Development of Chemometric Models Based on a LC-qToF-MS Approach to Verify the Geographic Origin of Virgin Olive Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
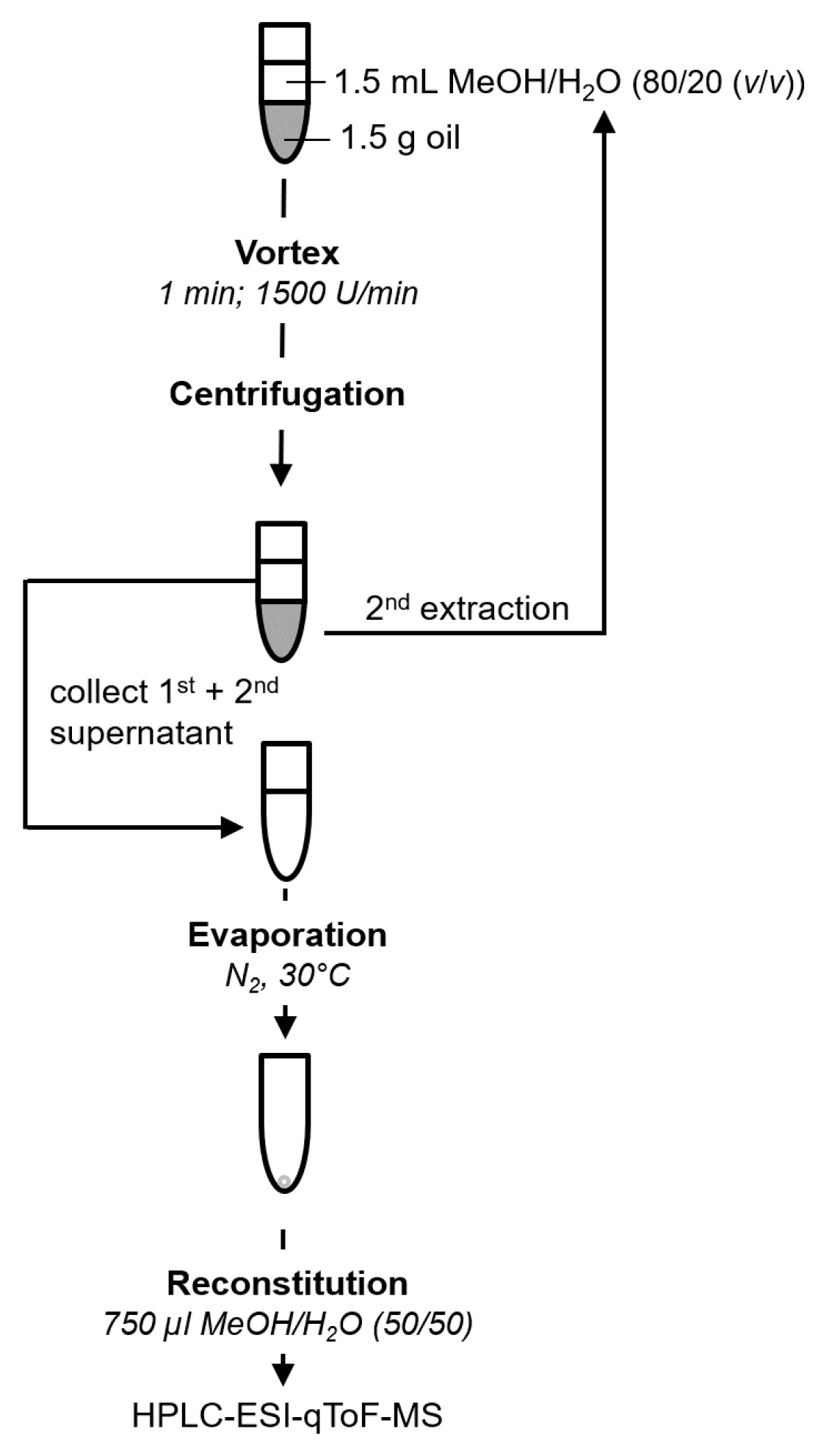
2.3. Sample Preparation
2.4. HPLC-ESI-qToF-MS Analysis
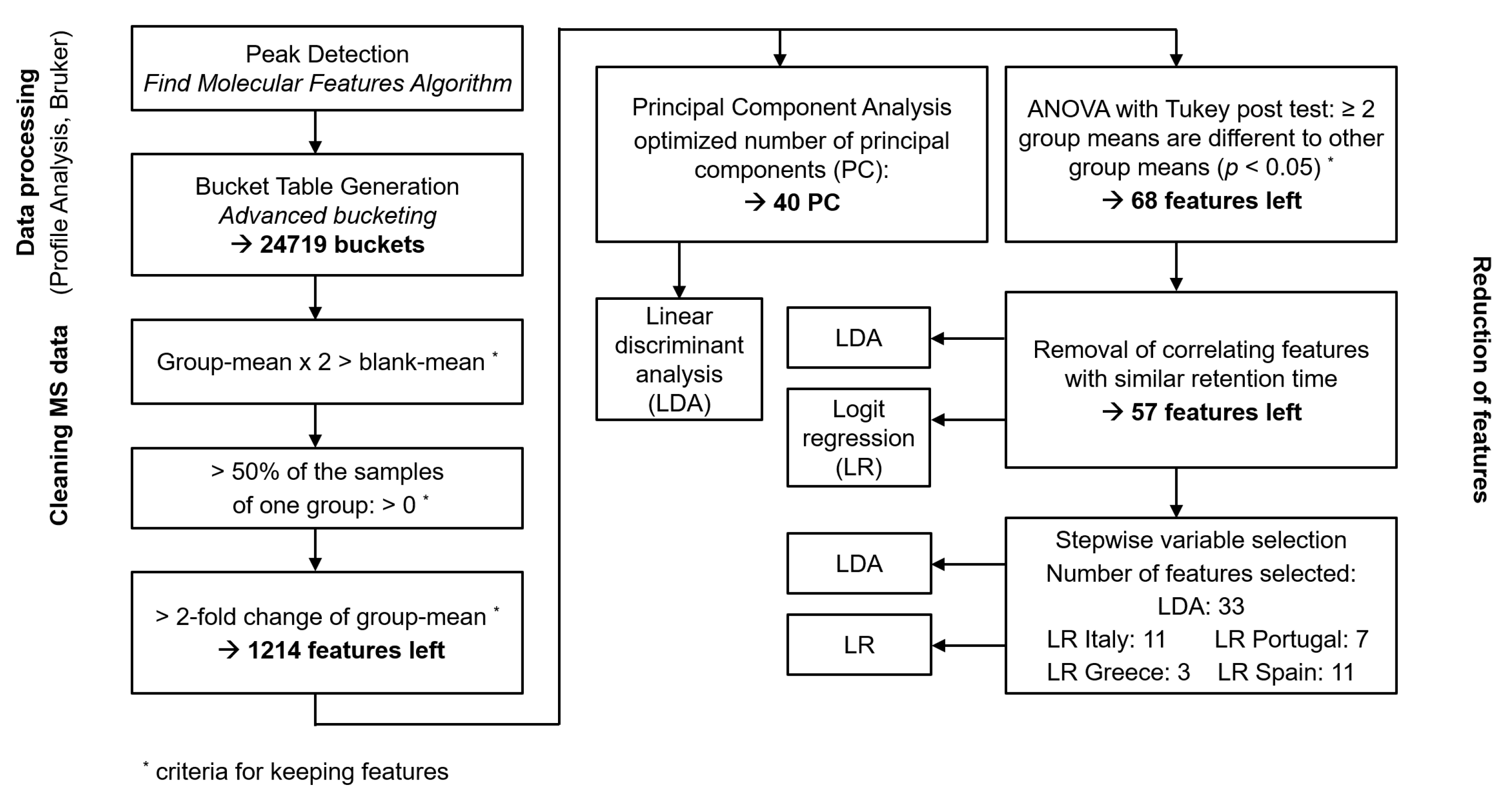
2.5. Data Processing
2.6. Chemometric Analysis
3. Results and Discussion
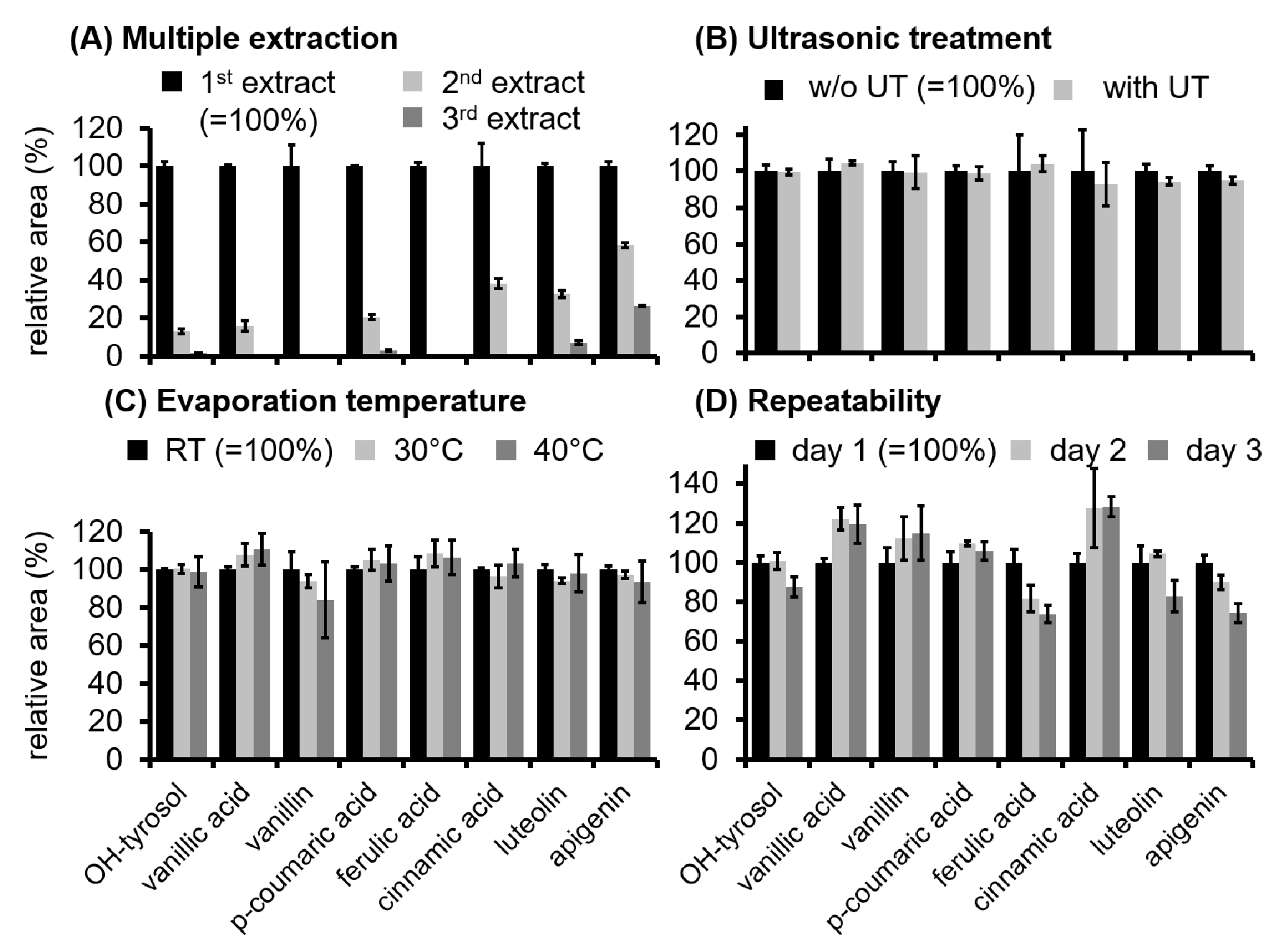
3.1. Optimization of the Sample Preparation
3.2. Data Processing
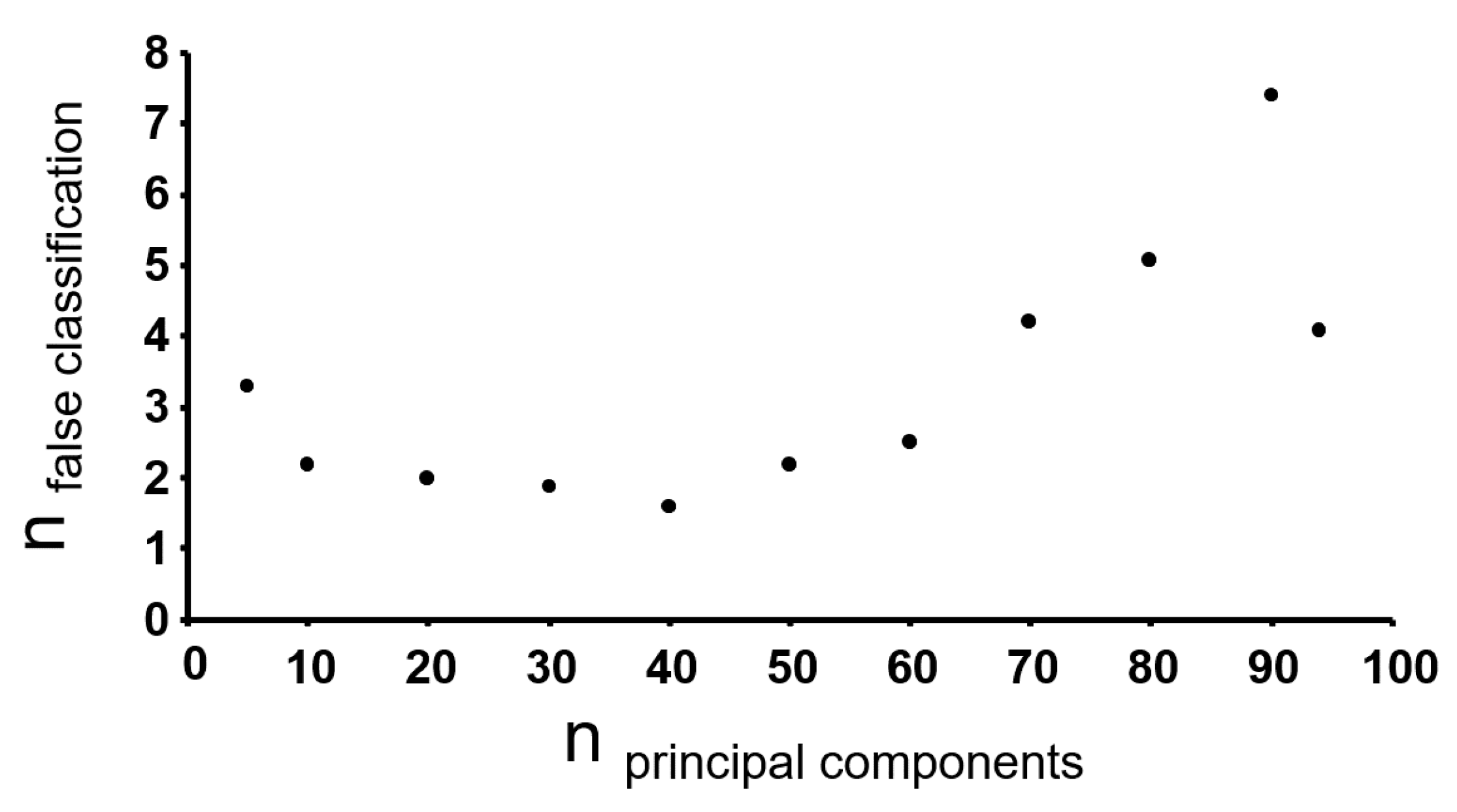
3.3. Chemometric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shears, P. Food fraud—A current issue but an old problem. Br. Food J. 2010, 112, 198–213. [Google Scholar] [CrossRef]
- Kulling, S.; Bunzel, D.; Frommherz, L.; Molkentin, J.; Lehmann, I.; Engert, S.; Steinberg, P. The Setup of the National Reference Centre for Authentic Food (NRZ-Authent) in Germany. Eur. J. Lipid Sci. Technol. 2019, 121, 1900023. [Google Scholar] [CrossRef]
- Matthäus, B.; Willenberg, I.; Engert, S.; Steinberg, P. The German National Reference Centre for Authentic Food (NRZ-Authent). OCL 2019, 26, 11. [Google Scholar] [CrossRef]
- Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on Official Controls and Other Official Activities Performed to Ensure the Application of Food and Feed Law, Rules on Animal Health and Welfare, Plant Health and Plant Protection Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32017R0625 (accessed on 22 January 2021).
- Bontempo, L.; Paolini, M.; Franceschi, P.; Ziller, L.; Garcia-Gonzalez, D.L.; Camin, F. Characterisation and attempted differentiation of European and extra-European olive oils using stable isotope ratio analysis. Food Chem. 2019, 276, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; Garcia-Gonzalez, D.L.; Moreda, W.; et al. Olive oil quality and authenticity: A review of current EU legislation, standards, relevant methods of analyses, their drawbacks andrecommendations for the future. Trends Food Sci. Technol. 2019, 105, 483–493. [Google Scholar] [CrossRef]
- Gertz, C.; Gertz, A.; Matthaus, B.; Willenberg, I. A Systematic Chemometric Approach to Identify the Geographical Origin of Olive Oils. Eur. J. Lipid Sci. Technol. 2019, 121, 1900281. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Aalizadeh, R.; Thomaidis, N.S. Application of an advanced and wide scope non-target screening workflow with LC-ESI-QTOF-MS and chemometrics for the classification of the Greek olive oil varieties. Food Chem. 2018, 256, 53–61. [Google Scholar] [CrossRef]
- Winkelmann, O.; Küchler, T. Reliable Classification of Olive Oil Origin Based on Minor Component Profile Using 1H-NMR and Multivariate Analysis. Eur. J. Lipid Sci. Technol. 2019, 121, 1900027. [Google Scholar] [CrossRef]
- International Olive Council (IOC). Trade Standard on Olive Oils and Olive-Pomace Oils COI/T.15/NC n. 3/rev. 15/2019, Madrid. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 22 January 2021).
- White, P.D.; Cundiff, E.W. Assessing the Quality of Industrial Products. J. Mark. 1978, 42, 80–86. [Google Scholar]
- Al-Sulaiti, K.I.; Baker, M.J. Country of origin effects: A literature review. Mark. Intell. Plan. 1998, 16, 150–199. [Google Scholar] [CrossRef]
- Caporale, G.; Policastro, S.; Carlucci, A.; Monteleone, E. Consumer expectations for sensory properties in virgin olive oils. Food Qual. Prefer. 2006, 17, 116–125. [Google Scholar] [CrossRef]
- IOC. EU Producer Prices. Available online: http://www.internationaloliveoil.org/estaticos/view/133-eu-producer-prices (accessed on 22 January 2021).
- Regulation (EU) No 29/2012 of 13 January 2012 on Marketing Standards for Olive Oil. 2012. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R0029&from=EN (accessed on 22 January 2021).
- Gil-Solsona, R.; Raro, M.; Sales, C.; Lacalle, L.; Díaz, R.; Ibáñez, M.; Beltran, J.; Sancho, J.V.; Hernández, F.J. Metabolomic approach for Extra virgin olive oil origin discrimination making use of ultra-high performance liquid chromatography—Quadrupole time-of-flight mass spectrometry. Food Control 2016, 70, 350–359. [Google Scholar] [CrossRef]
- Olmo-García, L.; Wendt, K.; Kessler, N.; Bajoub, A.; Fernández-Gutiérrez, A.; Baessmann, C.; Carrasco-Pancorbo, A. Exploring the Capability of LC-MS and GC-MS Multi-Class Methods to Discriminate Virgin Olive Oils from Different Geographical Indications and to Identify Potential Origin Markers. Eur. J. Lipid Sci. Technol. 2019, 121, 1800336. [Google Scholar] [CrossRef]
- Vera, D.N.; Jimenez-Carvelo, A.M.; Cuadros-Rodriguez, L.; Ruisanchez, I.; Callao, M.P. Authentication of the geographical origin of extra-virgin olive oil of the Arbequina cultivar by chromatographic fingerprinting and chemometrics. Talanta 2019, 203, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Agrimonti, C.; Marmiroli, N. Food Genomics for the Characterization of PDO and PGI Virgin Olive Oils. Eur. J. Lipid Sci. Technol. 2019, 121, 1800132. [Google Scholar] [CrossRef]
- Royer, A.; Gerard, C.; Naulet, N.; Lees, M.; Martin, G.J. Stable isotope characterization of olive oils. I—Compositional and carbon-13 profiles of fatty acids. J. Am. Oil Chem. Soc. 1999, 76, 357–363. [Google Scholar] [CrossRef]
- del Cano-Ochoa, S.; Ruiz-Aracama, A.; Guillen, M.D. Potential of Nuclear Magnetic Resonance for a Discriminant Characterization of PDO VOOs. Eur. J. Lipid Sci. Technol. 2019, 121, 1800137. [Google Scholar] [CrossRef]
- Longobardi, F.; Ventrella, A.; Napoli, C.; Humpfer, E.; Schütz, B.; Schäfer, H.; Kontominas, M.G.; Sacco, A. Classification of olive oils according to geographical origin by using 1H NMR fingerprinting combined with multivariate analysis. Food Chem. 2012, 130, 177–183. [Google Scholar] [CrossRef]
- Lukic, I.; Carlin, S.; Horvat, I.; Vrhovsek, U. Combined targeted and untargeted profiling of volatile aroma compounds with comprehensive two-dimensional gas chromatography for differentiation of virgin olive oils according to variety and geographical origin. Food Chem. 2019, 270, 403–414. [Google Scholar] [CrossRef]
- Galtier, O.; Dupuy, N.; Le Dréau, Y.; Ollivier, D.; Pinatel, C.; Kister, J.; Artaud, J. Geographic origins and compositions of virgin olive oils determinated by chemometric analysis of NIR spectra. Anal. Chim. Acta 2007, 595, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Luna, G.; Morales, M.T.; Aparicio, R. Characterisation of 39 varietal virgin olive oils by their volatile compositions. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; García-González, D.L.; Morales, M.T.; Lobo-Prieto, A.; Romero, I. Comparison of two analytical methods validated for the determination of volatile compounds in virgin olive oil: GC-FID vs GC-MS. Talanta 2018, 187, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Peršurić, Ž.; Saftić, L.; Mašek, T.; Kraljević Pavelić, S. Comparison of triacylglycerol analysis by MALDI-TOF/MS, fatty acid analysis by GC-MS and non-selective analysis by NIRS in combination with chemometrics for determination of extra virgin olive oil geographical origin. A case study. LWT 2018, 95, 326–332. [Google Scholar] [CrossRef]
- Quintanilla-Casas, B.; Bertin, S.; Leik, K.; Bustamante, J.; Guardiola, F.; Valli, E.; Bendini, A.; Toschi, T.G.; Tres, A.; Vichi, S. Profiling versus fingerprinting analysis of sesquiterpene hydrocarbons for the geographical authentication of extra virgin olive oils. Food Chem. 2020, 307, 125556. [Google Scholar] [CrossRef]
- Sales, C.; Cervera, M.I.; Gil, R.; Portolés, T.; Pitarch, E.; Beltran, J. Quality classification of Spanish olive oils by untargeted gas chromatography coupled to hybrid quadrupole-time of flight mass spectrometry with atmospheric pressure chemical ionization and metabolomics-based statistical approach. Food Chem. 2017, 216, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Bajoub, A.; Pacchiarotta, T.; Hurtado-Fernández, E.; Olmo-García, L.; García-Villalba, R.; Fernández-Gutiérrez, A.; Mayboroda, O.A.; Carrasco-Pancorbo, A. Comparing two metabolic profiling approaches (liquid chromatography and gas chromatography coupled to mass spectrometry) for extra-virgin olive oil phenolic compounds analysis: A botanical classification perspective. J. Chromatogr. A 2016, 1428, 267–279. [Google Scholar] [CrossRef]
- Alarcón Flores, M.I.; Romero-González, R.; Garrido Frenich, A.; Martínez Vidal, J.L. Analysis of phenolic compounds in olive oil by solid-phase extraction and ultra high performance liquid chromatography–tandem mass spectrometry. Food Chem. 2012, 134, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Gioacchini, A.M.; Roda, A.; Galletti, G.C.; Bocchini, P.; Manetta, A.C.; Baraldini, M. High-performance liquid chromatographic-electrospray mass spectrometric analysis of phenolic acids and aldehydes. J. Chromatogr. A 1996, 730, 31–37. [Google Scholar] [CrossRef]
- Nováková, L.; Vildová, A.; Mateus, J.P.; Gonçalves, T.; Solich, P. Development and application of UHPLC–MS/MS method for the determination of phenolic compounds in Chamomile flowers and Chamomile tea extracts. Talanta 2010, 82, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; León-Camacho, M.; Alcudia, F.; Cert, A. Determination of Phenols, Flavones, and Lignans in Virgin Olive Oils by Solid-Phase Extraction and High-Performance Liquid Chromatography with Diode Array Ultraviolet Detection. J. Agric. Food Chem. 2001, 49, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council: Determination of Biophenols in Olive Oils by HPLC, COI/T.20/Doc No 29/Rev. 1, Madrid. 2017. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 22 January 2021).
- Boccard, J.; Rudaz, S. Harnessing the complexity of metabolomic data with chemometrics. J. Chemom. 2014, 28, 1–9. [Google Scholar] [CrossRef]
- Vinaixa, M.; Samino, S.; Saez, I.; Duran, J.; Guinovart, J.J.; Yanes, O. A Guideline to Univariate Statistical Analysis for LC/MS-Based Untargeted Metabolomics-Derived Data. Metabolites 2012, 2, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson: Boston, MA, USA, 2007. [Google Scholar]
- Pohar, M.; Blas, M.; Turk, S. Comparison of Logistic Regression and Linear Discriminant Analysis: A Simulation Study. Metodološki Zv. 2004, 1, 143–161. [Google Scholar]
- Ricciutelli, M.; Marconi, S.; Boarelli, M.C.; Caprioli, G.; Sagratini, G.; Ballini, R.; Fiorini, D. Olive oil polyphenols: A quantitative method by high-performance liquid-chromatography-diode-array detection for their determination and the assessment of the related health claim. J. Chromatogr. A 2017, 1481, 53–63. [Google Scholar] [CrossRef] [PubMed]
| Overall Correct Classification Rate (%) * | ||
|---|---|---|
| Criteria Variable Selection | Training Set | Test Set |
| PCA | 98.2 | 83.3 |
| ANOVA with Tukey post-test/Removal of correlated features | 99.5 | 74.6 |
| Stepwise variable selection | 98.6 | 83.1 |
| Overall Correct Classification Rate (%) * | ||||
|---|---|---|---|---|
| Portugal | Italy | Greece | Spain | |
| ANOVA with Tukey post-test/Removal of correlated features | ||||
| Training Set | 100.0 | 100.0 | 100.0 | 100.0 |
| Test Set | 72.4 | 75.8 | 88.3 | 67.4 |
| Stepwise variable selection | ||||
| Training Set | 100.0 | 96.5 | 97.0 | 100.0 |
| Test Set | 90.4 | 86.2 | 93.8 | 88.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willenberg, I.; Parma, A.; Bonte, A.; Matthäus, B. Development of Chemometric Models Based on a LC-qToF-MS Approach to Verify the Geographic Origin of Virgin Olive Oil. Foods 2021, 10, 479. https://doi.org/10.3390/foods10020479
Willenberg I, Parma A, Bonte A, Matthäus B. Development of Chemometric Models Based on a LC-qToF-MS Approach to Verify the Geographic Origin of Virgin Olive Oil. Foods. 2021; 10(2):479. https://doi.org/10.3390/foods10020479
Chicago/Turabian StyleWillenberg, Ina, Alessandra Parma, Anja Bonte, and Bertrand Matthäus. 2021. "Development of Chemometric Models Based on a LC-qToF-MS Approach to Verify the Geographic Origin of Virgin Olive Oil" Foods 10, no. 2: 479. https://doi.org/10.3390/foods10020479
APA StyleWillenberg, I., Parma, A., Bonte, A., & Matthäus, B. (2021). Development of Chemometric Models Based on a LC-qToF-MS Approach to Verify the Geographic Origin of Virgin Olive Oil. Foods, 10(2), 479. https://doi.org/10.3390/foods10020479






