Dentex dentex Frauds: Establishment of a New DNA Barcoding Marker
Abstract
1. Introduction
2. Materials and Methods
2.1. Sparidae mtDNA Genome Data and Fish Samples
2.2. Total Genomic DNA Extraction
2.3. mtDNA Comparative Analysis and End-Point PCR
2.4. Real-Time PCR
3. Results
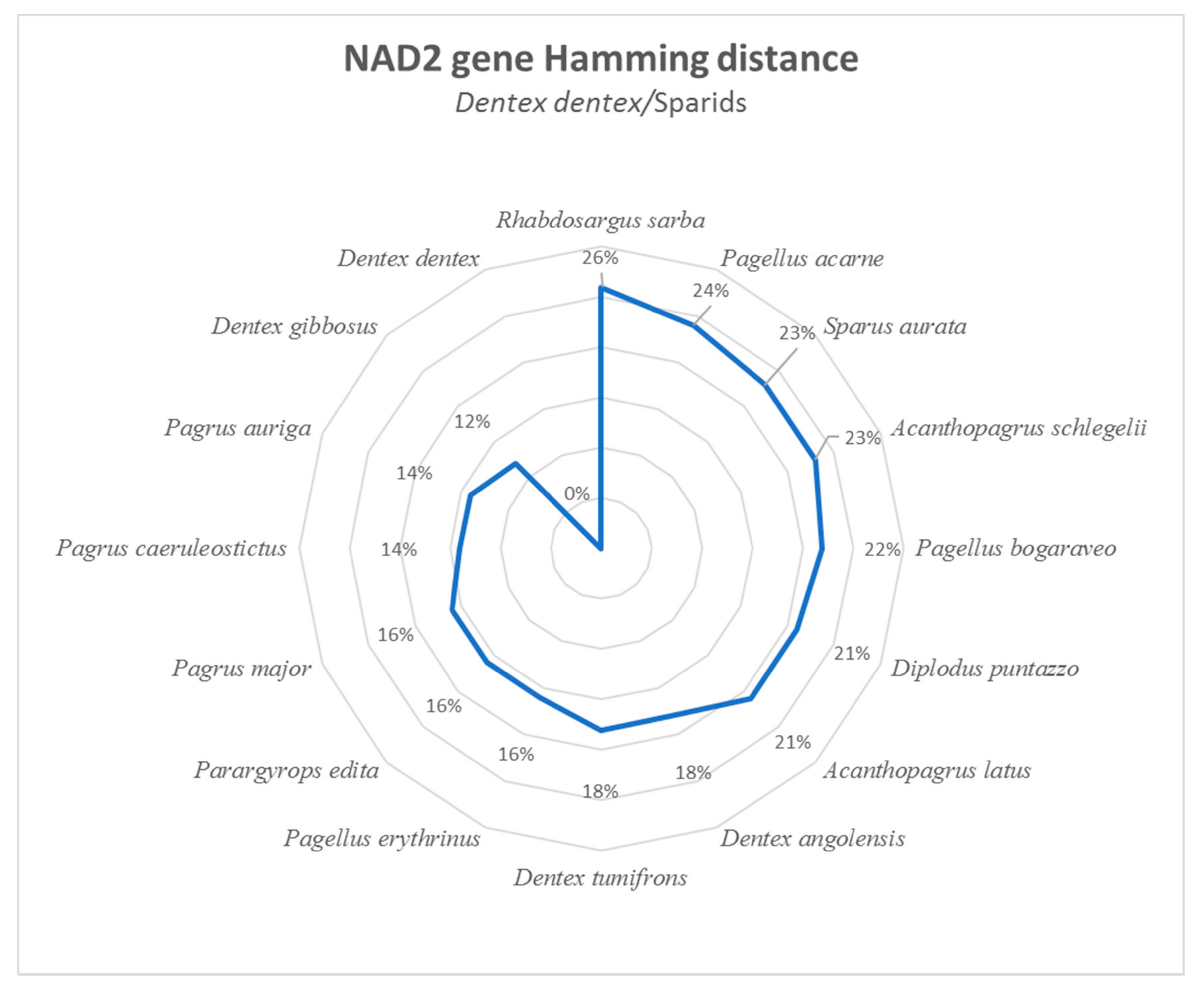
3.1. Sparidae mtDNA Analysis
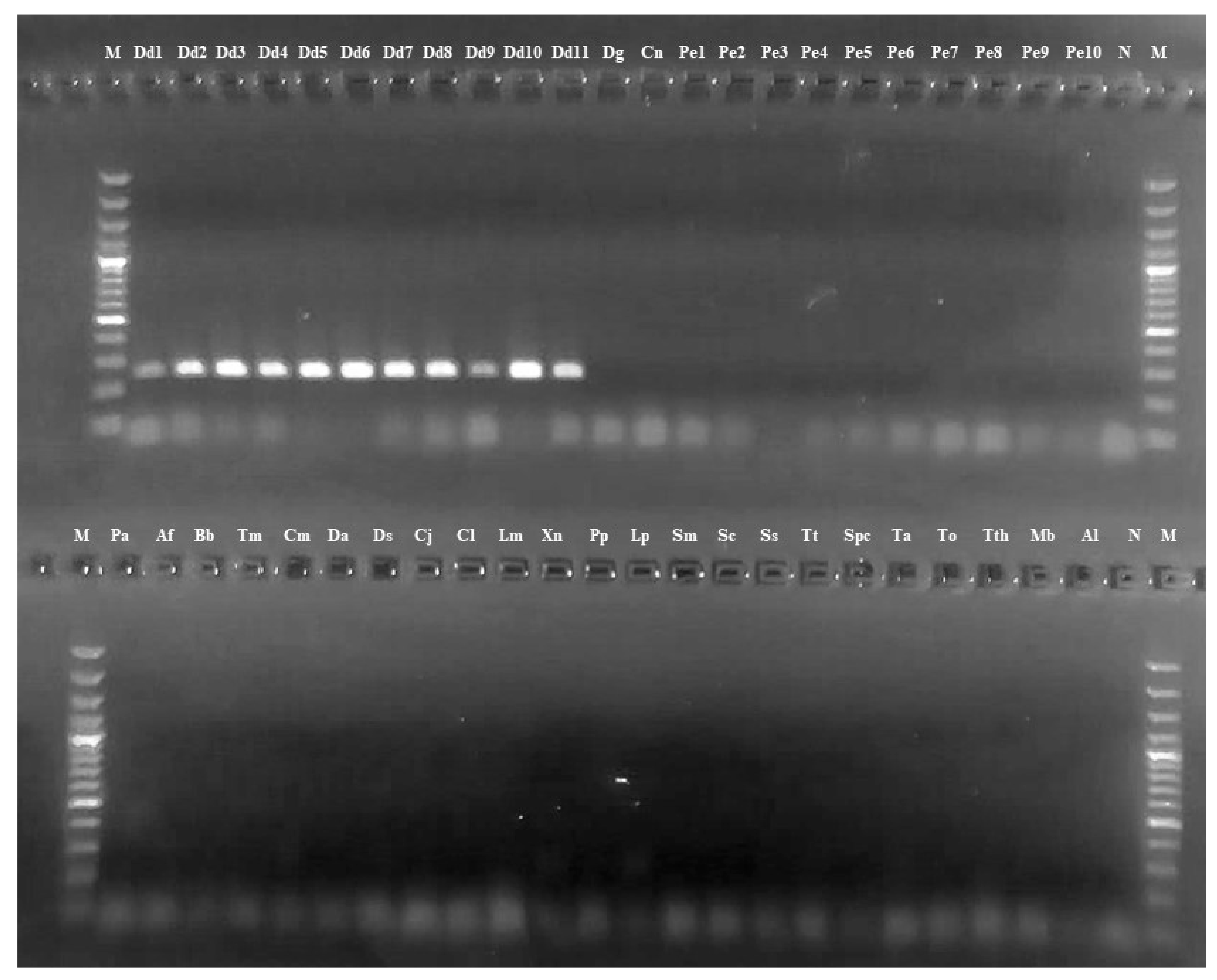
3.2. NAD2 Amplification and Analysis
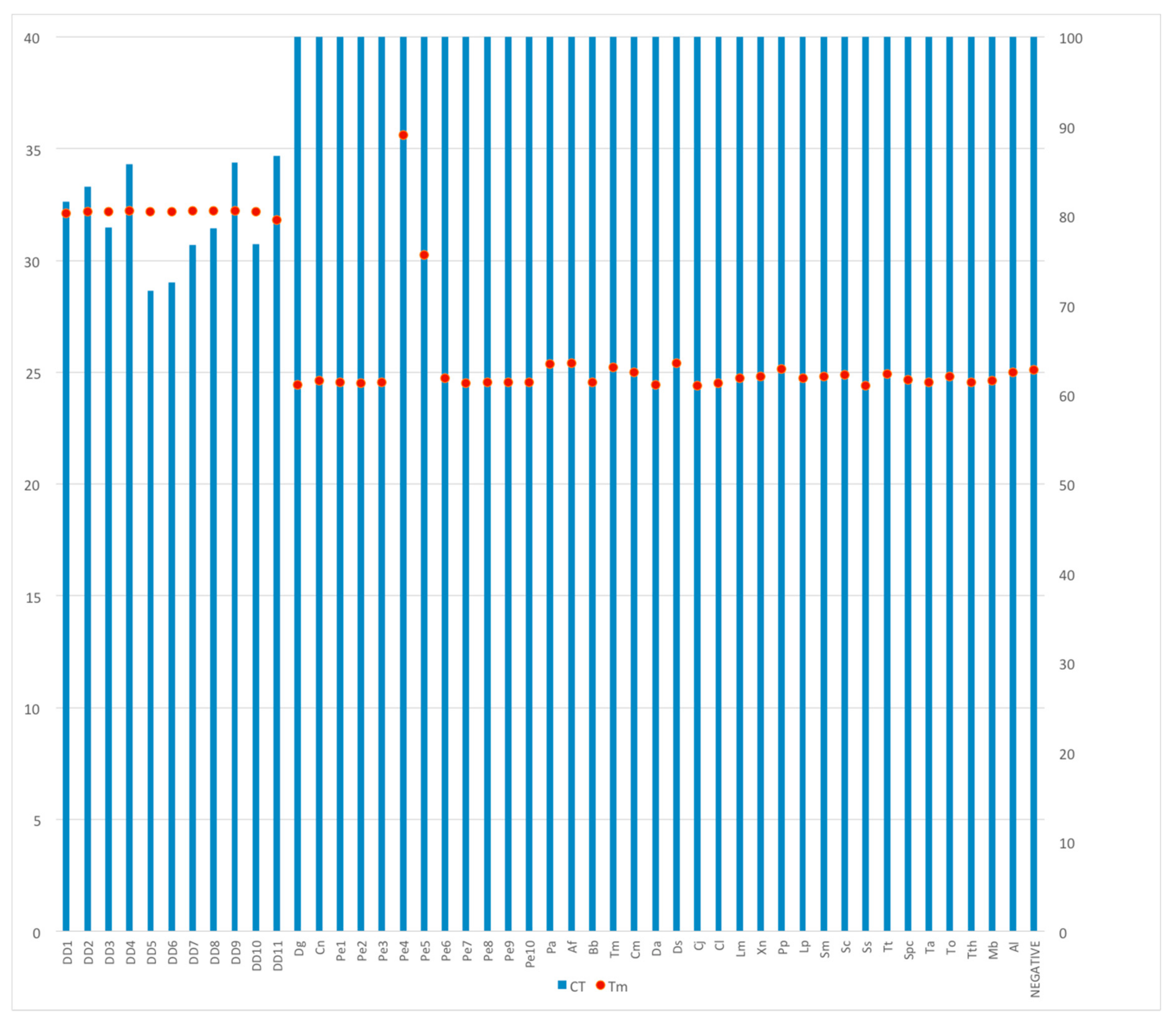
3.3. Real-Time PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wendołowicz, A.; Stefańska, E.; Ostrowska, L. Influence of selected dietary components on the functioning of the human nervous system. Rocz. Panstw. Zakl. Hig. 2018, 69, 15–21. [Google Scholar] [PubMed]
- Fishbase. Available online: https://www.fishbase.de/Summary/FamilySummary.php?ID=330 (accessed on 19 November 2020).
- Armani, A.; Guardone, L.; Castigliego, L.; D’Amico, P.; Messina, A.; Malandra, R.; Gianfaldoni, D.; Guidi, A. DNA and Mini-DNA Barcoding for the Identification of Porgies Species (Family Sparidae) of Commercial Interest on the International Market. Food Control 2015, 50, 589–596. [Google Scholar] [CrossRef]
- Cawthorn, D.M.; Duncan, J.; Kastern, C.; Francis, J.; Hoffman, L.C. Fish species substitution and misnaming in South Africa: An economic, safety and sustainability conundrum revisited. Food Chem. 2015, 185, 165–181. [Google Scholar] [CrossRef]
- Antonucci, F.; Costa, C.; Aguzzi, J.; Cataudella, S. Ecomorphology of morpho-functional relationships in the family of Sparidae: A quantitative statistic approach. J. Morphol. 2009, 270, 843–855. [Google Scholar] [CrossRef]
- Katavic, I.; Grubisic, L.; Skakelja, N. Growth performance of pink dentex as compared to four other sparids reared in marine cages in Croatia. Aquac. Int. 2000, 8, 455–461. [Google Scholar] [CrossRef]
- Acutis, P.L.; Bozzetta, E.; Gili, S.; Meistro, S.; Richelmi, G.; Riina, M.V. Ok il Pesce è Giusto. Guida Pratica All’Acquisto Consapevole del Pesce; Le Frodi nel Settore Ittico; IZSTo: Torino, Italy, 2015; Available online: http://www.izsto.it/images/stories/ok%20il%20pesce%20e%20giusto%20per%20sito.pdf (accessed on 30 September 2020).
- Messina, A. Identificazione di Sparidi di Interesse Commerciale Sui Mercati Internazionali Mediante DNA Barcoding. Master’s Thesis, Università di Pisa, Pisa, Italy, 2012. [Google Scholar]
- Malandra, R.; Renon, P. Le Principali Frodi dei Prodotti della Pesca; Libreria Universitaria Valdina: Milano, Italy, 1998; pp. 89–91, 105–106. [Google Scholar]
- Pardo, M.Á.; Jiménez, E.; Viðarsson, J.R.; Ólafsson, K.; Ólafsdóttir, G.; Daníelsdóttir, A.K.; Pérez-Villareal, B. DNA barcoding revealing mislabeling of seafood in European mass caterings. Food Control 2018, 7–16. [Google Scholar] [CrossRef]
- Pardo, M.Á.; Jiménez, E. DNA barcoding revealing seafood mislabeling in food services from Spain. J. Food Compos. Anal. 2020, 91, 103521. [Google Scholar] [CrossRef]
- Cramer, R. Advances in MALDI and Laser-Induced Soft Ionization Mass Spectrometry; Springer International Publishing: New York, NY, USA, 2016; pp. 273–274. [Google Scholar]
- Mazzeo, M.F.; Siciliano, R.A. Proteomics for the authentication of fish species. J. Proteom. 2016, 147, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Boffo, L.; Arcangeli, G.; Le Frodi Alimentari dei Prodotti Ittici. Il Pesce n.5 2018. Available online: http://www.ambienteamico.it/frodi.pdf (accessed on 16 September 2020).
- Trotta, M.; Schönhuth, S.; Pepe, T.; Cortesi, M.L.; Puyet, A.; Bautista, J.M. Multiplex PCR Method for use in real-time PCR for identification of fish fillets from grouper (Epinephelus and Mycteroperca Species) and common substitute species. J. Agric. Food Chem. 2005, 53, 2039–2045. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Chen, X.; Xiang, D.; Murphy, R.W.; Shen, Y. Detection of Potential Problematic Cytb Gene Sequences of Fishes in GenBank. Front. Genet. 2018, 9, 30. [Google Scholar] [CrossRef]
- Ceruso, M.; Mascolo, C.; Anastasio, A.; Pepe, T.; Sordino, P. Frauds and fish species authentication: Study of the complete mitochondrial genome of some Sparidae to provide specific barcode markers. Food Control. 2019, 103, 36–47. [Google Scholar] [CrossRef]
- Ceruso, M.; Venuti, I.; Osca, D.; Caputi, L.; Anastasio, A.; Crocetta, F.; Sordino, P.; Pepe, T. The complete mitochondrial genome of the sharpsnout seabream diplodus puntazzo (Perciformes: Sparidae). Mitochondrial DNA Part B 2020, 5, 2379–2381. [Google Scholar] [CrossRef] [PubMed]
- Ceruso, M.; Mascolo, C.; Palma, G.; Anastasio, A.; Pepe, T.; Sordino, P. The complete mitochondrial genome of the common dentex, Dentex dentex (Perciformes: Sparidae). Mitochondrial DNA Part B 2018, 3, 391–392. [Google Scholar] [CrossRef]
- Ceruso, M.; Mascolo, C.; Lowe, E.K.; Palma, G.; Anastasio, A.; Pepe, T.; Sordino, P. The complete mitochondrial genome of the common Pandora Pagellus erythrinus (Perciformes: Sparidae). Mitochondrial DNA Part B 2018, 3, 624–625. [Google Scholar] [CrossRef]
- Ceruso, M.; Mascolo, C.; De Luca, P.; Venuti, I.; Smaldone, G.; Biffali, E.; Anastasio, A.; Pepe, T.; Sordino, P. A Rapid Method for the Identification of Fresh and Processed Pagellus erythrinus Species against Frauds. Foods 2020, 9, 1397. [Google Scholar] [CrossRef]
- Mascolo, C.; Ceruso, M.; Palma, G.; Anastasio, A.; Sordino, P.; Pepe, T. The complete mitochondrial genome of the axillary seabream, Pagellus acarne (Perciformes: Sparidae). Mitochondrial DNA Part B 2018, 3, 434–435. [Google Scholar] [CrossRef]
- Mascolo, C.; Ceruso, M.; Palma, G.; Anastasio, A.; Sordino, P.; Pepe, T. The complete mitochondrial genome of the Pink dentex Dentex gibbosus (Perciformes: Sparidae). Mitochondrial DNA Part B 2018, 3, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, C.; Ceruso, M.; Chirollo, C.; Palma, G.; Anastasio, A.; Sordino, P.; Pepe, T. The complete mitochondrial genome of the Angolan dentex Dentex angolensis (Perciformes: Sparidae). Mitochondrial DNA Part B 2019, 4, 1245–1246. [Google Scholar] [CrossRef]
- Mascolo, C.; Ceruso, M.; Sordino, P.; Palma, G.; Anastasio, A.; Pepe, T. Comparison of mitochondrial DNA enrichment and sequencing methods from fish tissue. Food. Chem. 2019, 294, 333–338. [Google Scholar] [CrossRef]
- Marko, P.B.; Lee, S.C.; Rice, A.M.; Gramling, J.M.; Fitzhenry, T.M.; McAalister, J.S.; Harper, G.R.; Moran, A.L. Mislabelling of a depleted reef fish. Nature 2004, 430, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucl. Acids Symp. 1999, 41, 95–98. [Google Scholar]
- Kumar, A.; Chordia, N. Methods in molecular biology. In Silico PCR Primer Designing and Validation; Basu, C., Ed.; Humana Press: New York, NY, USA, 2015; Volume 1275, pp. 143–151. [Google Scholar]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-based registry for all animal species: The barcode index number (BIN) System. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef] [PubMed]
- Day, J. Phylogenetic relationships of the Sparidae (Teleostei: Percoidei) and implications for convergent trophic evolution. Biol. J. Linn. Soc. Lond. 2002, 76, 269–301. [Google Scholar] [CrossRef][Green Version]
- Orrell, T.M.; Carpenter, K.E.; Musick, J.A.; Graves, J.E. Phylogenetic and biogeographic analysis of the Sparidae (Perciformes: Percoidei) from Cytochrome b sequences. Copeia 2002, 3, 618–631. [Google Scholar] [CrossRef]
- Chiba, S.N.; Iwatsuki, Y.; Yoshino, T.; Hanzawa, N. Comprehensive phylogeny of the family Sparidae (Perciformes: Teleostei) inferred from mitochondrial gene analyses. Genes Genet. Syst. 2009, 84, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Fisheries and Aquaculture Software. FishStat Plus—Universal Software for Fishery Statistical Time Series. In FAO Fisheries Division; FAO: Rome, Italy, 2017; Available online: http://www.fao.org/fishery/species/3182/en (accessed on 19 November 2020).
- 24FISH srl. Dentice Pescato All’amo. Available online: https://www.24.fish/prodotto/dentice-pescato-allamo/ (accessed on 16 September 2020).
- Pleadin, J.; Lesic, T.; Kresic, G.; Baric, R.; Bogdanovic, T.; Oraic, D.; Vulic, A.; Legac, A.; Zrncic, S. Nutritional quality of different fish species farmed in the Adriatic Sea. Ital. J. Food Sci. 2017, 29, 537–549. [Google Scholar]
- Manzoni, P. Enciclopedia Illustrata Delle Specie Ittiche Marine; Edizioni De Agostini: Milano, Italy, 1993; pp. 77–89. [Google Scholar]

| Dentex dentex | |||
|---|---|---|---|
| Abbreviation | Sampling Area | Latitude | Longitude |
| Dd1 | GSA 5-Balearic Island | 39.808662 | 3.740905 |
| Dd2 | GSA 7-Gulf of Lions | 42.519035 | 3.534141 |
| Dd3 | GSA 10-South Tyrrhenian | 38.771348 | 14.946960 |
| Dd4 | GSA 10-Central Tyrrhenian | 40.409446 | 13.798889 |
| Dd5 | GSA 11.2-Sardinia (East) | 40.044575 | 9.841622 |
| Dd6 | GSA 17-Northern Adriatic | 44.980473 | 13.137520 |
| Dd7 | GSA 18-Southern Adriatic Sea | 40.668284 | 18.290108 |
| Dd8 | GSA 19-Western Ionian Sea | 40.044587 | 17.147530 |
| Dd9 | GSA 21-Southern Ionian Sea | 31.475938 | 18.685467 |
| Dd10 | GSA 27-Levante | 35.338496 | 35.708826 |
| Dd11 | GSA 10-Ponza Island, Central Tyrrhenian | 40.874620 | 12.985808 |
| N° | Scientific Name | Family | Common Name | Abbreviation |
|---|---|---|---|---|
| 1 | Arnoglossus laterna | Bothidae | Mediterranean scaldfish | Al |
| 2 | Aulopus filamentosus | Aulopidae | Royal flagfin | Af |
| 3 | Boops boops | Sparidae | Bogue | Bb |
| 4 | Cepola macrophthalma | Cepolidae | Red bandfish | Cm |
| 5 | Cheimerius nufar | Sparidae | Santer seabream | Cn |
| 6 | Chelidonichthys lucerna | Triglidae | Tub gurnard | Cl |
| 7 | Coris julis | Labridae | Rainbow wrasse | Cj |
| 8 | Dentex gibbosus | Sparidae | Pink dentex | Dg |
| 9 | Diplodus annularis | Sparidae | Annular seabream | Da |
| 10 | Diplodus sargus | Sparidae | White seabream | Ds |
| 11 | Lithognathus mormyrus | Sparidae | Sand steenbras | Lm |
| 12 | Lophius piscatorius | Lophiidae | Angler (= Monk) | Lp |
| 13 | Mullus barbatus | Mullidae | Red mullet | Mb |
| 14 | Pagellus acarne | Sparidae | Axillary seabream | Pa |
| 15 | Pagellus erythrinus | Sparidae | Common pandora | Pe |
| 16 | Pleuronectes platessa | Pleuronectidae | European plaice | Pp |
| 17 | Sebastes capensis | Sebastidae | Cape redfish | Sc |
| 18 | Scophthalmus maximus | Scophthalmidae | Turbot | Sm |
| 19 | Solea solea | Soleidae | Common sole | Ss |
| 20 | Spondyliosoma cantharus | Sparidae | Black seabream | Spc |
| 21 | Thunnus thynnus | Scombridae | Atlantic bluefin tuna | Tth |
| 22 | Thunnus albacares | Scombridae | Yellowfin tuna | Ta |
| 23 | Thunnus obesus | Scombridae | Bigeye tuna | To |
| 24 | Trachurus trachurus | Carangidae | Atlantic horse mackerel | Tt |
| 25 | Trisopterus minutus | Gadidae | Poor cod | Tm |
| 26 | Xyrichtys novacula | Labridae | Pearly razorfish | Xn |
| N° | Primer Name | 5′ → 3′ Sequence | Tm (°C) | CG (%) | nt | A | T | C | G |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Fw108 | CACCCTAGCTATCCTCCCCCTCATAGC | 72.4 | 59.3 | 27 | 5 | 6 | 14 | 2 |
| Rev330 | AATAACTTCGGGGAGTCACGAGTGTAGG | 71.1 | 50.0 | 28 | 8 | 6 | 4 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceruso, M.; Mascolo, C.; De Luca, P.; Venuti, I.; Biffali, E.; Ambrosio, R.L.; Smaldone, G.; Sordino, P.; Pepe, T. Dentex dentex Frauds: Establishment of a New DNA Barcoding Marker. Foods 2021, 10, 580. https://doi.org/10.3390/foods10030580
Ceruso M, Mascolo C, De Luca P, Venuti I, Biffali E, Ambrosio RL, Smaldone G, Sordino P, Pepe T. Dentex dentex Frauds: Establishment of a New DNA Barcoding Marker. Foods. 2021; 10(3):580. https://doi.org/10.3390/foods10030580
Chicago/Turabian StyleCeruso, Marina, Celestina Mascolo, Pasquale De Luca, Iolanda Venuti, Elio Biffali, Rosa Luisa Ambrosio, Giorgio Smaldone, Paolo Sordino, and Tiziana Pepe. 2021. "Dentex dentex Frauds: Establishment of a New DNA Barcoding Marker" Foods 10, no. 3: 580. https://doi.org/10.3390/foods10030580
APA StyleCeruso, M., Mascolo, C., De Luca, P., Venuti, I., Biffali, E., Ambrosio, R. L., Smaldone, G., Sordino, P., & Pepe, T. (2021). Dentex dentex Frauds: Establishment of a New DNA Barcoding Marker. Foods, 10(3), 580. https://doi.org/10.3390/foods10030580











