55. [Google Scholar] [CrossRef] [PubMed]
- Birch, A.N.; Peterson, M.A.; Hansen, A.S. Aroma of wheat bread crumb. Cereal Chem. 2014, 91, 105–114. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2014, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Hansen, B. Flavour of Sourdough wheat bread crumb. Z. Lebensm. Unters. Forsch. 1996, 202, 244–249. [Google Scholar] [CrossRef]
- Drapron, R.; Richard-Molard, D. Influence de Divers Procedes Technologiques sur la Formations de l’ Arộme du Pain. Repercussions sur sa Qualité. In Le Pain; Buré, J., Ed.; Actes du Researche Scientifique: Paris, France, 1979; pp. 143–161. [Google Scholar]
- Maga, J.A. Bread Flavor. Crit. Rev. Food Technol. 1974, 5, 55–142. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.A.; Guichard, E.; Cayot, N. Flavor control in baked cereal products. Food Rev. Int. 2006, 22, 335–379. [Google Scholar] [CrossRef]
- Hansen, A.; Schieberle, P. Generation of aroma compounds during sourdough fermentation: Applied and fundamental aspects. Trends Food Sci. Technol. 2005, 16, 85–94. [Google Scholar] [CrossRef]
- Kaseleht, K.; Paalme, T.; Mihhalevski, A.; Sarand, I. Analysis of volatile compounds produced by different species of lactobacilli in rye sourdough using multiple headspace extraction. Int. J. Food Sci. Technol. 2011, 46, 1940–1946. [Google Scholar] [CrossRef]
- Spicher, G.; Stephan, H. Handbuch Sauerteig: Biologie, Biochemie, Technologie; B.B.V. Wirstchaft Informationen GmbH: Hamburg, Germany, 1987. [Google Scholar]
- Seitz, L.M.; Chung, O.I.; Rengarajan, R. Volatiles in selected commercial breads. Cereal Chem. 1998, 75, 847–853. [Google Scholar] [CrossRef]
- De Vuyst, L.; Neysens, P. The sourdough microflora: Biodiversity and metabolic interactions. Trends Food Sci. Technol. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Cho, H.; Peterson, D.G. Chemistry of bread aroma. Food Sci. Biotechnol. 2010, 19, 575–582. [Google Scholar] [CrossRef]
- Plessas, S.; Bekatorou, A.; Gallanagh, J.; Nigam, P.; Koutinas, A.A.; Psarianos, C. Evolution of aroma volatiles during storage of sourdough breads made by mixed cultures of Kluyveromyces marxianus and Lactobacillus delbrueckii ssp. bulgaricus or Lactobacillus helveticus. Food Chem. 2008, 107, 883–889. [Google Scholar]
- Maga, J.A. Pyrazine update. Food Rev. Intl. 1992, 8, 479–558. [Google Scholar] [CrossRef]
- Soukoulis, C.; Yonekura, L.; Gan, H.H.; Behboudi-Jobbehdar, S.; Parmenter, C.D.J.; Fisk, I.D. Probiotic edible films as a new strategy for developing functional bakery products: The case of pan bread. Food Hydrocoll. 2014, 39, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, Y.; Tang, K.; Hu, Y.; Xu, X.; Gänzle, M.G. Effect of Mixed Cultures of Yeast and Lactobacilli on the Quality of Wheat Sourdough Bread. Front. Microbiol. 2019, 10, 2113. [Google Scholar] [CrossRef]
- Rinaldi, M.; Paciulli, M.; Caligiani, A.; Scazzina, F.; Chiavaro, E. Sourdough fermentation and chestnut flour in gluten-free bread: A shelf life evaluation. Food Chem. 2016, 224, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Casado, A.; Álvarez, A.; Gonzalez, L.; Fernandez, D.; Marcos, J.L.; Tornadijo, M.E. Effect of Fermentation on Microbiological, Physicochemical and Physical Characteristics of Sourdough and Impact of its Use on Bread Quality. Czech J. Food Sci. 2017, 35, 496–506. [Google Scholar]
- Hadaegh, H.; Seyyedain Ardabili, S.M.; Tajabadi Ebrahimi, M.; Chamani, M.; Azizi Nezhad, R. The Impact of Different Lactic Acid Bacteria Sourdoughs on the Quality Characteristics of Toast Bread. J. Food Qual. 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Edeghor, U.; Lennox, J.; Etta-Abgo, B.; Aminadokiari, D. Bread fermentation using synergistic activity between lactic acid bacteria (Lactobacillus bulgaricus) and baker’s yeast (Sacchromyces cerevisae). Pak. J. Food Sci. 2016, 26, 46–53. [Google Scholar]
- Quintero Lira, A.; Alvarado-Resendiz, M.G.; Soto Simental, S.; Piloni Martini, J.; Reyes-Santamaria, M.I.; Guemes-Vera, N. Use of Lactobacillus from Pulque in Sourdough. Adv. Microbiol. 2014, 4, 969–977. [Google Scholar] [CrossRef][Green Version]
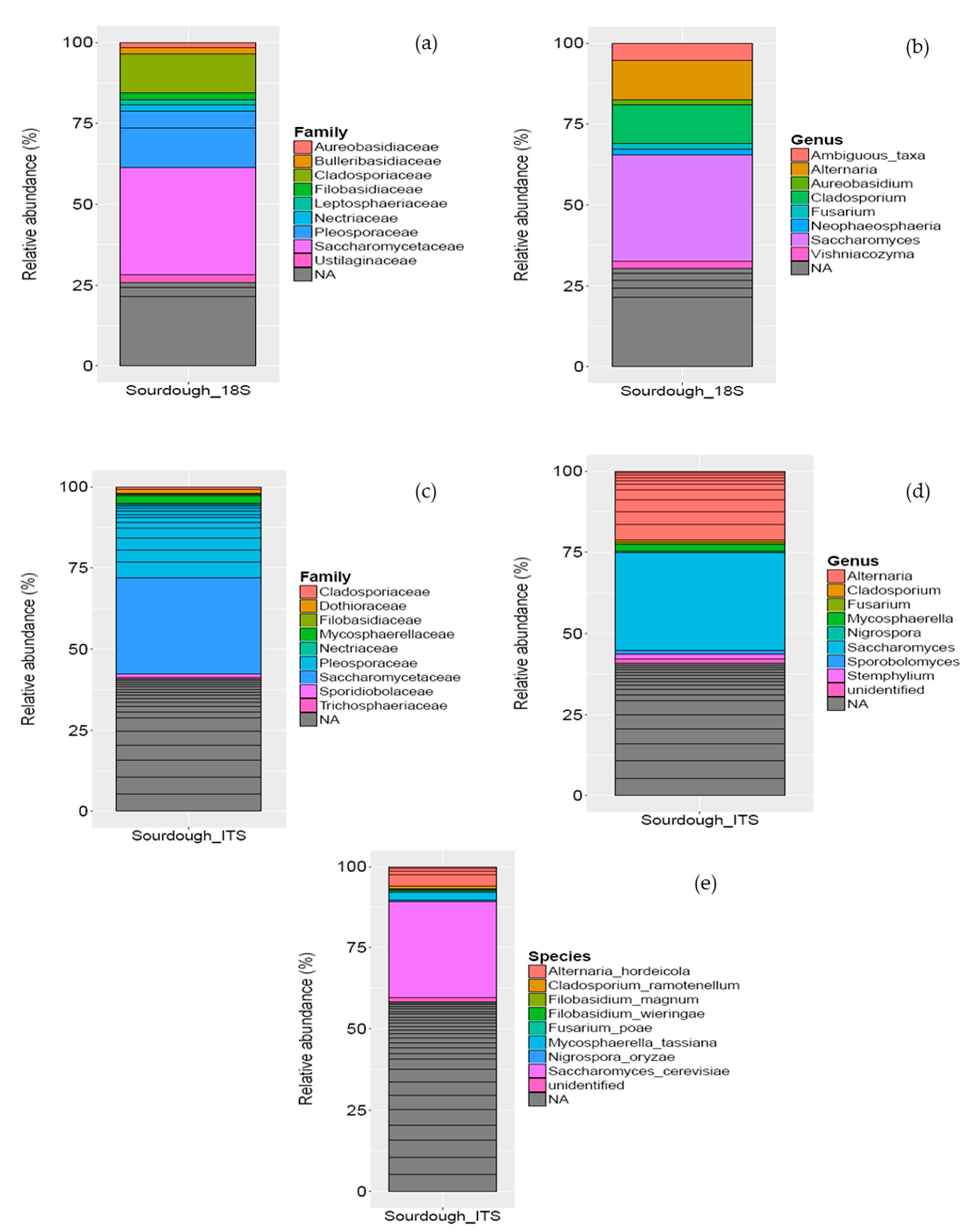
| Amplicon | Number of Raw Reads | Number of Reads after Quality Filtering | Number of Reads after Chimera Filtering | Number of OTUs Identified |
|---|---|---|---|---|
| 16S | 107.167 | 72.435 | 70.145 | 61 |
| 18S | 143.801 | 92.318 | 88.189 | 13 |
| ITS | 76.328 | 42.361 | 14.139 | 75 |
| Day 0 | Day 4 | Day 8 | Day 12 | Day 16 | Day 18 | KIEx | KILt | |
|---|---|---|---|---|---|---|---|---|
| Alcohols | ||||||||
| Ethanol | 335.50 ± 1.28 c | 343.40 ± 2.20 c | 219.30 ± 3.50 b | 214.70 ± 2.96 b | 212.80 ± 3.08 b | 190.50 ± 7.40 a | <500 | <500 |
| 2-Methyl-1-propanol | 6.13 ± 0.07 c | 5.33 ± 0.43 bc | 4.74 ± 0.12 ab | 4.20 ± 0.15 a | 4.24 ± 0.58 a | 4.16 ± 0.11 a | 623 | 625 |
| 3-Methyl-1-butanol | 73.45 ± 1.00 a | 69.69 ± 5.87 a | 47.49 ± 3.83 b | 40.79 ± 0.41 ab | 38.22 ± 0.25 a | 38.11 ± 0.16 a | 734 | 743 |
| 2-Methyl-1-butanol | 14.20 ± 2.10 d | 12.86 ± 1.36 d | 9.54 ± 0.59 a | 7.25 ± 0.22 a | 8.28 ± 0.31 a | 7.79 ± 0.25 a | 738 | 748 |
| 1-Pentanol | 7.01 ± 0.16 b | 6.70 ± 0.39 b | 4.69 ± 0.23 a | 4.67 ± 0.23 a | 4.50 ± 0.06 a | 3.86 ± 0.82 a | 765 | 766 |
| 1-Hexanol | 28.44 ± 1.66 c | 28.03 ± 2.01 c | 18.68 ± 1.97 ab | 19.46 ± 1.01 ab | 21.42 ± 1.29 b | 15.80 ± 0.56 a | 867 | 862 |
| 1-Octen-3-ol | n.d. | n.d. | n.d. | n.d. | n.d. | 57.53 ± 30.10 b | 981 | 978 |
| Sum | 464.73 ± 1.28 | 466.01 ± 2.75 | 304.74 ± 2.28 | 291.07 ± 1.30 | 289.46 ± 1.40 | 317.75 ± 3.05 | ||
| Aldehydes | ||||||||
| 3-Methyl-1-butanal | 15.85 ± 0.44 d | 4.03 ± 0.47 c | 3.35 ± 0.01 bc | 1.92 ± 0.44 a | 2.56 ± 0.13 ab | 2.90 ± 0.46 ab | 656 | 650 |
| Hexanal | 26.63 ± 1.38 d | 14.69 ± 2.65 c | 11.28 ± 0.17 bc | 10.86 ± 0.20 b | 12.46 ± 0.45 bc | 6.23 ± 1.17 a | 802 | 798 |
| Furfural | 10.70 ± 1.60 c | 9.78 ± 1.51 c | 3.38 ± 0.11 b | 1.08 ± 0.54 ab | n.d. | n.d. | 840 | 831 |
| Heptanal | 8.61 ± 0.18 c | 5.71 ± 1.35 b | 2.80 ± 0.32 a | 3.59 ± 0.24 a | 2.99 ± 0.17 a | 2.20 ± 0.27 a | 904 | 901 |
| Benzaldehyde | 10.15 ± 0.87 c | 9.39 ± 0.79 c | 3.92 ± 1.86 b | 6.58 ± 0.35 c | 4.40 ± 0.79 bc | n.d. | 980 | 970 |
| Octabal | 2.95 ± 0.43 c | 2.90 ± 0.67 bc | 1.60 ± 0.13 a | 1.93 ± 0.12 ab | 2.32 ± 0.36 abc | 4.04 ± 0.06 d | 1007 | 1004 |
| Nonanal | n.d. | 2.55 ± 0.39 ab | 3.12 ± 0.29 b | 9.42 ± 2.37 c | 12.41 ± 1.03 d | n.d. | 1109 | 1099 |
| Sum | 74.89 ± 0.96 | 49.05 ± 1.34 | 29.45 ± 0.72 | 35.28 ± 0.95 | 37.14 ± 0.59 | 15.37 ± 0.64 | ||
| Ketones | ||||||||
| 2-Pentyl-furanone | 3.15 ± 2.23 a | 2.62 ± 0.61 a | 1.18 ± 0.37 a | 2.19 ± 0.04a | 1.94 ± 0.02 a | 6.61 ± 0.64 b | 993 | 989 |
| Esters | ||||||||
| Butanoic acid, methyl ester | 4.36 ± 0.29 d | 1.95 ± 0.21 a | 2.12 ± 0.07 ab | 2.61 ± 0.20 bc | 2.09 ± 0.18 a | 3.12 ± 0.08 c | 721 | 735 |
| Sum | 4.36 ± 0.29 | 1.95 ± 0.21 | 2.12 ± 0.07 | 2.61 ± 0.20 | 2.09 ± 0.18 | 3.12 ± 0.08 | ||
| Organic Acids | ||||||||
| Acetic acid | 115.90 ± 6.10 abc | 99.30 ± 14.5 a | 131.00 ± 6.68 bc | 137.14 ± 5.90 c | 122.90 ± 6.90 bc | 113.24 ± 2.40 ab | 581 | 608 |
| Ηydrocarbons | ||||||||
| Heptane | 6.09 ± 0.31 b | 4.24 ± 0.09 a | 4.17 ± 0.27 a | 3.59 ± 0.04 a | 3.87 ± 0.19 a | 4.75 ± 1.28 ab | 698 | 700 |
| Nonane | 2.37 ± 0.09 c | 1.45 ± 0.08 b | 1.20 ± 0.05 ab | 1.49 ± 0.27 b | 0.86 ± 0.04 a | 2.55 ± 0.07 c | 901 | 900 |
| Decane | n.d. | 3.02 ± 0.49 ab | 1.70 ± 0.62 b | 3.29 ± 0.16 c | 2.05 ± 0.02 bc | 32.14 ± 0.95 d | 1001 | 1000 |
| Dodecane | n.d. | n.d. | 0.88 ± 0.44 a | n.d. | n.d. | 9.82 ± 0.84 b | 1202 | 1200 |
| Tetradecane | n.d. | n.d. | 3.51 ± 0.51 c | 1.86 ± 0.37 b | n.d. | n.d. | 1403 | 1400 |
| dl-Limonene | 1.30 ± 0.35 a | 1.22 ± 0.35 a | 0.97 ± 0.01 a | 1.40 ± 0.27 a | 4.87 ± 0.81 b | 3.78 ± 0.43 b | 1043 | 1039 |
| gamma-Terpinene | 4.33 ± 1.69 c | 2.20 ± 0.62 b | n.d. | n.d. | n.d. | n.d. | 1070 | 1062 |
| Sum | 14.09 ± 0.88 | 12.13 ± 0.39 | 12.43 ± 0.39 | 11.63 ± 0.25 | 11.65 ± 0.42 | 53.04 ± 0.82 | ||
| Aromatic compounds | ||||||||
| 2-Pentyl-furan | 19.82 ± 0.22 d | 12.85 ± 1.74 c | 5.08 ± 0.29 a | 5.26 ± 0.26 a | 4.10 ± 0.34 a | 7.99 ± 0.77 b | 994 | 992 |
| p-Cymene | 2.97 ± 0.58 c | 2.71 ± 0.70 bc | 1.89 ± 0.23 abc | 1.82 ± 0.25 ab | 0.85 ± 0.11 a | 10.70 ± 0.29 d | 1037 | 1026 |
| Sum | 22.79 ± 0.44 | 15.56 ± 1.32 | 6.79 ± 0.26 | 7.08 ± 0.25 | 4.95 ± 0.25 | 18.69 ± 0.58 | ||
| Sulfur compounds | ||||||||
| Dimethyl-disulfide | n.d. | n.d. | 0.62 ± 0.44 b | n.d. | n.d. | 0.46 ± 0.32 ab | 751 | 746 |
| Compounds | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | KIEx | KILt |
|---|---|---|---|---|---|---|---|
| Alcohols | |||||||
| Ethanol | 447.60 ± 6.30 d | 462.20 ± 15.97 d | 312.43 ± 2.96 c | 274.20 ± 7.63 b | 11.86 ± 0.63 a | - | - |
| 1-Propanol | 1.42 ± 0.36 b | 1.42 ± 0.27 b | 1.19 ± 0.04 b | n.d. | n.d. | 552 | 554 |
| 2-Methyl-1-propanol | 37.26 ± 1.37 c | 35.49 ± 2.35 c | 22.98 ± 2.49 b | 22.18 ± 1.38 b | 14.02 ± 0.59 a | 623 | 625 |
| 3-Methyl-1-butanol | 169.70 ± 5.97 d | 169.60 ± 5.72 d | 90.09 ± 4.55 c | 67.98 ± 2.49 b | 3.87 ± 0.18 a | 734 | 743 |
| 2-Methyl-1-butanol | 88.46 ± 2.69 d | 89.62 ± 3.11 d | 46.86 ± 5.95 c | 37.29 ± 0.11 b | 7.86 ± 0.69 a | 738 | 748 |
| 1-Pentanol | 4.01 ± 0.65 c | 4.11 ± 0.65 c | 2.36 ± 0.17 b | n.d. | n.d. | 765 | 766 |
| 1-Hexanol | 18.29 ± 0.04 d | 17.31 ± 1.07 d | 9.97 ± 2.70 c | 4.28 ± 0.83 b | n.d. | 867 | 862 |
| 1-Octen-3-ol | n.d. | n.d. | 4.70 ± 2.00 b | n.d. | 88.35 ± 2.42 c | 981 | 978 |
| Sum | 766.74 ± 3.48 | 779.75 ± 6.60 | 490.58 ± 3.21 | 405.93 ± 3.67 | 125.96 ± 1.19 | ||
| Aldehydes | |||||||
| 3-Methyl-1-butanal | 2.49 ± 0.19 c | 1.24 ± 0.09 b | 0.98 ± 0.16 b | n.d. | 2.77 ± 0.10 c | 656 | 650 |
| Hexanal | 15.04 ± 0.51 d | 11.74 ± 0.32 c | 1.27 ± 0.51 b | n.d. | n.d. | 802 | 798 |
| Heptanal | 6.94 ± 0.45 b | 7.29 ± 0.91 b | 0.46 ± 0.02 a | n.d. | n.d. | 904 | 901 |
| Benzaldehyde | 4.12 ± 0.76 a | 5.22 ± 1.48 a | n.d. | n.d. | n.d. | 980 | 970 |
| Octanal | 4.03 ± 0.73 d | 2.68 ± 0.09 cd | 2.06 ± 0.37 bc | 1.16 ± 0.82 ab | n.d. | 1007 | 1004 |
| Nonanal | 3.16 ± 0.26 b | 6.82 ± 0.35 c | n.d. | n.d. | n.d. | 1109 | 1099 |
| Sum | 35.78 ± 0.52 | 34.99 ± 0.74 | 4.77 ± 0.33 | 1.16 ± 0.82 | 2.77 ± 0.10 | ||
| Ketones | |||||||
| 2,3-Butanedione | 3.94 ± 0.28 b | 4.31 ± 0.30 b | 4.16 ± 0.41 b | 5.67 ± 0.32 c | 1.84 ± 0.42 a | 584 | 584 |
| 3-Hydroxy-2-butanone | 3.68 ± 0.17 b | 3.41 ± 0.38 b | 3.34 ± 0.41 b | 3.11 ± 0.07 b | n.d. | 710 | 708 |
| 2-Pentyl-furanone | 3.12 ± 0.09 ab | 2.76 ± 0.05 b | 3.61 ± 0.51 c | 2.78 ± 0.11 b | n.d. | 993 | 989 |
| Sum | 10.74 ± 0.20 | 10.48 ± 0.28 | 11.11 ± 0.45 | 11.56 ± 0.20 | 1.84 ± 0.42 | ||
| Esters | |||||||
| Formic acid, methyl ester | n.d. | n.d. | n.d. | n.d. | 18.78 ± 0.39 b | - | - |
| Acetic acid, ethyl ester | n.d. | 2.04 ± 1.04 b | 0.43 ± 0.30 a | n.d. | n.d. | 610 | 614 |
| Butanoic acid, methyl ester | 3.39 ± 0.52 ab | 2.84 ± 0.36 b | 4.13 ± 0.70 c | 2.70 ± 0.10 b | 1.41 ± 0.08 a | 721 | 735 |
| Sum | 3.39 ± 0.52 | 4.88 ± 0.78 | 4.56 ± 0.34 | 2.70 ± 0.10 | 20.19 ± 0.28 | ||
| Hydrocarbons | |||||||
| Hexane | 4.04 ± 0.07 a | 3.78 ± 0.24 a | 3.92 ± 0.32 a | 6.12 ± 0.12 c | 5.54 ± 0.11 b | 594 | 600 |
| Heptane | 6.72 ± 0.52 c | 5.12 ± 0.06 ab | 4.79 ± 0.11 a | 5.83 ± 0.28 b | 4.52 ± 0.27 a | 698 | 700 |
| Octane | n.d. | n.d. | 0.78 ± 0.03 b | 1.01 ± 0.07 c | 1.67 ± 0.00 d | 801 | 800 |
| Nonane | 2.94 ± 0.30 c | 1.94 ± 0.20 b | 1.42 ± 0.19 b | 1.34 ±0.12 ab | 0.77 ± 0.27 a | 901 | 900 |
| Decane | 5.12 ± 0.30 b | 3.56 ± 0.18 a | 4.77 ± 0.40 b | 3.39 ± 0.08 a | 6.14 ± 0.04 c | 1001 | 1000 |
| Dodecane | 6.16 ± 0.61 b | 12.22 ± 0.77 c | 11.19 ± 0.99 c | 6.43 ± 0.45 b | 2.82 ± 0.09 a | 1202 | 1200 |
| Tetradecane | 1.92 ± 0.02 a | 14.73 ± 2.60 c | 20.4 ± 1.03 d | 15.31 ± 1.21 c | 7.17 ± 1.80 b | 1403 | 1400 |
| dl-Limonene | 3.29 ± 0.15 b | 2.93 ± 0.53 b | 2.18 ± 0.01 a | 4.68 ± 0.04 c | 2.12 ± 0.07 a | 1043 | 1039 |
| Caryophyllene | n.d. | n.d. | n.d. | n.d. | 5.49 ± 1.04 b | 1459 | 1440 |
| gamma-Terpinene | n.d. | n.d. | n.d. | n.d. | 3.08 ± 0.78 b | 1070 | 1062 |
| Sum | 30.19 ± 0.34 | 44.28 ± 1.05 | 49.45 ± 0.54 | 44.11 ± 0.47 | 39.32 ± 0.71 | ||
| Aromatic compounds | |||||||
| 2-Pentyl-furan | 7.70 ± 0.61 c | 4.28 ± 0.20 b | 3.66 ± 0.53 b | n.d. | n.d. | 994 | 992 |
| p-Cymene | 4.01 ± 0.09 ab | 10.29 ± 6.28 b | 3.18 ± 0.29 ab | 2.65 ± 0.29 a | 3.20 ± 0.31 ab | 1037 | 1026 |
| Sum | 11.71 ± 0.44 | 14.56 ± 4.44 | 6.84 ± 0.43 | 2.65 ± 0.29 | 3.20 ± 0.31 | ||
| Sulfur compounds | |||||||
| Dimethyl-disulfide | n.d. | n.d. | 2.70 ± 0.09 c | 1.34 ± 0.04 b | n.d. | 751 | 746 |
| a. Hardness | b. Springiness | ||||||
|---|---|---|---|---|---|---|---|
| Yeast Bread | Sourdough Bread | Yeast Bread | Sourdough Bread | ||||
| Day | (N) | Day | (N) | Day | (mm) | Day | (mm) |
| 0 | 4.06 ± 0.59 a | 0 | 7.15 ± 1.94 a | 0 | 3.76 ± 0.92a | 0 | 1.74 ± 0.39 a |
| 2 | 4.76 ± 2.58 ab | 2 | 18.37 ± 1.88 ab | 2 | 4.53 ± 0.85a | 2 | 1.91 ± 0.60 a |
| 4 | 11.78 ± 3.53 bc | 4 | 26.21 ± 6.84 abc | 4 | 5.07 ± 0.70a | 4 | 2.00 ± 0.15 ab |
| 6 | 11.88 ± 3.42 bc | 6 | 49.34 ± 11.42 cd | 6 | 3.42 ± 0.62a | 6 | 2.07 ± 0.12 ab |
| 8 | 12.52 ± 3.02 c | 8 | 39.43 ± 7.67 bcd | 8 | 4.72 ± 0.51a | 8 | 2.61 ± 0.41 ab |
| 10 | 35.40 ± 5.89 bcd | 10 | 3.28 ± 0.30 b | ||||
| 12 | 53.78 ± 10.07 d | 12 | 2.51 ± 0.49 ab | ||||
| 14 | 52.65 ± 10.68 cd | 14 | 2.93 ± 0.36 ab | ||||
| 16 | 58.60 ± 10.83 d | 16 | 2.76 ± 0.35 ab | ||||
| 18 | 48.09 ± 16.20 cd | 18 | 2.21 ± 0.61 ab | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsi, P.; Kosma, I.S.; Michailidou, S.; Argiriou, A.; Badeka, A.V.; Kontominas, M.G. Characterization of Artisanal Spontaneous Sourdough Wheat Bread from Central Greece: Evaluation of Physico-Chemical, Microbiological, and Sensory Properties in Relation to Conventional Yeast Leavened Wheat Bread. Foods 2021, 10, 635. https://doi.org/10.3390/foods10030635
Katsi P, Kosma IS, Michailidou S, Argiriou A, Badeka AV, Kontominas MG. Characterization of Artisanal Spontaneous Sourdough Wheat Bread from Central Greece: Evaluation of Physico-Chemical, Microbiological, and Sensory Properties in Relation to Conventional Yeast Leavened Wheat Bread. Foods. 2021; 10(3):635. https://doi.org/10.3390/foods10030635
Chicago/Turabian StyleKatsi, Pavlina, Ioanna S. Kosma, Sofia Michailidou, Anagnostis Argiriou, Anastasia V. Badeka, and Michael G. Kontominas. 2021. "Characterization of Artisanal Spontaneous Sourdough Wheat Bread from Central Greece: Evaluation of Physico-Chemical, Microbiological, and Sensory Properties in Relation to Conventional Yeast Leavened Wheat Bread" Foods 10, no. 3: 635. https://doi.org/10.3390/foods10030635
APA StyleKatsi, P., Kosma, I. S., Michailidou, S., Argiriou, A., Badeka, A. V., & Kontominas, M. G. (2021). Characterization of Artisanal Spontaneous Sourdough Wheat Bread from Central Greece: Evaluation of Physico-Chemical, Microbiological, and Sensory Properties in Relation to Conventional Yeast Leavened Wheat Bread. Foods, 10(3), 635. https://doi.org/10.3390/foods10030635