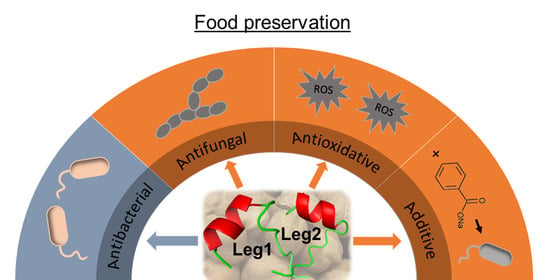
Antioxidative, Antifungal and Additive Activity of the Antimicrobial Peptides Leg1 and Leg2 from Chickpea
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis
2.2. Bacterial and Fungal Strains and Culture Conditions
2.3. Checkerboard Assay to Test for Combinatorial Effects of Leg1/Leg2 and Sodium Benzoate
2.4. Antifungal Susceptibility Tests
2.5. Flow Cytometry Measurement of Antifungal Activity
2.6. Assays for Antioxidative Activity
2.7. Cell Culture
2.8. MTT Cytotoxicity Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Cooperative Effects of Leg1 and Leg2 with Sodium Benzoate on Bacterial Growth
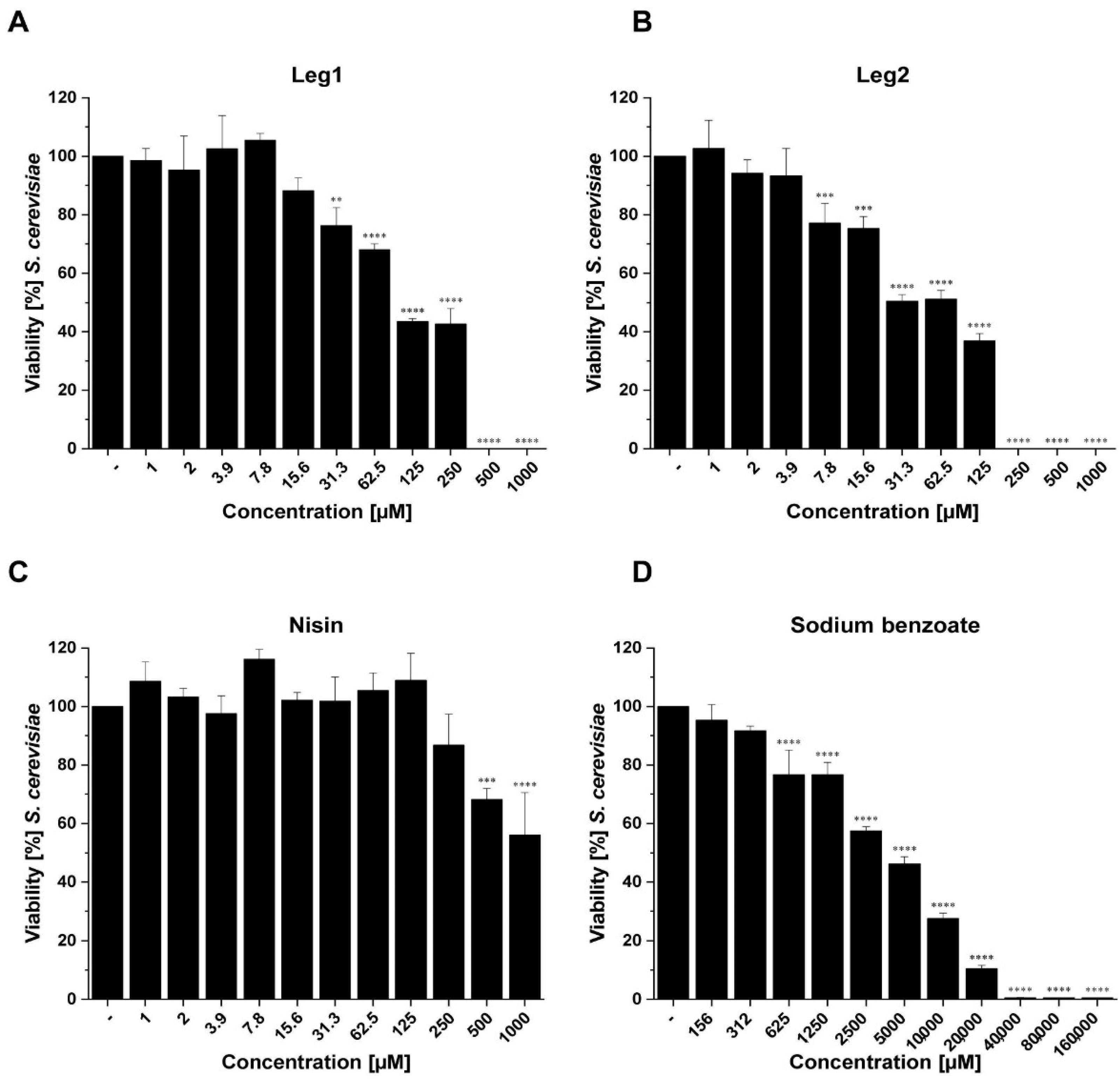
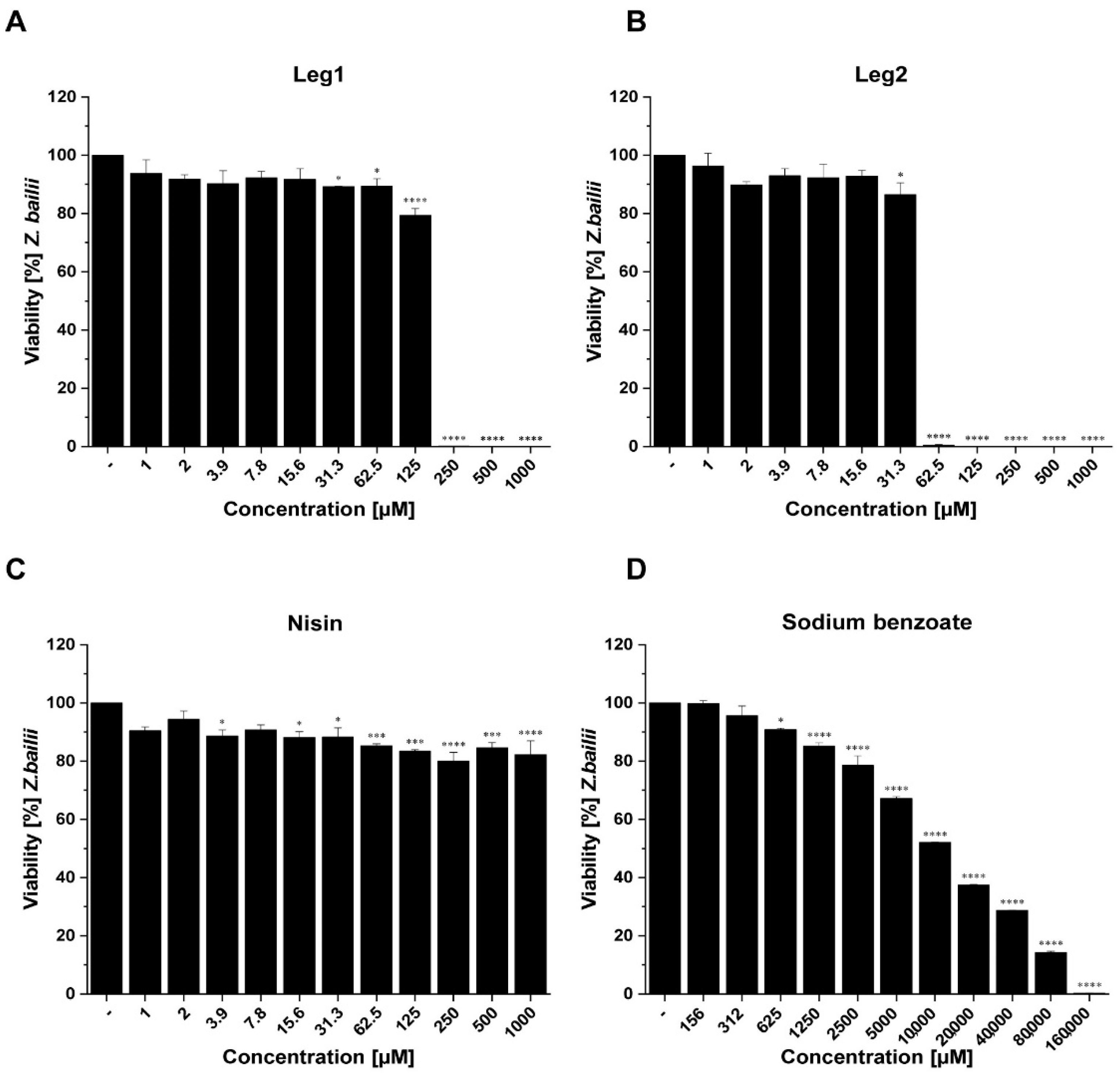
3.2. Antifungal Activity of Leg1 and Leg2
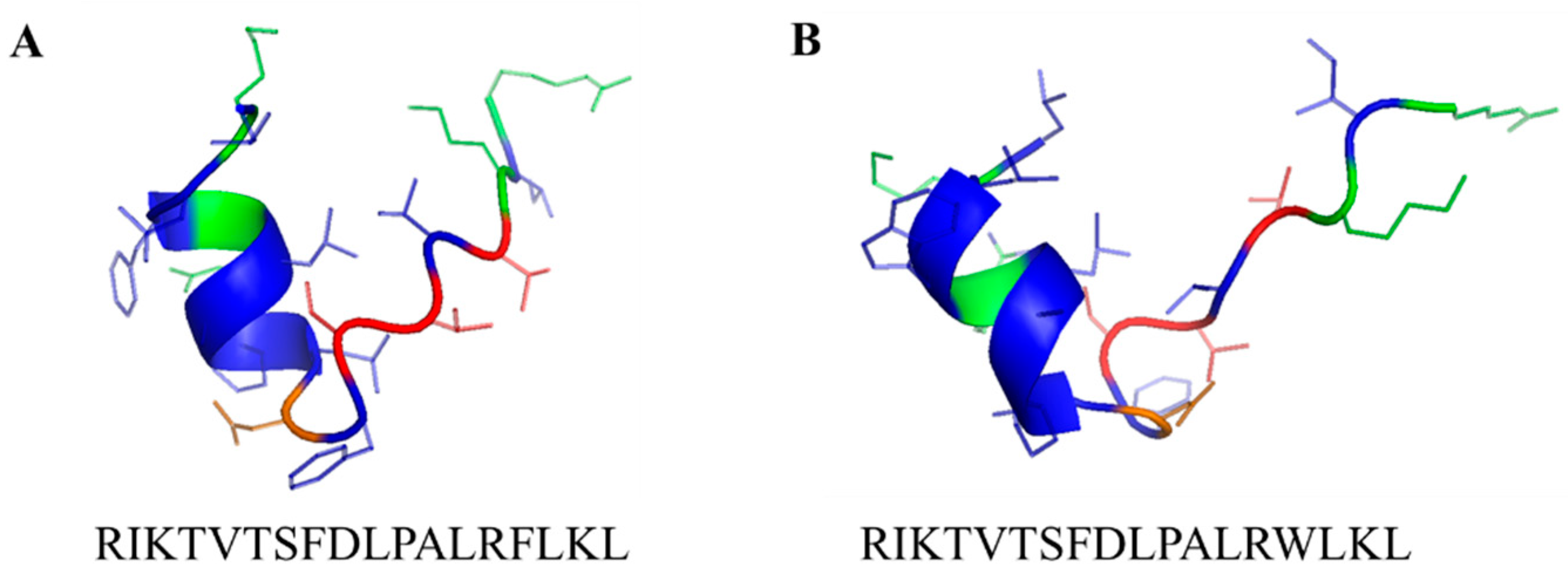
3.3. Antioxidative Activity of Leg1/Leg2
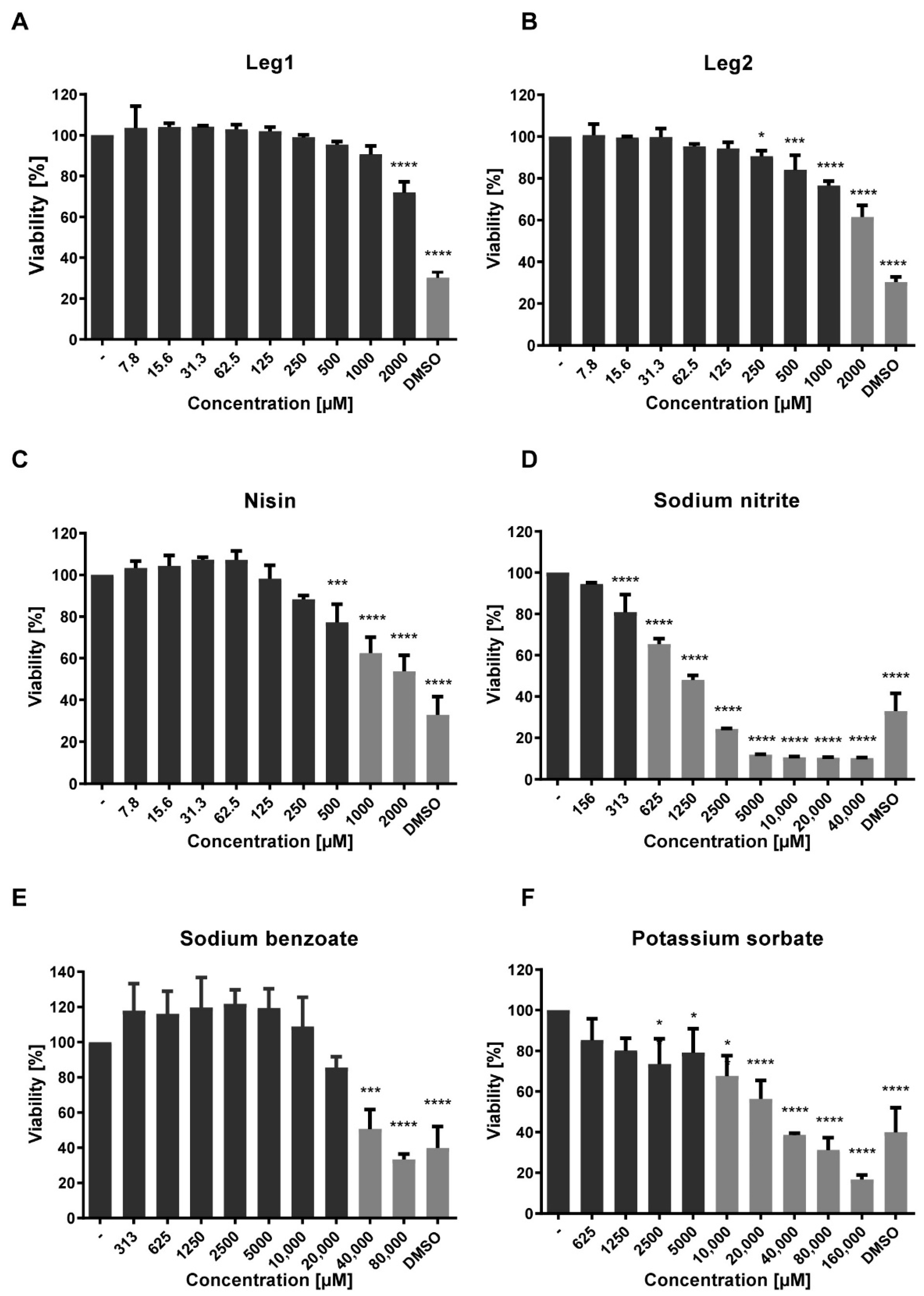
3.4. Cytotoxicity against Caco-2 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalabasmaz, S.; Pischetsrieder, M. Peptidomics in Food. In Comprehensive Foodomics; Cifuentes, A., Ed.; Elsevier: Cambridge, MA, USA, 2021; pp. 651–665. [Google Scholar]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and Recent Advances in Peptide and Protein Drug Delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Gaikwad, S.; Kovics, G. Antimicrobial Peptides as Natural Bio-Preservative to Enhance the Shelf-Life of Food. J. Food Sci. Technol. 2016, 53, 3381–3394. [Google Scholar] [CrossRef]
- Heymich, M.-L.; Friedlein, U.; Trollmann, M.; Schwaiger, K.; Böckmann, R.A.; Pischetsrieder, M. Generation of Antimicrobial Peptides Leg1 and Leg2 from Chickpea Storage Protein, Active against Food Spoilage Bacteria and Foodborne Pathogens. Food Chem. 2021, 347, 128917. [Google Scholar] [CrossRef]
- van der Weerden, N.L.; Hancock, R.E.; Anderson, M.A. Permeabilization of Fungal Hyphae by the Plant Defensin Nad1 Occurs through a Cell Wall-Dependent Process. J. Biol. Chem. 2010, 285, 37513–37520. [Google Scholar] [CrossRef]
- Thomas, D.S. Yeasts as Spoilage Organisms in Beverages. In The Yeasts, 2nd ed.; Rose, A.H., Harrison, J.S., Eds.; Academic Press: London, UK, 1993; pp. 517–561. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 2nd ed.; Springer: London, UK, 1997. [Google Scholar]
- James, S.A.; Stratford, M. Spoilage Yeasts with Emphasis on the Genus Zygosaccharomyces. In Yeasts in Food; Boekhout, T., Robert, V., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2003; pp. 171–191. [Google Scholar]
- Stratford, M.; Steels, H.; Nebe-von-Caron, G.; Novodvorska, M.; Hayer, K.; Archer, D.B. Extreme Resistance to Weak-Acid Preservatives in the Spoilage Yeast Zygosaccharomyces Bailii. Int. J. Food Microbiol. 2013, 166, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Steels, H.; James, S.A.; Roberts, I.N.; Stratford, M. Sorbic Acid Resistance: The Inoculum Effect. Yeast 2000, 16, 1173–1183. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. Subcommittee on Antifungal Susceptiblitiy Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing. In Eucast Definitive Document E.Def 7.3.1 Method for the Determination of Broth Dilution Minimum Inhibitory Concentratins of Antifungal Agents for Yeasts; ESCMID European Committee for Antimicrobial Susceptiblity Testing—EUCAST: Växjö, Sweden, 2017. [Google Scholar]
- Arendrup, M.C.; Guinea, J.; Cuenca-Estrella, M.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Howard, S.J. Subcommittee on Antifungal Susceptiblitiy Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing. In Eucast Definitive Document E.Def 9.3 Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds Version 9.3; The European Committee on Antimicrobial Susceptibility Testing—EUCAST: Växjö, Sweden, 2015. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre Plate-Based Antibacterial Assay Incorporating Resazurin as an Indicator of Cell Growth, and Its Application in the in Vitro Antibacterial Screening of Phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-Antioxidant Activity Relationship of Methoxy, Phenolic Hydroxyl, and Carboxylic Acid Groups of Phenolic Acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid Content of Several Vegetables and Their Antioxidant Activity. J. Sci. Food Agr. 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Brul, S.; Coote, P. Preservative Agents in Foods. Mode of Action and Microbial Resistance Mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef]
- Mohammadi Azad, Z.; Moravej, H.; Fasihi-Ramandi, M.; Masjedian, F.; Nazari, R.; Mirnejad, R.; Moosazadeh Moghaddam, M. In Vitro Synergistic Effects of a Short Cationic Peptide and Clinically Used Antibiotics against Drug-Resistant Isolates of Brucella Melitensis. J. Med. Microbiol. 2017, 66, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Kampshoff, F.; Willcox, M.D.P.; Dutta, D. A Pilot Study of the Synergy between Two Antimicrobial Peptides and Two Common Antibiotics. Antibiotics 2019, 8, 60. [Google Scholar] [CrossRef]
- Lopez-Exposito, I.; Pellegrini, A.; Amigo, L.; Recio, I. Synergistic Effect between Different Milk-Derived Peptides and Proteins. J. Dairy Sci. 2008, 91, 2184–2189. [Google Scholar] [CrossRef]
- Stanojevic, D.; Comic, L.; Stefanovic, O.; Solujic-Sukdolak, S. Antimicrobial Effects of Sodium Benzoate, Sodium Nitrite and Potassium Sorbate and Their Synergistic Action in Vitro. Bulg. J. Agric. Sci. 2009, 15, 308–312. [Google Scholar]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Georgopapadakou, N.H.; Walsh, T.J. Antifungal Agents: Chemotherapeutic Targets and Immunologic Strategies. Antimicrob. Agents Chemother. 1996, 40, 279–291. [Google Scholar] [CrossRef]
- Letscher-Bru, V.; Herbrecht, R. Caspofungin: The First Representative of a New Antifungal Class. J. Antimicrob. Chemother. 2003, 51, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Free, S.J. Fungal Cell Wall Organization and Biosynthesis. Adv. Genet. 2013, 81, 33–82. [Google Scholar] [CrossRef]
- Gastebois, A.; Clavaud, C.; Aimanianda, V.; Latge, J.P. Aspergillus Fumigatus: Cell Wall Polysaccharides, Their Biosynthesis and Organization. Future Microbiol. 2009, 4, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Lesage, G.; Bussey, H. Cell Wall Assembly in Saccharomyces Cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef]
- Grillitsch, K.; Tarazona, P.; Klug, L.; Wriessnegger, T.; Zellnig, G.; Leitner, E.; Feussner, I.; Daum, G. Isolation and Characterization of the Plasma Membrane from the Yeast Pichia Pastoris. Biochim. Biophys. Acta 2014, 1838, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- James, S.A.; Collins, M.D.; Roberts, I.N. Genetic Interrelationship among Species of the Genus Zygosaccharomyces as Revealed by Small-Subunit Rrna Gene Sequences. Yeast 1994, 10, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P. Discussion of Teleomorphic and Anamorphic Ascomycetous Yeasts and Yeast-Like Taxa. In The Yeasts. A Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Burlington: London, UK; Elsevier Science: San Diego, CA, USA, 2011; pp. 293–307. [Google Scholar]
- Nguyen, T.H.; Fleet, G.H.; Rogers, P.L. Composition of the Cell Walls of Several Yeast Species. Appl. Microbiol. Biotechnol. 1998, 50, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Hazan, R.; Levine, A.; Abeliovich, H. Benzoic Acid, a Weak Organic Acid Food Preservative, Exerts Specific Effects on Intracellular Membrane Trafficking Pathways in Saccharomyces Cerevisiae. Appl. Environ. Microbiol. 2004, 70, 4449–4457. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.V.; Delves-Broughton, J. Nisin. In Antimicrobials in Food; Davidson, P.M., Sofos, J.N., Branen, A.L., Eds.; CRC Press, Taylor & Francis Publishing Group: Boca-Raton, FL, USA, 2005; pp. 237–274. [Google Scholar]
- Dielbandhoesing, S.K.; Zhang, H.; Caro, L.H.; van der Vaart, J.M.; Klis, F.M.; Verrips, C.T.; Brul, S. Specific Cell Wall Proteins Confer Resistance to Nisin Upon Yeast Cells. Appl. Environ. Microbiol. 1998, 64, 4047–4052. [Google Scholar] [CrossRef]
- Gossler-Schofberger, R.; Hesser, G.; Muik, M.; Wechselberger, C.; Jilek, A. An Orphan Dermaseptin from Frog Skin Reversibly Assembles to Amyloid-Like Aggregates in a Ph-Dependent Fashion. FEBS J. 2009, 276, 5849–5859. [Google Scholar] [CrossRef]
- Raghuraman, H.; Chattopadhyay, A. Effect of Ionic Strength on Folding and Aggregation of the Hemolytic Peptide Melittin in Solution. Biopolymers 2006, 83, 111–121. [Google Scholar] [CrossRef]
- Rao, B.D.; Chakraborty, H.; Keller, S.; Chattopadhyay, A. Aggregation Behavior of Phlip in Aqueous Solution at Low Concentrations: A Fluorescence Study. J. Fluoresc. 2018, 28, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, N.K.; Parente, R.A.; Szoka, F.C., Jr.; Nadasdi, L.; Pongracz, K. Ph-Dependent Bilayer Destabilization by an Amphipathic Peptide. Biochemistry 1987, 26, 2964–2972. [Google Scholar] [CrossRef]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Power, O.; Jakeman, P.; FitzGerald, R.J. Antioxidative Peptides: Enzymatic Production, in Vitro and in Vivo Antioxidant Activity and Potential Applications of Milk-Derived Antioxidative Peptides. Amino Acids 2013, 44, 797–820. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Nino, A.; Rodriguez-Serrano, G.M.; Gonzalez-Olivares, L.G.; Contreras-Lopez, E.; Regal-Lopez, P.; Cepeda-Saez, A. Sequence Identification of Bioactive Peptides from Amaranth Seed Proteins (Amaranthus Hypochondriacus Spp.). Molecules 2019, 24, 3033. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative Properties of Histidine-Containing Peptides Designed from Peptide Fragments Found in the Digests of a Soybean Protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, C.F.; Fashakin, J.B.; Fagbemi, T.N.; Aluko, R.E. Effect of Peptide Size on Antioxidant Properties of African Yam Bean Seed (Sphenostylis Stenocarpa) Protein Hydrolysate Fractions. Int. J. Mol. Sci. 2011, 12, 6685–6702. [Google Scholar] [CrossRef]
- Sangtitanu, T.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Karnchanatat, A. Peptides Obtained from Edible Mushrooms: Hericium Erinaceus Offers the Ability to Scavenge Free Radicals and Induce Apoptosis in Lung Cancer Cells in Humans. Food Funct. 2020, 11, 4927–4939. [Google Scholar] [CrossRef]
- Wu, D.; Sun, N.; Ding, J.; Zhu, B.; Lin, S. Evaluation and Structure-Activity Relationship Analysis of Antioxidant Shrimp Peptides. Food Funct. 2019, 10, 5605–5615. [Google Scholar] [CrossRef]
- Bueno-Gavilá, E.; Abellán, A.; Girón-Rodríguez, F.; Cayuela, J.M.; Tejada, L. Bioactivity of Hydrolysates Obtained from Chicken Egg Ovalbumin Using Artichoke (Cynara Scolymus L.) Proteases. Foods 2021, 10, 246. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Karas, M.; Stanikowski, P.; Rutkowska, B.; Dziedzic, M.; Zielinska, E.; Szychowski, K.A.; Binduga, U.E.; Rybczynska-Tkaczyk, K.; Baraniak, B. Characterisation of Biologically Active Hydrolysates and Peptide Fractions of Vacuum Packaging String Bean (Phaseolus Vulgaris L.). Foods 2020, 9, 842. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 Cell Line as a Model of the Intestinal Barrier: Influence of Cell and Culture-Related Factors on Caco-2 Cell Functional Characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- International Organization for Standardization. Iso 10993-5:2009, Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; Vernier: Geneva, Switzerland, 2009. [Google Scholar]
- Ahmadi, S.; Ghollasi, M.; Hosseini, H.M. The Apoptotic Impact of Nisin as a Potent Bacteriocin on the Colon Cancer Cells. Microb. Pathog. 2017, 111, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Lewies, A.; Wentzel, J.F.; Miller, H.C.; Du Plessis, L.H. The Antimicrobial Peptide Nisin Z Induces Selective Toxicity and Apoptotic Cell Death in Cultured Melanoma Cells. Biochimie 2018, 144, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Zainodini, N.; Hassanshahi, G.; Hajizadeh, M.; Khanamani Falahati-Pour, S.; Mahmoodi, M.; Mirzaei, M.R. Nisin Induces Cytotoxicity and Apoptosis in Human Asterocytoma Cell Line (Sw1088). Asian Pac. J. Cancer Prev. 2018, 19, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Vaucher, R.A.; Teixeira, M.L.; Brandelli, A. Investigation of the Cytotoxicity of Antimicrobial Peptide P40 on Eukaryotic Cells. Curr. Microbiol. 2010, 60, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, Z.; Bian, Y.; Hu, G.; Wang, J.; Zhou, Y. Molecular Dynamics Simulations of Human Antimicrobial Peptide Ll-37 in Model Popc and Popg Lipid Bilayers. Int. J. Mol. Sci. 2018, 19, 1186. [Google Scholar] [CrossRef]

| Peptide | MICA/MICAB (Peptide) [µM] | MICB/MICBA (SB) [µM] | FIC 1 |
|---|---|---|---|
| Leg1 | 62.5/31.3 | 80,000/10,000 | 0.625 |
| Leg2 | 62.5/31.3 | 80,000/10,000 | 0.625 |
| Nisin | 125/15.6 | 80,000/40,000 | 0.625 |
| Peptide | MICA/MICAB (Peptide) [µM] | MICB/MICBA (SB) [µM] | FIC 1 |
|---|---|---|---|
| Leg1 | 15.6/7.8 | 80,000/10,000 | 0.75 |
| Leg2 | 15.6/7.8 | 80,000/10,000 | 0.75 |
| Nisin | 7.8/2.0 | 80,000/40,000 | 0.75 |
| Concentration [µM] | Antioxidant Activity (%) | ||||||
|---|---|---|---|---|---|---|---|
| 1000 | 500 | 250 | 125 | 62.5 | 31.3 | 15.6 | |
| Leg1 (RIKTVTSFDLPALRFLKL) | 29.3 ± 3.7 | 6.0 ± 8.2 | 0.30 ± 0.5 | - 1 | - | - | - |
| Leg2 (RIKTVTSFDLPALRWLKL) | 91.5 ± 8.3 | 72.2 ± 12.8 | 46.2 ± 16.6 | 20.9 ± 22.6 | 26.7 ± 1.3 | 17.7 ± 2.3 | 5.3 ± 3.3 |
| Tyrosine | 50.3 ± 6.0 | 52.4 ± 4.5 | 42.3 ± 11.6 | 31.2 ± 4.5 | 40.1 ± 7.5 | 10.4 ± 12.2 | - |
| Histidine | 34.5 ± 9.1 | 32.8 ± 7.3 | 30.0 ± 3.2 | 17.9 ± 11.9 | 19.1 ± 3.7 | 6.1 ± 4.0 | 0.2 ± 6.4 |
| Cysteine | 93.6 ± 1.9 | 82.6 ± 8.9 | 95.7 ± 1.5 | 94.1 ± 2.2 | 90.2 ± 8.8 | 88.5 ± 7.2 | 72.8 ± 11.6 |
| Tryptophan | 60.8 ± 11.1 | 38.6 ± 2.5 | 22.8 ± 4.4 | 15.2 ± 7.9 | 17.9 ± 5.8 | 19.5 ± 5.6 | 17.6 ± 5.7 |
| Proline | 28.7 ± 9.4 | 25.6 ± 10.9 | 23.3 ± 5.9 | 19.0 ± 11.6 | 8.5 ± 8.5 | - | - |
| Phenylalanine | 29.0 ± 6.2 | 20.5 ± 3.9 | 18.1 ± 8.4 | 19.3 ± 14.2 | 10.6 ± 6.4 | - | - |
| Ascorbic acid | 91.2 ± 2.4 | 82.2 ± 8.2 | 74.3 ± 13.2 | 79.2 ± 15.4 | 70.1 ± 4.2 | 71.9 ± 6.9 | 58.9 ± 1.9 |
| Concentration [µM] | Antioxidant Activity (%) | ||||||
|---|---|---|---|---|---|---|---|
| 1000 | 500 | 250 | 125 | 62.5 | 31.3 | 15.6 | |
| Leg1 (RIKTVTSFDLPALRFLKL) | 70.3 ± 2.2 | 64.7 ± 3.0 | 56.3 ± 2.2 | 42.6 ± 3.0 | 26.0 ± 2.5 | 19.3 ± 1.4 | 8.3 ± 1.8 |
| Leg2 (RIKTVTSFDLPALRWLKL) | 86.3 ± 2.2 | 84.2 ± 3.3 | 79.5 ± 0.3 | 60.2 ± 2.4 | 52.4 ± 2.8 | 30.0 ± 1.7 | 20.3 ± 10.2 |
| Tyrosine | 87.1 ± 0.7 | 82.7 ± 0.7 | 75.1 ± 0.8 | 57.1 ± 1.9 | 40.9 ± 8.0 | 14.6 ± 2.8 | 11.1± 1.0 |
| Histidine | 85.4 ± 5.7 | 81.1 ± 0.9 | 30.0 ± 6.3 | 17.3 ± 3.2 | 19.0 ± 7.0 | 6.1 ± 2.6 | - 1 |
| Cysteine | 90.2 ± 0.1 | 83.5 ± 0.1 | 75.4 ± 0.3 | 62.6 ± 0.4 | 47.5 ± 0.5 | 32.0 ± 1.8 | 20.0 ± 1.4 |
| Tryptophan | 92.7 ± 0.3 | 88.2 ± 1.0 | 82.2 ± 1.9 | 69.9 ± 5.4 | 55.7 ± 6.9 | 24.7 ± 6.2 | 10.8 ± 10.7 |
| Proline | 12.2 ± 4.2 | 28.2 ± 5.2 | 1.7 ± 4.8 | 33.6 ± 4.9 | 14.3 ± 1.3 | - | - |
| Phenylalanine | 22.8 ± 2.9 | 19.7 ± 0.5 | 14.1± 1.1 | 2.7 ± 3.1 | - | - | - |
| Ascorbic acid | 94.7 ± 0.1 | 92.0 ± 0.3 | 83.6 ± 0.6 | 74.6 ± 0.4 | 60.2 ± 1.7 | 40.8 ± 2.1 | 28.4 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heymich, M.-L.; Nißl, L.; Hahn, D.; Noll, M.; Pischetsrieder, M. Antioxidative, Antifungal and Additive Activity of the Antimicrobial Peptides Leg1 and Leg2 from Chickpea. Foods 2021, 10, 585. https://doi.org/10.3390/foods10030585
Heymich M-L, Nißl L, Hahn D, Noll M, Pischetsrieder M. Antioxidative, Antifungal and Additive Activity of the Antimicrobial Peptides Leg1 and Leg2 from Chickpea. Foods. 2021; 10(3):585. https://doi.org/10.3390/foods10030585
Chicago/Turabian StyleHeymich, Marie-Louise, Laura Nißl, Dominik Hahn, Matthias Noll, and Monika Pischetsrieder. 2021. "Antioxidative, Antifungal and Additive Activity of the Antimicrobial Peptides Leg1 and Leg2 from Chickpea" Foods 10, no. 3: 585. https://doi.org/10.3390/foods10030585
APA StyleHeymich, M.-L., Nißl, L., Hahn, D., Noll, M., & Pischetsrieder, M. (2021). Antioxidative, Antifungal and Additive Activity of the Antimicrobial Peptides Leg1 and Leg2 from Chickpea. Foods, 10(3), 585. https://doi.org/10.3390/foods10030585