Use of Chia by-Products Obtained from the Extraction of Seeds Oil for the Development of New Biodegradable Films for the Agri-Food Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Defatting of Chia Seeds and Mucilage Extraction
2.3. Preparation of the Chia Films
2.4. Characterization of Chia Biofilms
2.4.1. Moisture Content and Water Solubility (WS)
2.4.2. Water Vapor Permeability (WVP)
2.4.3. Color and Opacity
2.4.4. Light Transmittance
2.4.5. Thickness and Mechanical Properties
2.5. Statistical Analysis
3. Results
3.1. Mucilage Extraction Yields
3.2. Moisture Content, Water Solubility, and Thickness
3.3. Water Vapor Permeability (WVP)
3.4. Color
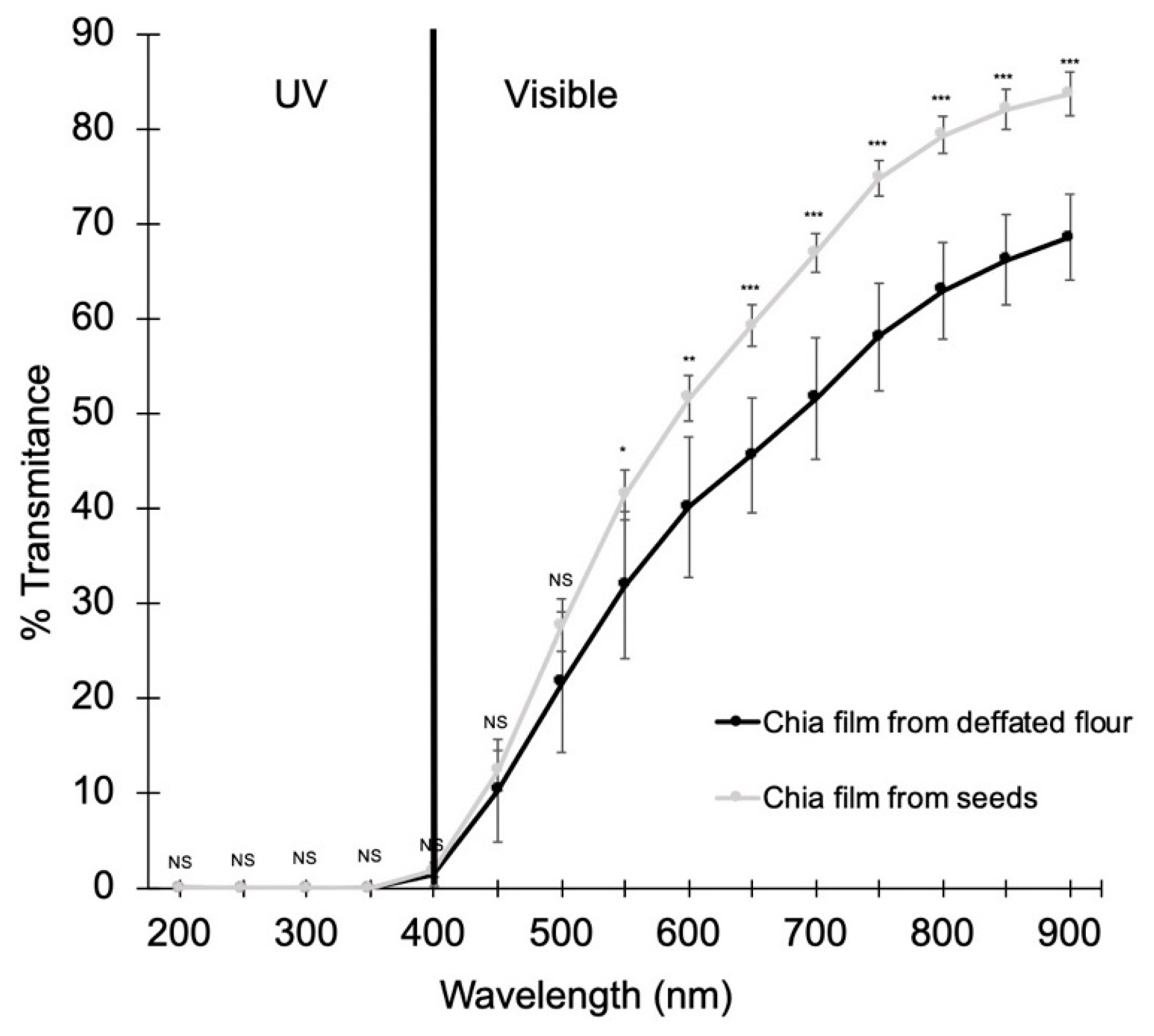
3.5. Optical Properties
3.6. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giteru, S.G.; Oey, I.; Ali, M.A.; Johnson, S.K.; Fang, Z. Effect of kafirin-based films incorporating citral and quercetin on storage of fresh chicken fillets. Food Control 2017, 80, 37–44. [Google Scholar] [CrossRef]
- Eurostat Packaging Waste Statistics 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Packaging_waste_statistics (accessed on 15 December 2020).
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; Garrigós, M.D.; Jiménez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef]
- Hernandez-Izquierdo, V.M.; Krochta, J.M. Thermoplastic processing of proteins for film formation—A review. J. Food Sci. 2008, 73, 30–39. [Google Scholar] [CrossRef]
- Soukoulis, C.; Gaiani, C.; Hoffmann, L. Plant seed mucilage as emerging biopolymer in food industry applications. Curr. Opin. Food Sci. 2018, 22, 28–42. [Google Scholar] [CrossRef]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Natural pectin polysaccharides as edible coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef]
- Cuomo, F.; Iacovino, S.; Messia, M.C.; Sacco, P.; Lopez, F. Protective action of lemongrass essential oil on mucilage from chia (Salvia hispanica) seeds. Food Hydrocoll. 2020, 105, 105860. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Romani, V.P.; da Silva Filipini, G.; Martins, V.G. Chia seeds to develop new biodegradable polymers for food packaging: Properties and biodegradability. Polym. Eng. Sci. 2020, 60, 2214–2223. [Google Scholar] [CrossRef]
- Lin, K.Y.; Daniel, J.R.; Whistler, R.L. Structure of chia seed polysaccharide exudate. Carbohydr. Polym. 1994, 23, 13–18. [Google Scholar] [CrossRef]
- Muñoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia seeds: Microstructure, mucilage extraction and hydration. J. Food Eng. 2012, 108, 216–224. [Google Scholar] [CrossRef]
- Campos, B.E.; Dias Ruivo, T.; da Silva Scapim, M.R.; Madrona, G.S.; Bergamasco, R.D.C. Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT Food Sci. Technol. 2016, 65, 874–883. [Google Scholar] [CrossRef]
- Chaves, M.A.; Piati, J.; Malacarne, L.T.; Gall, R.E.; Colla, E.; Bittencourt, P.R.S.; de Souza, A.H.P.; Gomes, S.T.M.; Matsushita, M. Extraction and application of chia mucilage (Salvia hispanica L.) and locust bean gum (Ceratonia siliqua L.) in goat milk frozen dessert. J. Food Sci. Technol. 2018, 55, 4148–4158. [Google Scholar] [CrossRef] [PubMed]
- Zettel, V.; Hitzmann, B. Applications of chia (Salvia hispanica L.) in food products. Trends Food Sci. Technol. 2018, 80, 43–50. [Google Scholar] [CrossRef]
- Saha, A.; Tyagi, S.; Gupta, R.K.; Tyagi, Y.K. Natural gums of plant origin as edible coatings for food industry applications. Crit. Rev. Biotechnol. 2017, 37, 959–973. [Google Scholar] [CrossRef]
- Muñoz-Tébar, N.; De la Vara, J.A.; Ortiz de Elguea-Culebras, G.; Cano, E.L.; Molina, A.; Carmona, M.; Berruga, M.I. Enrichment of sheep cheese with chia (Salvia hispanica L.) oil as a source of omega-3. LWT 2019, 108. [Google Scholar] [CrossRef]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; Rios, A.D.O.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef]
- Costa, M.J.; Cerqueira, M.A.; Ruiz, H.A.; Fougnies, C.; Richel, A.; Vicente, A.A.; Teixeira, J.A.; Aguedo, M. Use of wheat bran arabinoxylans in chitosan-based films: Effect on physicochemical properties. Ind. Crops Prod. 2015, 66, 305. [Google Scholar] [CrossRef]
- Khazaei, N.; Esmaiili, M.; Djomeh, Z.E.; Ghasemlou, M.; Jouki, M. Characterization of new biodegradable edible film made from basil seed (Ocimum basilicum L.) gum. Carbohydr. Polym. 2014, 102, 199–206. [Google Scholar] [CrossRef]
- ASTM D882-10. Standard Test Method for Tensile Properties of Thin Plastic Sheeting 2010. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/D882-10.htm (accessed on 15 January 2020).
- Segura-Campos, M.R.; Ciau-Solís, N.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Chemical and functional properties of chia seed (Salvia hispanica L.) gum. Int. J. Food Sci. 2014, 2014. [Google Scholar] [CrossRef]
- Dick, M.; Pagno, C.H.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; Rios, A.; de Oliveira Rios, A.; Flôres, S.H. Edible films based on chia flour: Development and characterization. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Physical, barrier and antioxidant properties of a novel plasticized edible film from quince seed mucilage. Int. J. Biol. Macromol. 2013, 62, 500–507. [Google Scholar] [CrossRef]
- González Sandoval, D.; Luna Sosa, B.; Martínez-Ávila, G.C.G.; Rodríguez Fuentes, H.; Avendaño Abarca, V.H.; Rojas, R. Formulation and characterization of edible films based on organic mucilage from Mexican Opuntia ficus-indica. Coatings 2019, 9, 506. [Google Scholar] [CrossRef]
- Ortiz de Elguea-Culebras, G.; Bourbon, A.I.; Costa, M.J.; Muñoz-Tebar, N.; Carmona, M.; Molina, A.; Sánchez-Vioque, R.; Berruga, M.I.; Vicente, A.A. Optimization of a chitosan solution as potential carrier for the incorporation of Santolina chamaecyparissus L. solid by-product in an edible vegetal coating on ‘Manchego’ cheese. Food Hydrocoll. 2019, 89. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Mohammad, A.A.; Zahedi, Y. Characterisation of a new biodegradable edible film based on sage seed gum: Influence of plasticiser type and concentration. Food Hydrocoll. 2015, 43, 290–298. [Google Scholar] [CrossRef]
- Muñoz, L.A.; Aguilera, J.M.; Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Characterization and microstructure of films made from mucilage of Salvia hispanica and whey protein concentrate. J. Food Eng. 2012, 111, 511–518. [Google Scholar] [CrossRef]
- Salazar Vega, I.M.; Quintana Owen, P.; Segura Campos, M. Physicochemical, thermal, mechanical, optical, and barrier characterization of chia (Salvia hispanica L.) mucilage-protein concentrate biodegradable films. J. Food Sci. 2020, 85, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M.; Khazaei, N.; Ghasemlou, M.; Hadinezhad, M. Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr. Polym. 2013, 96, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Kalbasi-Ashtari, A.; Oromiehie, A.; Yarmand, M.S.; Jahandideh, F. Development and characterization of a novel biodegradable edible film obtained from psyllium seed (Plantago ovata Forsk). J. Food Eng. 2012, 109, 745–751. [Google Scholar] [CrossRef]
- Sadeghi-varkani, A.; Emam-djomeh, Z.; Askari, G. Physicochemical and Microstructural Properties of a Novel Edible Film Synthesized from Balangu Seed Mucilage. Int. J. Biol. Macromol. 2017, 108, 1110–1119. [Google Scholar] [CrossRef]
- Kurt, A.; Kahyaoglu, T. Characterization of a new biodegradable edible film made from salep glucomannan. Carbohydr. Polym. 2014, 104, 50–58. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.; Mauri, A.N.; Menegalli, F.C.; Sobral, P.J.A.; Añón, M.C. Contribution of the starch, protein, and lipid fractions to the physical, thermal, and structural properties of amaranth (Amaranthus caudatus) flour films. J. Food Sci. 2007, 72, 293–300. [Google Scholar] [CrossRef]
- Araújo, A.; Galvão, A.; Filho, C.S.; Mendes, F.; Oliveira, M.; Barbosa, F.; Filho, M.S.; Bastos, M. Okra mucilage and corn starch bio-based film to be applied in food. Polym. Test. 2018, 71, 352–361. [Google Scholar] [CrossRef]
- Mujtaba, M.; Koc, B.; Martinez Salaberria, A.; Ilk, S.; Cansaran-Duman, D.; Akyuz, L.; Cakmak, Y.; Kaya, M.; Khawar, K..; Labidi, J.; et al. Production of novel chia-mucilage nanocomposite films with starch nanocrystals; An inclusive biological and physicochemical perspective. Int. J. Biol. Macromol. 2019, 133, 663–673. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Barrier, mechanical and optical properties of plasticized yam starch films. Carbohydr. Polym. 2004, 56, 129–135. [Google Scholar] [CrossRef]
- Chambi, H.; Grosso, C. Effect of surfactants on the functional properties of gelatin-polysaccharide-based films. Eur. Food Res. Technol. 2011, 232, 63–69. [Google Scholar] [CrossRef]
- Shiku, Y.; Hamaguchi, P.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effect of surimi quality on properties of edible films based on Alaska pollack. Food Chem. 2004, 86, 493–499. [Google Scholar] [CrossRef]
- Zavareze, E.; Pinto, V.Z.; Klein, B.; El Halal, S.L.; Elias, M.C.; Prentice-Hernández, C.; Dias, A.R. Development of oxidised and heat-moisture treated potato starch film. Food Chem. 2012, 132, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Beikzadeh, S.; Khezerlou, A.; Jafari, S.M.; Pilevar, Z.; Mortazavian, A.M. Seed mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Adv. Colloid Interface Sci. 2020, 280, 102164. [Google Scholar] [CrossRef]

| Parameters | Chia Film from Defatted Flour | Chia Film from Seeds | p-Value 1 |
|---|---|---|---|
| % Moisture | 22.44 ± 1.19 | 24.73 ± 1.83 | * |
| % Water solubility | 64.45 ± 3.90 | 82.56 ± 4.82 | *** |
| Thickness (mm) | 0.12 ± 0.01 | 0.09 ± 0.01 | *** |
| WVP × 10−10 (g s−1 m−1 Pa−1) | 0.58 ± 0.03 | 0.33 ± 0.03 | *** |
| Parameters | Chia Film from Defatted Flour | Chia Film from Seeds | p-Value 1 |
|---|---|---|---|
| L* | 56.42 ± 4.15 | 52.58 ± 2.67 | NS |
| a* | 5.45 ± 0.32 | 8.92 ± 0.72 | *** |
| b* | 37.39 ± 4.16 | 38.98 ± 0.60 | NS |
| ∆E | 54.4 ± 5.71 | 58.77 ± 2.12 | NS |
| WI | 42.31 ± 5.71 | 37.94 ± 2.12 | NS |
| % Opacity | 26.87 ± 0.75 | 25.21 ± 0.99 | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Tebar, N.; Molina, A.; Carmona, M.; Berruga, M.I. Use of Chia by-Products Obtained from the Extraction of Seeds Oil for the Development of New Biodegradable Films for the Agri-Food Industry. Foods 2021, 10, 620. https://doi.org/10.3390/foods10030620
Muñoz-Tebar N, Molina A, Carmona M, Berruga MI. Use of Chia by-Products Obtained from the Extraction of Seeds Oil for the Development of New Biodegradable Films for the Agri-Food Industry. Foods. 2021; 10(3):620. https://doi.org/10.3390/foods10030620
Chicago/Turabian StyleMuñoz-Tebar, Nuria, Ana Molina, Manuel Carmona, and María Isabel Berruga. 2021. "Use of Chia by-Products Obtained from the Extraction of Seeds Oil for the Development of New Biodegradable Films for the Agri-Food Industry" Foods 10, no. 3: 620. https://doi.org/10.3390/foods10030620
APA StyleMuñoz-Tebar, N., Molina, A., Carmona, M., & Berruga, M. I. (2021). Use of Chia by-Products Obtained from the Extraction of Seeds Oil for the Development of New Biodegradable Films for the Agri-Food Industry. Foods, 10(3), 620. https://doi.org/10.3390/foods10030620






