Physicochemical Changes of Deep-Fat-Fried Chicken Drumsticks Treated with Quercetin-in-Edible Coating during Storage Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Chicken Sample Preparation
2.2. Protein Isolation from Chicken By-Product
2.2.1. Recovery and Brine Washing of Chicken By-Product
2.2.2. Isoelectric Solubilization/Precipitation and Quantification of Protein Content
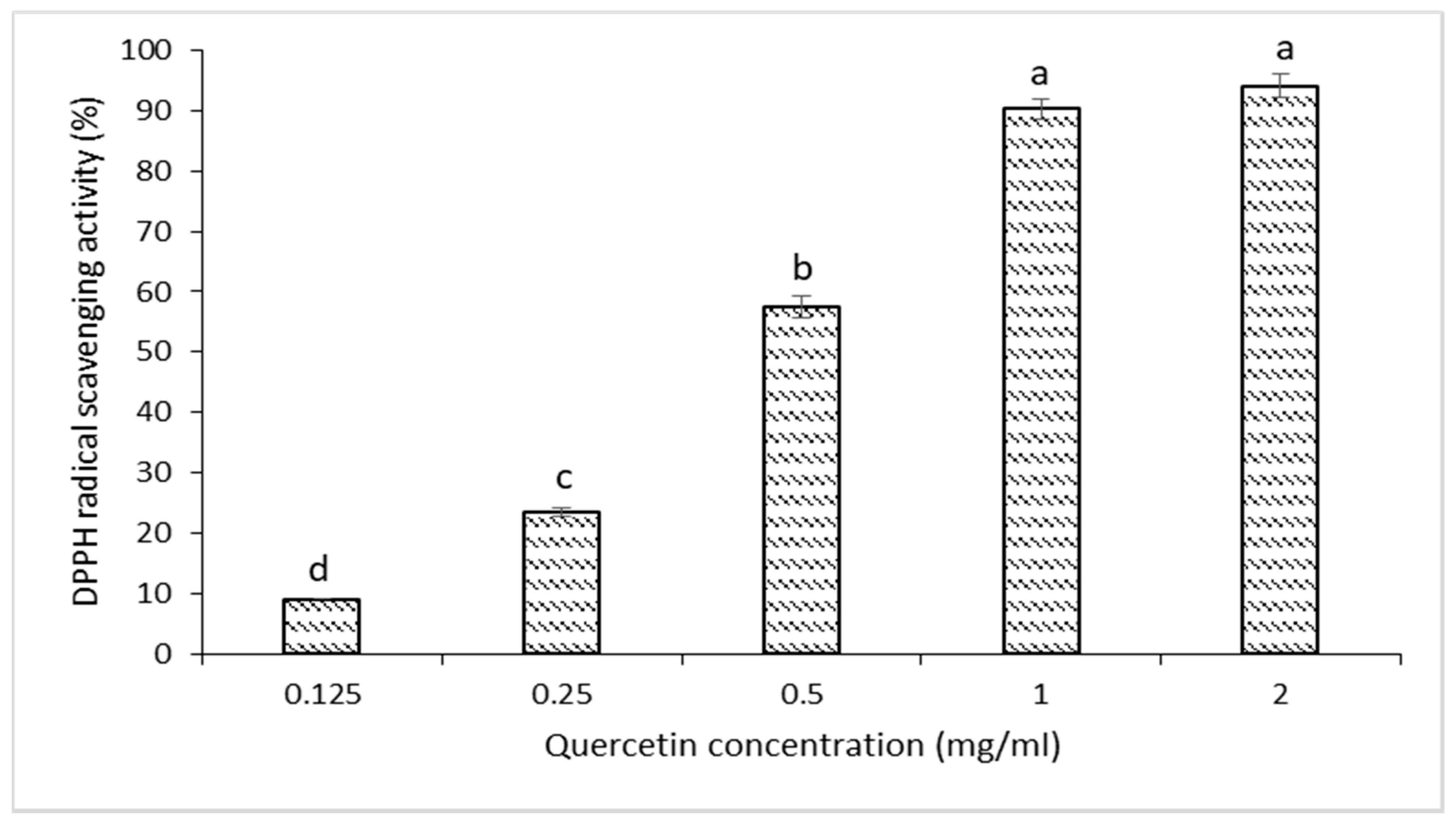
2.3. DPPH Free-Radical Scavenging Activity of Quercetin
2.4. Preparation of Bioactive Edible Coating
2.5. Sample Preparation for Frying
2.6. Deep-Frying
2.7. Storage Condition
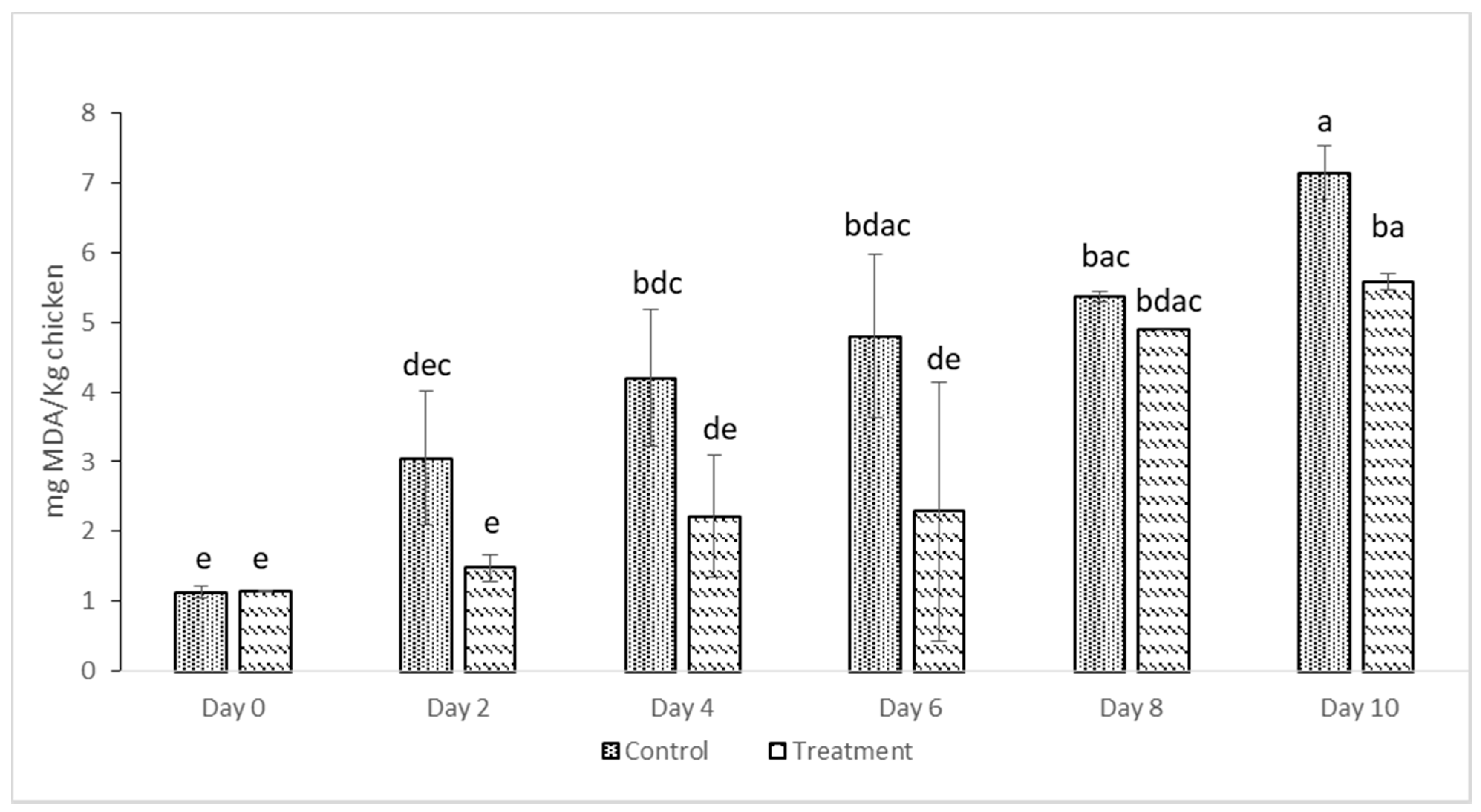
2.8. TBARS Value
2.9. Color Properties
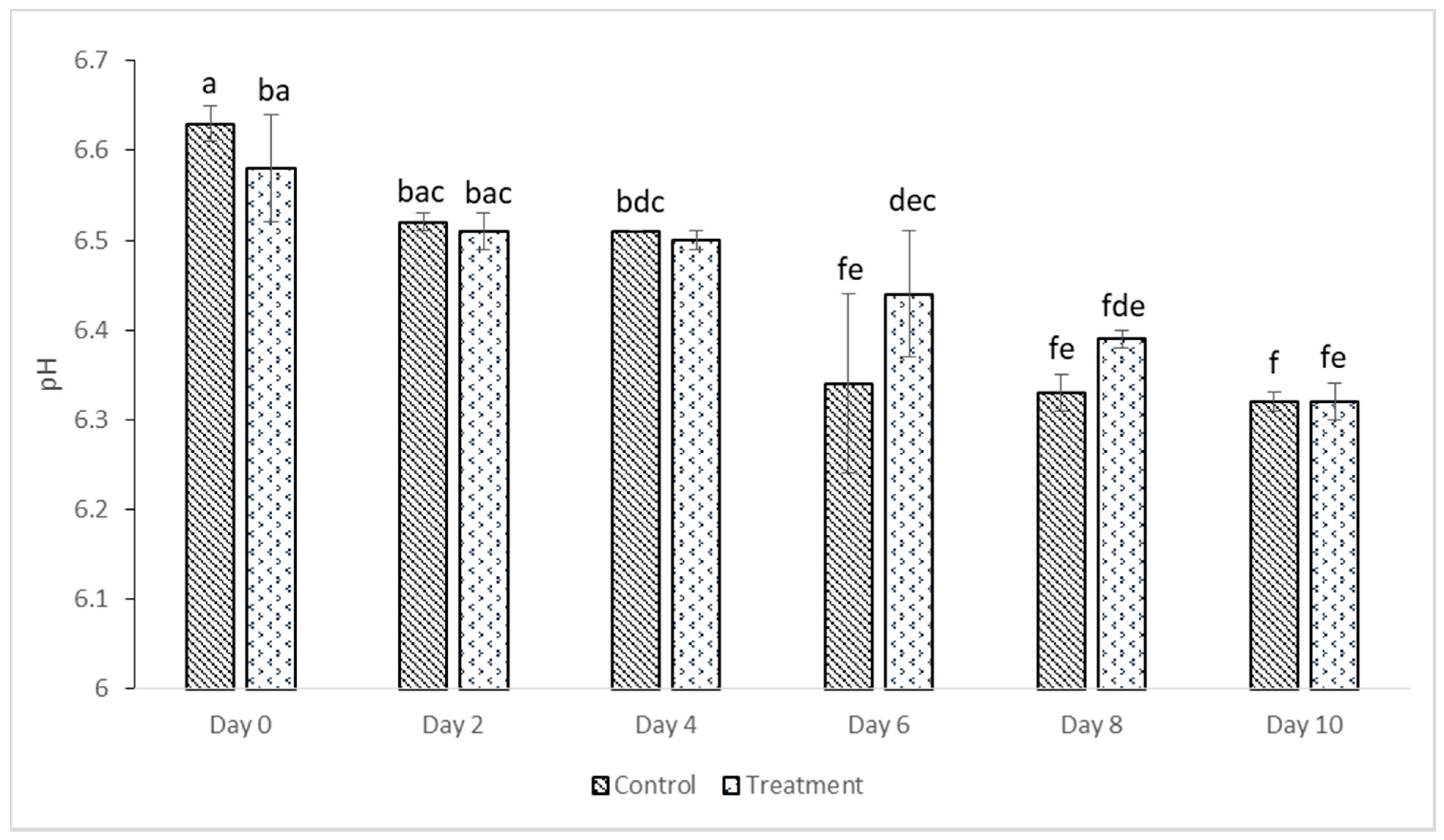
2.10. Determination of pH
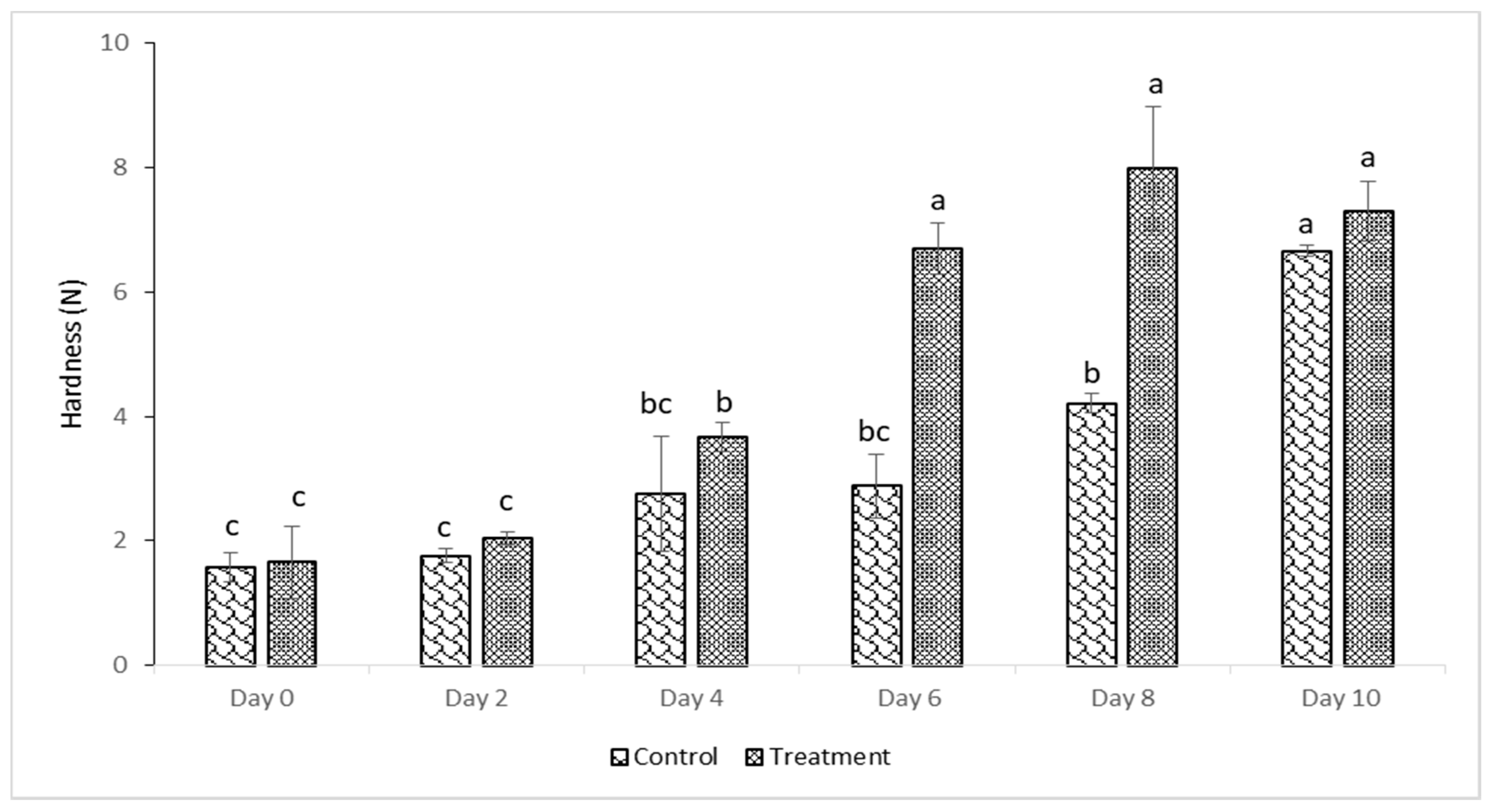
2.11. Textural Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Value of Quercetin
3.2. Oxidative Stability of Deep-Fat Fried Chicken
3.3. Instrumental Color
3.4. Texture Properties
3.5. pH Value
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donfrancesco, C.; Noce, C.L.; Brignoli, O.; Riccardi, G.; Ciccarelli, P.; Dima, F.; Palmieri, L.; Giampaoli, S. Italian network for obesity and cardiovascular disease surveillance: A pilot project. BMC Fam. Pract. 2008, 9, 53. [Google Scholar] [CrossRef]
- Pedreschi, F.; Moyano, P. Oil uptake and texture development in fried potato slices. J. Food Eng. 2005, 70, 557–563. [Google Scholar] [CrossRef]
- Soriguer, F.; Rojo-Martínez, G.; Dobarganes, M.C.; Almeida, J.M.G.; Esteva, I.; Beltrán, M.; De Adana, M.S.R.; Tinahones, F.; Gómez-Zumaquero, J.M.; García-Fuentes, E.; et al. Hypertension is related to the degradation of dietary frying oils. Am. J. Clin. Nutr. 2003, 78, 1092–1097. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Mittal, G.; Williams, R.; Mittal, G. Water and Fat Transfer Properties of Polysaccharide Films on Fried Pastry Mix. LWT 1999, 32, 440–445. [Google Scholar] [CrossRef]
- Azahrani, M.H.; Ananey-Obiri, D.; Matthews, L.; Tahergorabi, R. Development of low-fat fried fish using a two-prong strategy. CyTA J. Food 2019, 17, 882–891. [Google Scholar] [CrossRef]
- Ananey-Obiri, D.; Matthews, L.; Tahergorabi, R. Chicken processing by-product: A source of protein for fat uptake reduction in deep-fried chicken. Food Hydrocoll. 2020, 101, 105500. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef]
- Ahmad Mir, S.; Ahmad Masoodi, F.; Raja, J. Influence of natural antioxidants on microbial load, lipid oxidation and sensorial quality of rista—A traditional meat product of India. Food Biosci. 2017, 20, 79–87. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E.; Siebenhandl, S.; Bauer, F. The antioxidative properties of Holy basil and Galangal in cooked ground pork. Meat Sci. 2006, 72, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Karre, L.; Lopez, K.; Getty, K.J. Natural antioxidants in meat and poultry products. Meat Sci. 2013, 94, 220–227. [Google Scholar] [CrossRef]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Terao, J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Pobiega, K.; Przybył, J.L.; Żubernik, J.; Gniewosz, M. Prolonging the Shelf Life of Cherry Tomatoes by Pullulan Coating with Ethanol Extract of Propolis During Refrigerated Storage. Food Bioprocess Technol. 2020, 13, 1447–1461. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Przybył, J.L.; Bączek, K.; Żubernik, J.; Witrowa-Rajchert, D.; Gniewosz, M. Growth Biocontrol of Foodborne Pathogens and Spoilage Microorganisms of Food by Polish Propolis Extracts. Molecules 2019, 24, 2965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, L.; Zhang, L.; Guo, Y.; Qi, X.; He, L. Effects of quercetin on postharvest blue mold control in kiwifruit. Sci. Hortic. 2018, 228, 18–25. [Google Scholar] [CrossRef]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.N.; Ziaullah; Rupasinghe, H.P.V. Long Chain Fatty Acid Acylated Derivatives of Quercetin-3-O-Glucoside as Antioxidants to Prevent Lipid Oxidation. Biomolecules 2014, 4, 980–993. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Cha, Y.-J.; Lee, K.-H.; Yim, J.-E. Onion peel extract reduces the percentage of body fat in overweight and obese subjects: A 12-week, randomized, double-blind, placebo-controlled study. Nutr. Res. Pract. 2016, 10, 175–181. [Google Scholar] [CrossRef]
- Ananey-Obiri, D.; Matthews, L.; Azahrani, M.H.; Ibrahim, S.A.; Galanakis, C.M.; Tahergorabi, R. Application of protein-based edible coatings for fat uptake reduction in deep-fat fried foods with an emphasis on muscle food proteins. Trends Food Sci. Technol. 2018, 80, 167–174. [Google Scholar] [CrossRef]
- Odila Pereira, J.; Soares, J.; Sousa, S.; Madureira, A.R.; Gomes, A.; Pintado, M. Edible films as carrier for lactic acid bacteria. LWT 2016, 73, 543–550. [Google Scholar] [CrossRef]
- Pothakamury, U.R.; Barbosa-Cánovas, G.V. Fundamental aspects of controlled release in foods. Trends Food Sci. Technol. 1995, 6, 397–406. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Songtipya, P. Characteristics of film based on protein isolate from red tilapia muscle with negligible yellow discoloration. Int. J. Biol. Macromol. 2011, 48, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Tahergorabi, R.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Effect of isoelectric solubilization/precipitation and titanium dioxide on whitening and texture of proteins recovered from dark chicken-meat processing by-products. LWT 2011, 44, 896–903. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free Radical Scavenging Properties of Wheat Extracts. J. Agric. Food Chem. 2002, 50, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.S.; Browse, D.J.; Reading, S.; Benjamin, I. A simple and accurate mathematical method for calculation of the EC50. J. Pharmacol. Toxicol. Methods 1999, 41, 55–58. [Google Scholar] [CrossRef]
- Shaltout, F.; Marionette, Z.N.; Shakran, A. Quality of battered and breaded chicken meat products. Glob. J. Agric. Food Saf. Sci. 2014, 1, 283–299. [Google Scholar]
- Witte, V.C.; Krause, G.F.; Bailey, M.E. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J. Food Sci. 1970, 35, 582–585. [Google Scholar] [CrossRef]
- Alakhrash, F.; Anyanwu, U.; Tahergorabi, R. Physicochemical properties of Alaska pollock (Theragra chalcograma) surimi gels with oat bran. LWT 2016, 66, 41–47. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, H.-W.; Hwang, K.-E.; Song, D.-H.; Choi, M.-S.; Ham, Y.-K.; Choi, Y.-S.; Lee, J.-W.; Lee, S.-K.; Kim, C.-J. Combined Effect of Kimchi Powder and Onion Peel Extract on Quality Characteristics of Emulsion Sausages Prepared with Irradiated Pork. Food Sci. Anim. Resour. 2015, 35, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Jung, S.; Yong, H.I.; Bae, Y.S.; Kang, S.N.; Kim, I.S.; Jo, C. Improvement of microbiological safety and sensorial quality of pork jerky by electron beam irradiation and by addition of onion peel extract and barbecue flavor. Radiat. Phys. Chem. 2014, 98, 22–28. [Google Scholar] [CrossRef]
- Montero, P.; Giménez, B.; Perez-Mateos, M.; Gómez-Guillén, M.C. Oxidation stability of muscle with quercetin and rosemary during thermal and high-pressure gelation. Food Chem. 2005, 93, 17–23. [Google Scholar] [CrossRef]
- Lombard, K.; Peffley, E.; Geoffriau, E.; Thompson, L.; Herring, A. Quercetin in onion (Allium cepa L.) after heat-treatment simulating home preparation. J. Food Compos. Anal. 2005, 18, 571–581. [Google Scholar] [CrossRef]
- Crozier, A.; Lean, M.E.J.; McDonald, M.S.; Black, C. Quantitative Analysis of the Flavonoid Content of Commercial Tomatoes, Onions, Lettuce, and Celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Ewald, C.; Fjelkner-Modig, S.; Johansson, K.; Sjöholm, I.; Åkesson, B. Effect of processing on major flavonoids in processed onions, green beans, and peas. Food Chem. 1999, 64, 231–235. [Google Scholar] [CrossRef]
- Hirota, S.; Shimoda, T.; Takahama, U. Tissue and Spatial Distribution of Flavonol and Peroxidase in Onion Bulbs and Stability of Flavonol Glucosides during Boiling of the Scales. J. Agric. Food Chem. 1998, 46, 3497–3502. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Domestic Processing of Onion Bulbs (Allium cepa) and Asparagus Spears (Asparagus officinalis): Effect on Flavonol Content and Antioxidant Status. J. Agric. Food Chem. 2001, 49, 3216–3222. [Google Scholar] [CrossRef]
- Price, K.R.; Bacon, J.R.; Rhodes, M.J. Effect of storage and domestic processing on the content and composition of flavonol glucosides in onion (Allium cepa). J. Agric. Food Chem. 1997, 45, 938–942. [Google Scholar] [CrossRef]
- Sohaib, M.; Butt, M.S.; Anjum, F.M.; Khan, M.I.; Shahid, M. Augmentation of Oxidative Stability, Descriptive Sensory Attributes and Quality of Meat Nuggets from Broilers by Dietary Quercetin and ALPHA-Tocopherol Regimens. J. Food Process. Preserv. 2015, 40, 373–385. [Google Scholar] [CrossRef]
- Berri, C.; Debut, M.; Santé-Lhoutellier, V.; Arnould, C.; Boutten, B.; Sellier, N.; Baéza, E.; Jehl, N.; Jégo, Y.; Duclos, M.; et al. Variations in chicken breast meat quality: Implications of struggle and muscle glycogen content at death. Br. Poult. Sci. 2005, 46, 572–579. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.L.C.; de Carvalho, S.M.; de Araújo Soares, R.; Andrade, M.A.; das Graças Cardoso, M.; Ramos, E.M.; Piccoli, R.H. Antioxidant effects of Satureja montana L. essential oil on TBARS and color of mortadella-type sausages formulated with different levels of sodium nitrite. LWT 2012, 45, 204–212. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture Measurement of Individual Cooked Dry Beans By The Puncture Test. J. Food Sci. 1972, 37, 751–753. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Cava, R. Effect of natural and synthetic antioxidants on protein oxidation and colour and texture changes in refrigerated stored porcine liver pâté. Meat Sci. 2006, 74, 396–403. [Google Scholar] [CrossRef]
- Arshad, M.S.; Anjum, F.M.; Khan, M.I.; Shahid, M.; Akhtar, S.; Sohaib, M. Wheat germ oil enrichment in broiler feed with α-lipoic acid to enhance the antioxidant potential and lipid stability of meat. Lipids Health Dis. 2013, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-E.; Choi, Y.-S.; Choi, S.-M.; Kim, H.-W.; Choi, J.-H.; Lee, M.-A.; Kim, C.-J. Antioxidant action of ganghwayakssuk (Artemisia princeps Pamp.) in combination with ascorbic acid to increase the shelf life in raw and deep fried chicken nuggets. Meat Sci. 2013, 95, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, J.; Sayas-Barberá, E.; Muñoz, T.; Sendra, E.; Navarro, C.; Pérez-Alvarez, J.A. Effect of packaging conditions on shelf-life of ostrich steaks. Meat Sci. 2008, 78, 143–152. [Google Scholar] [CrossRef]
| Days of Storage | L* | a* | b* | |||
|---|---|---|---|---|---|---|
| Control | Treatment | Control | Treatment | Control | Treatment | |
| 0 | 52.89 ± 3.89 a | 52.23 ± 0.53 ab | 7.51 ± 0.91 a | 8.88 ± 0.52 a | 3.05 ± 0.71 c | 6.86 ± 3.65 bc |
| 2 | 48.71 ± 0.92 abcd | 50.54 ± 0.97 abc | 8.10 ± 0.48 a | 8.69 ± 1.45 a | 9.25 ± 1.08 ab | 9.64 ± 0.99 ab |
| 4 | 47.92 ± 1.57 abcd | 49.36 ± 0.82 abc | 8.03 ± 1.26 a | 9.33 ± 0.91 a | 12.17 ± 3.24 ab | 14.31 ± 2.39 a |
| 6 | 48.51 ± 1.55 abcd | 48.40 ± 2.22 abcd | 7.89 ± 0.07 a | 8.56 ± 2.15 a | 12.51 ± 0.33 ab | 12.64 ± 3.17 ab |
| 8 | 46.19 ± 1.15 cd | 47.33 ± 2.25 bcd | 7.38 ± 0.50 a | 7.78 ± 1.24 a | 11.17 ± 1.70 ab | 13.52 ± 0.90 a |
| 10 | 45.75 ± 0.98 cd | 43.91 ± 1.62 d | 7.17 ± 0.56 a | 7.36 ± 1.40 a | 12.24 ± 1.64 ab | 11.47 ± 2.13 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adrah, K.; Ananey-Obiri, D.; Tahergorabi, R. Physicochemical Changes of Deep-Fat-Fried Chicken Drumsticks Treated with Quercetin-in-Edible Coating during Storage Time. Foods 2021, 10, 467. https://doi.org/10.3390/foods10020467
Adrah K, Ananey-Obiri D, Tahergorabi R. Physicochemical Changes of Deep-Fat-Fried Chicken Drumsticks Treated with Quercetin-in-Edible Coating during Storage Time. Foods. 2021; 10(2):467. https://doi.org/10.3390/foods10020467
Chicago/Turabian StyleAdrah, Kelvin, Daniel Ananey-Obiri, and Reza Tahergorabi. 2021. "Physicochemical Changes of Deep-Fat-Fried Chicken Drumsticks Treated with Quercetin-in-Edible Coating during Storage Time" Foods 10, no. 2: 467. https://doi.org/10.3390/foods10020467
APA StyleAdrah, K., Ananey-Obiri, D., & Tahergorabi, R. (2021). Physicochemical Changes of Deep-Fat-Fried Chicken Drumsticks Treated with Quercetin-in-Edible Coating during Storage Time. Foods, 10(2), 467. https://doi.org/10.3390/foods10020467







