The Impact of Terroir on the Flavour of Single Malt Whisk(e)y New Make Spirit
Abstract
1. Introduction
2. Methods
2.1. Chemicals
2.2. Experimental Conditions
2.3. New Make Spirit Production
2.4. Gas Chromatography Olfactometry (GCO) Method
2.5. Panel Training for GCO Sessions
2.6. Olfactory Tests in GCO
2.7. Sensory Evaluation of New Make Spirits
2.8. Statistical Analysis
3. Results
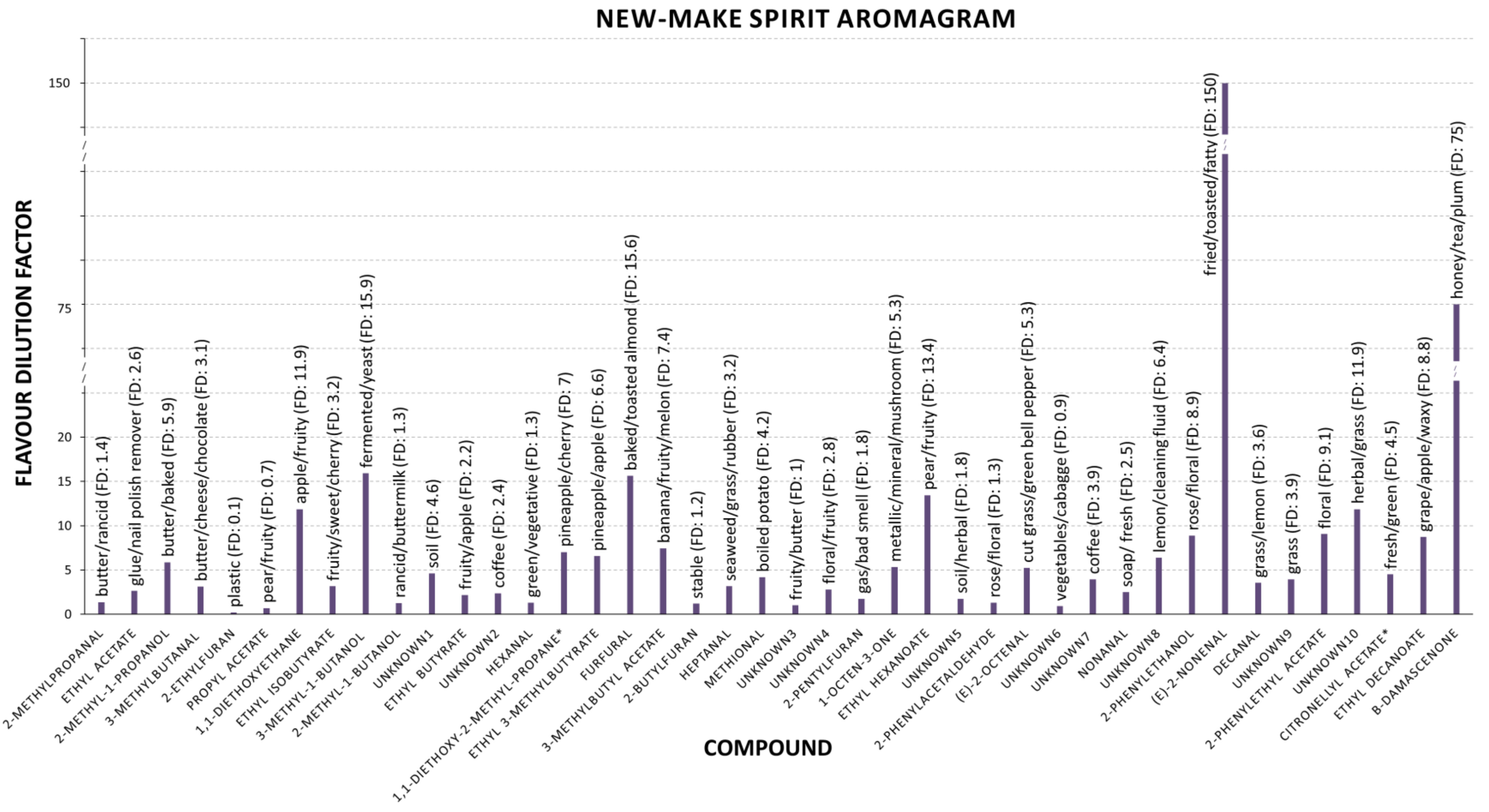
3.1. Aroma Active Compounds
3.1.1. Chemical Odours
3.1.2. Earthy odours
3.1.3. Fatty Odours
3.1.4. Floral Odours
3.1.5. Fruity Odours
3.1.6. Grassy and Vegetal Odours
3.1.7. Roasty Odours
3.1.8. Key Aroma Active Compounds
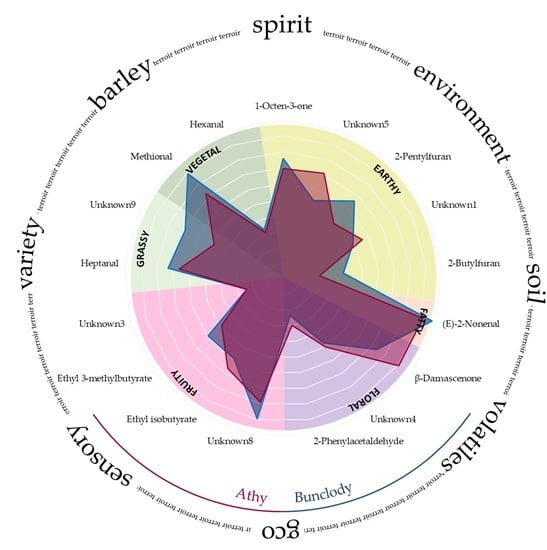
3.2. Sensory Evaluation of New Make Spirits
3.3. Chemometric Analysis of Sensory and Aroma Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- gov.ie—Geographical Indications—Spirit Drinks. Available online: https://www.gov.ie/en/publication/75770-geographical-indications-spirit-drinks/#technical-files (accessed on 2 January 2021).
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef]
- Bravi, E.; Marconi, O.; Perretti, G.; Fantozzi, P. Influence of barley variety and malting process on lipid content of malt. Food Chem. 2012, 135, 1112–1117. [Google Scholar] [CrossRef]
- Dong, L.; Piao, Y.; Zhang, X.; Zhao, C.; Hou, Y.; Shi, Z. Analysis of volatile compounds from a malting process using headspace solid-phase micro-extraction and GC–MS. Food Res. Int. 2013, 51, 783–789. [Google Scholar] [CrossRef]
- Smit, B.A.; Engels, W.J.M.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef]
- Ledauphin, J.; Guichard, H.; Saint-Clair, J.-F.; Picoche, B.; Barillier, D. Chemical and Sensorial Aroma Characterization of Freshly Distilled Calvados. 2. Identification of Volatile Compounds and Key Odorants. J. Agric. Food Chem. 2003, 51, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.D.S.; Marques, J.C.; Perestrelo, R.M.D.S.; Rodrigues, F.; Oliveira, L.; Andrade, P.; Caldeira, M. Comparative study of the whisky aroma profile based on headspace solid phase microextraction using different fibre coatings. J. Chromatogr. A 2007, 1150, 198–207. [Google Scholar] [CrossRef]
- Apostolopoulou, A.; Flouros, A.; Demertzis, P.; Akrida-Demertzi, K. Differences in concentration of principal volatile constituents in traditional Greek distillates. Food Control. 2005, 16, 157–164. [Google Scholar] [CrossRef]
- Arnold, R.J.; Ochoa, A.; Kerth, C.R.; Miller, R.K.; Murray, S.C. Assessing the impact of corn variety and Texas terroir on flavor and alcohol yield in new-make bourbon whiskey. PLoS ONE 2019, 14, e0220787. [Google Scholar] [CrossRef]
- Cacho, J.; Moncayo, L.; Palma, J.C.; Ferreira, V.; Culleré, L. Characterization of the aromatic profile of the Italia variety of Peruvian pisco by gas chromatography-olfactometry and gas chromatography coupled with flame ionization and mass spectrometry detection systems. Food Res. Int. 2012, 49, 117–125. [Google Scholar] [CrossRef]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T.F. Nature’s Chemical Signatures in Human Olfaction: A Foodborne Perspective for Future Biotechnology. Angew. Chem. Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; De Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas Chromatography Analysis with Olfactometric Detection (GC-O) as a Useful Methodology for Chemical Characterization of Odorous Compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef] [PubMed]
- Guichard, H.; Lemesle, S.; Ledauphin, J.; Barillier, D.; Picoche, B. Chemical and Sensorial Aroma Characterization of Freshly Distilled Calvados. 1. Evaluation of Quality and Defects on the Basis of Key Odorants by Olfactometry and Sensory Analysis. J. Agric. Food Chem. 2003, 51, 424–432. [Google Scholar] [CrossRef]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef]
- Poisson, L.; Schieberle, P. Characterization of the Most Odor-Active Compounds in an American Bourbon Whisky by Application of the Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2008, 56, 5813–5819. [Google Scholar] [CrossRef]
- Poisson, L.; Schieberle, P. Characterization of the Key Aroma Compounds in an American Bourbon Whisky by Quantitative Measurements, Aroma Recombination, and Omission Studies. J. Agric. Food Chem. 2008, 56, 5820–5826. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Lablanquie, O.; Cantagrel, R.; Ledauphin, J.; Payot, T.; Fournier, N.; Guichard, E. Determination of Key Odorant Compounds in Freshly Distilled Cognac Using GC-O, GC-MS, and Sensory Evaluation. J. Agric. Food Chem. 2004, 52, 5670–5676. [Google Scholar] [CrossRef]
- Biernacka, P.; Wardencki, W. Volatile composition of raw spirits of different botanical origin. J. Inst. Brew. 2012, 118, 393–400. [Google Scholar] [CrossRef]
- Cortés-Diéguez, S.; Rodríguez, R.; Salgado, J.M.; Domínguez, J.M. Comparative study between Italian and Spanish grape marc spirits in terms of major volatile compounds. Food Control. 2011, 22, 673–680. [Google Scholar] [CrossRef]
- Herb, D.; Filichkin, T.; Fisk, S.; Helgerson, L.; Hayes, P.; Meints, B.; Jennings, R.; Monsour, R.; Tynan, S.; Vinkemeier, K.; et al. Effects of Barley (Hordeum Vulgare L.) Variety and Growing Environment on Beer Flavor. J. Am. Soc. Brew. Chem. 2017, 75, 345–353. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Seguin, G. The concept of terroir in viticulture. J. Wine Res. 2006, 17, 1–10. [Google Scholar] [CrossRef]
- Lucini, L.; Rocchetti, G.; Trevisan, M. Extending the concept of terroir from grapes to other agricultural commodities: An overview. Curr. Opin. Food Sci. 2020, 31, 88–95. [Google Scholar] [CrossRef]
- gov.ie-Rural Environment & Sustainability-Nitrates. Available online: https://www.gov.ie/en/publication/c9563-rural-environment-sustainability-nitrates/ (accessed on 26 January 2021).
- Vene, K.; Seisonen, S.; Koppel, K.; Leitner, E.; Paalme, T. A Method for GC–Olfactometry Panel Training. Chemosens. Percept. 2013, 6, 179–189. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Printza, A.; Genetzaki, S.; Mamali, K.; Kekes, G.; Constantinidis, J. Cultural adaptation of an olfactory identification test: The Greek version of Sniffin’ Sticks. Rhinol. J. 2008, 46, 292–296. [Google Scholar]
- Feng, Y.; Cai, Y.; Sun-Waterhouse, D.; Cui, C.; Su, G.; Lin, L.; Zhao, M. Approaches of aroma extraction dilution analysis (AEDA) for headspace solid phase microextraction and gas chromatography–olfactometry (HS-SPME–GC–O): Altering sample amount, diluting the sample or adjusting split ratio? Food Chem. 2015, 187, 44–52. [Google Scholar] [CrossRef]
- ISO. ISO 13299:2016—Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. Available online: https://www.iso.org/standard/58042.html (accessed on 26 January 2021).
- Falcão, L.D.; De Revel, G.; Rosier, J.P.; Bordignon-Luiz, M.T. Aroma impact components of Brazilian Cabernet Sauvignon wines using detection frequency analysis (GC–olfactometry). Food Chem. 2008, 107, 497–505. [Google Scholar] [CrossRef]
- Sefton, M.A.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Occurrence, Sensory Impact, Formation, and Fate of Damascenone in Grapes, Wines, and Other Foods and Beverages. J. Agric. Food Chem. 2011, 59, 9717–9746. [Google Scholar] [CrossRef]
- Guyot-Declerck, C.; François, N.; Ritter, C.; Govaerts, B.; Collin, S. Influence of pH and ageing on beer organoleptic properties. A sensory analysis based on AEDA data. Food Qual. Prefer. 2005, 16, 157–162. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdieu, D. Identification of substances responsible for the “sawdust” aroma in oak wood. J. Sci. Food Agric. 1998, 76, 179–188. [Google Scholar] [CrossRef]
- Svoboda, Z.; Mikulíková, R.; Běláková, S.; Benešová, K.; Marová, I.; Nesvadba, Z. Optimization of Modern Analytical SPME and SPDE Methods for Determination of Trans-2-nonenal in Barley, Malt and Beer. Chromatographia 2011, 73, 157–161. [Google Scholar] [CrossRef]
- Wanikawa, A.; Hosoi, K.; Kato, T.; Nakagawa, K.-I. Identification of green note compounds in malt whisky using multidimensional gas chromatography. Flavour Fragr. J. 2002, 17, 207–211. [Google Scholar] [CrossRef]
- Campo, E.; Cacho, J.; Ferreira, V. The Chemical Characterization of the Aroma of Dessert and Sparkling White Wines (Pedro Ximénez, Fino, Sauternes, and Cava) by Gas Chromatography−Olfactometry and Chemical Quantitative Analysis. J. Agric. Food Chem. 2008, 56, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hou, Y.; Li, F.; Piao, Y.; Zhang, X.; Li, C.; Zhao, C. Characterization of volatile aroma compounds in different brewing barley cultivars. J. Sci. Food Agric. 2014, 95, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Caldeira, M.; Câmara, J. Development of a dynamic headspace solid-phase microextraction procedure coupled to GC–qMSD for evaluation the chemical profile in alcoholic beverages. Anal. Chim. Acta 2008, 609, 82–104. [Google Scholar] [CrossRef]
- Fitzgerald, G.; James, K.J.; MacNamara, K.; Stack, M.A. Characterisation of whiskeys using solid-phase microextraction with gas chromatography–mass spectrometry. J. Chromatogr. A 2000, 896, 351–359. [Google Scholar] [CrossRef]
- González-Arjona, D.; González-Gallero, V.; Pablos, F.; González, A.G. Authentication and differentiation of irish whiskeys by higher-alcohol congener analysis. Anal. Chim. Acta 1999, 381, 257–264. [Google Scholar] [CrossRef]
- Russell, I.; Stewart, G. Distilling Yeast and Fermentation, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780124017351. [Google Scholar]
- Jeleń, H.H.; Majcher, M.; Szwengiel, A. Key odorants in peated malt whisky and its differentiation from other whisky types using profiling of flavor and volatile compounds. LWT 2019, 107, 56–63. [Google Scholar] [CrossRef]
- Singer, D.D. The proportion of 2-methylbutanol and 3-methylbutanol in some brandies and whiskies as determined by direct gas chromatography. Analyst 1966, 91, 790–794. [Google Scholar] [CrossRef]
- Ferreira, I.F.M.; Guido, L.F. Impact of Wort Amino Acids on Beer Flavour: A Review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Śliwińska, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The analysis of raw spirits–A review of methodology. J. Inst. Brew. 2016, 122, 5–10. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical Characterization of the Aroma of Grenache Rosé Wines: Aroma Extract Dilution Analysis, Quantitative Determination, and Sensory Reconstitution Studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Pineau, B.; Barbe, J.-C.; Van Leeuwen, C.; Dubourdieu, D. Which Impact for β-Damascenone on Red Wines Aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Tolle, S.; Gök, R.; Winterhalter, P. Characterisation of odour-active compounds in aged rum. Food Chem. 2012, 132, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Pineau, B.; Barbe, J.-C.; Van Leeuwen, C.; Dubourdieu, D. Examples of Perceptive Interactions Involved in Specific “Red-” and “Black-berry” Aromas in Red Wines. J. Agric. Food Chem. 2009, 57, 3702–3708. [Google Scholar] [CrossRef] [PubMed]
- Demyttenaere, J.C.; Martı#xnez, J.I.S.; Verhé, R.; Sandra, P.; De Kimpe, N.; Sanchezmartinez, J. Analysis of volatiles of malt whisky by solid-phase microextraction and stir bar sorptive extraction. J. Chromatogr. A 2003, 985, 221–232. [Google Scholar] [CrossRef]
- Kłosowski, G.; Czupryński, B. Kinetics of acetals and esters formation during alcoholic fermentation of various starchy raw materials with application of yeasts Saccharomyces cerevisiae. J. Food Eng. 2006, 72, 242–246. [Google Scholar] [CrossRef]
- Piggott, J.R.; Conner, J.M. Whiskies; Springer: Berlin/Heidelberg, Germany, 2003; pp. 239–262. [Google Scholar]
- San-Juan, F.; Ferreira, V.; Cacho, J.; Escudero, A. Quality and Aromatic Sensory Descriptors (Mainly Fresh and Dry Fruit Character) of Spanish Red Wines can be Predicted from their Aroma-Active Chemical Composition. J. Agric. Food Chem. 2011, 59, 7916–7924. [Google Scholar] [CrossRef]
- Prentice, R.D.M.; McKernan, G.; Bryce, J.H. A Source of Dimethyl Disulfide and Dimethyl Trisulfide in Grain Spirit Produced with a Coffey Still. J. Am. Soc. Brew. Chem. 1998, 56, 99–103. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Quek, A.Y.H. Evaluation of Beer Fermentation with a Novel Yeast Williopsis saturnus. Food Technol. Biotechnol. 2016, 54, 403–412. [Google Scholar] [CrossRef]
- Balcerek, M.; Patelski, P.; Strąk, E.; Dziekońska-Kubczak, U.; Pielech-Przybylska, K. The effect of distillation conditions and alcohol content in ‘heart’ fractions on the concentration of aroma volatiles and undesirable compounds in plum brandies. J. Inst. Brew. 2017, 123, 452–463. [Google Scholar] [CrossRef]
- Coldea, T.E.; Mudura, E.; Socaciu, C. Advances in Distilled Beverages Authenticity and Quality Testing. In Ideas and Applications Toward Sample Preparation for Food and Beverage Analysis; IntechOpen: London, UK, 2017; pp. 109–130. [Google Scholar]
- Yahya, H.; Linforth, R.S.; Cook, D.J. Flavour generation during commercial barley and malt roasting operations: A time course study. Food Chem. 2014, 145, 378–387. [Google Scholar] [CrossRef]
- De Rosso, M.; Cancian, D.; Panighel, A.; Vedova, A.D.; Flamini, R. Chemical compounds released from five different woods used to make barrels for aging wines and spirits: Volatile compounds and polyphenols. Wood Sci. Technol. 2009, 43, 375–385. [Google Scholar] [CrossRef]
- Aylott, R.I.; Clyne, A.H.; Fox, A.P.; Walker, D.A. Analytical strategies to confirm Scotch whisky authenticity. Analyst 1994, 119, 1741–1746. [Google Scholar] [CrossRef]
- Ebeler, S.E.; Terrien, M.B.; Butzke, C.E. Analysis of brandy aroma by solid-phase microextraction and liquid-liquid extraction. J. Sci. Food Agric. 2000, 80, 625–630. [Google Scholar] [CrossRef]
- Lee, K.-Y.M.; Paterson, A.; Piggott, J.R.; Richardson, G.D. Origins of Flavour in Whiskies and a Revised Flavour Wheel: A Review. J. Inst. Brew. 2001, 107, 287–313. [Google Scholar] [CrossRef]
- De Schutter, D.P.; Saison, D.; Delvaux, F.; Derdelinckx, G.; Rock, J.-M.; Neven, H.; Delvaux, F.R. Optimisation of wort volatile analysis by headspace solid-phase microextraction in combination with gas chromatography and mass spectrometry. J. Chromatogr. A 2008, 1179, 75–80. [Google Scholar] [CrossRef]
- Martins, C.; Brandão, T.; Almeida, A.; Rocha, S.M. Insights on beer volatile profile: Optimization of solid-phase microextraction procedure taking advantage of the comprehensive two-dimensional gas chromatography structured separation. J. Sep. Sci. 2015, 38, 2140–2148. [Google Scholar] [CrossRef]
- Windes, S.; Bettenhausen, H.M.; Van Simaeys, K.R.; Clawson, J.; Fisk, S.; Heuberger, A.L.; Lim, J.; Queisser, S.H.; Shellhammer, T.H.; Hayes, P.M. Comprehensive Analysis of Different Contemporary Barley Genotypes Enhances and Expands the Scope of Barley Contributions to Beer Flavor. J. Am. Soc. Brew. Chem. 2020, 1–25. [Google Scholar] [CrossRef]
- Barley–International. Available online: https://www.barleyhub.org/barley/ (accessed on 26 January 2021).
| Compound | Cas No | RT 1 | LRI | Chemical Class | Descriptor | Odour Type | MF% Average |
|---|---|---|---|---|---|---|---|
| Nonanal | 124-19-6 | 18.07 | 1147.4 | Aldehyde | soap/fresh | chemical | 42.4 |
| Ethyl acetate | 141-78-6 | 4.30 | 642.2 | Ester | glue/nail polish remover | chemical | 12.0 |
| 2-Ethylfuran | 3208-16-0 | 5.94 | 717.1 | Furan | plastic | chemical | 12.0 |
| 1-Octen-3-one | 4312-99-6 | 14.70 | 1018.5 | Ketone | metallic/mineral/mushroom | earthy | 72.6 |
| Unknown5 | - | 16.27 | 1073.0 | - | soil/herbal | earthy | 61.9 |
| 2-Pentylfuran | 3777-69-3 | 14.36 | 1008.7 | Furan | gas/bad smell | earthy | 56.6 |
| Unknown1 | - | 8.25 | 796.5 | - | soil | earthy | 52.1 |
| 2-Butylfuran | 4466-24-4 | 12.20 | 932.7 | Furan | stable | earthy | 30.4 |
| (E)-2-Nonenal | 18829-56-6 | 19.80 | 1219.8 | Aldehyde | fried/toasted/fatty | fatty | 94.2 |
| 3-Methyl-1-butanol (Isoamyl alcohol) | 123-51-3 | 8.08 | 789.1 | Alcohol | fermented/yeast | fatty | 87.1 |
| 3-Methylbutanal (Isovaleraldehyde) | 590-86-3 | 5.33 | 693.4 | Aldehyde | butter/cheese/chocolate | fatty | 60.0 |
| 2-Methyl-1-butanol | 137-32-6 | 8.15 | 792.5 | Alcohol | rancid/buttermilk | fatty | 47.9 |
| 2-Methyl-1-propanol (Isobutyl alcohol) | 78-83-1 | 5.15 | 685.9 | Alcohol | butter/baked | fatty | 40.9 |
| 2-Methylpropanal (Isobutyraldehyde) | 78-84-2 | 3.20 | 598.6 | Aldehyde | butter/rancid | fatty | 16.8 |
| β-Damascenone | 23726-93-4 | 23.03 | 1452.7 | Ketone | honey/tea/plum | floral | 82.3 |
| 2-Phenylethanol | 60-12-8 | 19.30 | 1194.5 | Alcohol | rose/floral | floral | 77.9 |
| 2-Phenylethyl acetate | 103-45-7 | 21.30 | 1311.3 | Ester | floral | floral | 58.8 |
| Unknown4 | - | 13.96 | 991.7 | - | floral/fruity | floral | 48.8 |
| 2-Phenylacetaldehyde | 122-78-1 | 17.16 | 1108.2 | Aldehyde | rose/floral | floral | 26.6 |
| Ethyl hexanoate | 123-66-0 | 14.89 | 1025.3 | Ester | pear/fruity | fruity | 84.9 |
| Unknown8 | - | 19.22 | 1191.5 | - | lemon/cleaning fluid | fruity | 84.2 |
| 1,1-Diethoxy-2-methyl-propane * | 1741-41-9 | 10.55 | 874.3 | Acetal | pineapple/cherry | fruity | 72.1 |
| 3-Methylbutyl acetate (Isoamyl acetate) | 123-92-2 | 11.50 | 905.5 | Ester | banana/fruity/melon | fruity | 70.4 |
| Ethyl isobutyrate | 97-62-1 | 7.90 | 784.9 | Ester | fruity/sweet/cherry | fruity | 62.7 |
| 1,1-Diethoxyethane | 105-57-7 | 6.81 | 746.6 | Acetal | apple/fruity | fruity | 59.4 |
| Ethyl butyrate | 105-54-4 | 9.16 | 827.7 | Ester | fruity/apple | fruity | 57.8 |
| Ethyl 3-methylbutyrate (Ethyl Isovalerate) | 108-64-5 | 10.70 | 879.6 | Ester | pineapple/apple | fruity | 54.0 |
| Ethyl decanoate | 110-38-3 | 22.73 | 1423.1 | Ester | grape/apple/waxy | fruity | 39.6 |
| Unknown3 | - | 13.80 | 985.7 | - | fruity/butter | fruity | 24.1 |
| Propyl acetate | 109-60-4 | 6.71 | 744.4 | Ester | pear/fruity | fruity | 20.3 |
| Unknown10 | - | 21.41 | 1318.0 | - | herbal/grass | grassy | 93.5 |
| Heptanal | 111-71-7 | 12.50 | 941.5 | Aldehyde | seaweed/grass/rubber | grassy | 69.5 |
| Unknown6 | - | 17.28 | 1114.5 | - | cut grass/green bell pepper | grassy | 61.9 |
| Unknown9 | - | 20.89 | 1278.5 | - | grass | grassy | 58.9 |
| Citronellyl acetate * | 150-84-5 | 22.26 | 1384.3 | Ester | fresh/green | grassy | 44.8 |
| Decanal | 112-31-2 | 20.20 | 1251.6 | Aldehyde | grass/lemon | grassy | 30.9 |
| Methional | 3268-49-3 | 13.31 | 968.8 | Aldehyde | boiled potato | vegetal | 80.9 |
| (E)-2-Octenal | 2548-87-0 | 17.35 | 1118.5 | Aldehyde | vegetable/cabagge | vegetal | 37.0 |
| Hexanal | 66-25-1 | 9.50 | 838.7 | Aldehyde | green/vegetative | vegetal | 32.2 |
| Furfural | 98-01-1 | 11.20 | 896.7 | Furan | baked/toasted almond | roasty | 85.5 |
| Unknown7 | - | 17.73 | 1132.5 | - | coffee | roasty | 62.5 |
| Unknown2 | - | 9.22 | 828.5 | - | coffee | roasty | 55.7 |
| Compound | Attribute | Odour Type | Variety MF% | Environment MF% | Season MF% | |||
|---|---|---|---|---|---|---|---|---|
| Laureate | Olympus | Athy | Bunclody | 2017 | 2018 | |||
| 1-Octen-3-one | metallic/mineral/mushroom | earthy | 72.4 a | 72.7 a | 75.8 a | 69.3 a | 63.6 b | 81.5 a |
| Unknown 5 | soil/herbal | earthy | 64.5 a | 59.3 a | 52.7 b | 71.1 a | 83.1 a | 40.6 b |
| 2-Pentylfuran | gas/bad smell | earthy | 62.7 a | 50.6 a | 66.3 a | 47.0 b | 69.5 a | 43.8 b |
| Unknown 1 | soil | earthy | 63.7 a | 40.6 a | 48.8 a | 55.5 a | 71.0 a | 33.3 b |
| 2-Butylfuran | stable | earthy | 29.8 a | 31.0 a | 38.0 a | 22.8 b | 14.4 b | 46.4 a |
| (E)-2-Nonenal | fried/toasted/fatty | fatty | 94.3 a | 94.1 a | 97.1 a | 91.2 b | 94.2 a | 94.2 a |
| β-Damascenone | honey/tea/plum | floral | 85.8 a | 78.7 a | 73.7 b | 90.8 a | 89.3 a | 75.3 a |
| Unknown 4 | floral/fruity | floral | 43.3 a | 54.3 a | 47.3 a | 50.3 a | 60.0 a | 37.7 b |
| 2-Phenylacetaldehyde | rose/floral | floral | 16.4 b | 36.8 a | 23.5 a | 29.7 a | 11.8 b | 41.4 a |
| Unknown 8 | lemon/cleaning fluid | fruity | 84.1 a | 84.3 a | 89.5 a | 79.0 a | 75.8 b | 92.7 a |
| Ethyl isobutyrate | fruity/sweet/cherry | fruity | 64.3 a | 61.1 a | 59.1 a | 66.3 a | 70.1 a | 55.3 b |
| Ethyl 3-methylbutyrate (Ethyl Isovalerate) | pineapple/apple | fruity | 49.3 a | 58.7 a | 59.4 a | 48.6 a | 70.1 a | 37.9 b |
| Unknown 3 | fruity/butter | fruity | 9.6 b | 38.5 a | 24.1 a | 24.0 a | 33.7 a | 14.4 a |
| Heptanal | seaweed/grass/rubber | grassy | 57.7 b | 81.3 a | 72.9 a | 66.0 a | 78.5 a | 60.5 a |
| Unknown 9 | grass | grassy | 61.1 a | 56.7 a | 69.1 a | 48.6 b | 60.7 a | 57.1 a |
| Methional | boiled potato | vegetal | 80.9 a | 80.9 a | 89.3 a | 72.5 b | 92.7 a | 69.1 b |
| Hexanal | green/vegetative | vegetal | 9.6 b | 54.7 a | 33.3 a | 31.1 a | 26.2 a | 38.1 a |
| Intensity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variety | Environment | Variety × Environment | |||||||||
| Sensory Attribute | Laureate | Olympus | Significant Level | Athy | Bunclody | Significant Level | Laureate × Athy | Laureate × Bunclody | Olympus × Athy | Olympus × Bunclody | Significant Level |
| Season 2017 | |||||||||||
| Pungent | 1.85 b | 2.50 a | *** | 2.25 a | 2.10 b | * | 1.85 b,c | 1.56 c | 2.66 a | 2.33 a,b | * |
| Feinty/Earthy | 0.74 a | 0.99 a | --- | 1.16 a | 0.57 b | *** | 0.87 b | 0.61 b | 1.45 a | 0.53 b | ** |
| Cereal/Grainy | 2.30 a | 2.46 a | --- | 2.42 a | 2.34 a | --- | 2.15 a | 2.46 a,b | 2.70 a | 2.22 b | ** |
| Malty/Biscuity | 2.47 a | 2.44 a | --- | 2.70 a | 2.21 b | ** | 2.63 a,b | 2.31 b | 2.76 a | 2.12 b | --- |
| Green/Grassy | 0.90 a | 0.80 a | --- | 0.49 b | 1.21 a | *** | 0.47 b | 1.33 a | 0.51 b | 1.08 a | --- |
| Floral | 1.38 a | 1.07 a | --- | 0.79 b | 1.66 a | *** | 0.87 c | 1.90 a | 0.71 c | 1.43 b | * |
| Fresh Fruit | 2.41 b | 2.98 a | ** | 2.55 b | 2.83 a | ** | 2.35 b | 2.47 b | 2.76 b | 3.20 a | * |
| Dried Fruit | 2.40 a | 2.60 a | --- | 2.25 b | 2.74 a | ** | 2.12 c | 2.67 a,b | 2.38 b,c | 2.81 a | --- |
| Soapy | 1.19 a | 1.22 a | --- | 1.29 a | 1.13 a | --- | 1.36 a | 1.02 a | 1.21 a | 1.23 a | --- |
| Solventy | 2.00 a | 2.20 a | --- | 1.91 b | 2.28 a | * | 1.85 a | 2.15 a | 1.98 a | 2.42 a | --- |
| Sweet | 2.15 a | 2.08 a | --- | 2.19 a | 2.05 a | --- | 2.17 a | 2.13 a | 2.21 a | 1.96 a | --- |
| Oily finish | 0.30 a | 0.45 a | --- | 0.53 a | 0.21 b | * | 0.42 a,b | 0.17 b | 0.65 a | 0.25 b | --- |
| Sour | 0.13 a | 0.04 a | --- | 0.15 a | 0.02 a | --- | 0.25 a | 0.01 a | 0.05 a | 0.03 a | --- |
| Sulphury | 0.16 a | 0.15 a | --- | 0.17 a | 0.15 a | --- | 0.10 a | 0.23 a | 0.25 a | 0.06 a | --- |
| Stale/Mouldy | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| Season 2018 | |||||||||||
| Pungent | 2.49 b | 2.77 a | * | 3.12 a | 2.14 b | *** | 2.77 b | 2.21 c | 3.46 a | 2.07 c | ** |
| Feinty/Earthy | 1.77 a | 1.94 a | --- | 2.22 a | 1.48 b | *** | 1.99 b | 1.54 c | 2.45 a | 1.43 c | * |
| Cereal/Grainy | 1.56 a | 1.68 a | --- | 1.77 a | 1.47 b | ** | 1.66 a,b | 1.45 b | 1.88 a | 1.48 b | --- |
| Malty/Biscuity | 2.64 b | 2.92 a | ** | 3.13 a | 2.43 b | *** | 3.08 a | 2.19 c | 3.18 a | 2.66 b | --- |
| Green/Grassy | 1.52 a | 1.30 a | --- | 1.32 a | 1.50 a | --- | 1.47 a | 1.57 a | 1.18 a | 1.42 a | --- |
| Floral | 1.92 a | 1.33 b | ** | 1.02 b | 2.24 a | *** | 1.33 c | 2.52 a | 0.71 d | 1.96 b | * |
| Fresh Fruit | 2.87 b | 3.21 a | ** | 2.62 b | 3.46 a | *** | 2.49 c | 3.26 b | 2.76 c | 3.66 a | * |
| Dried Fruit | 2.49 a | 2.22 a | --- | 2.15 b | 2.56 a | ** | 2.38 a | 2.60 a | 1.92 b | 2.51 a | --- |
| Soapy | 1.87 a | 1.65 a | --- | 1.69 a | 1.83 a | --- | 1.89 a | 1.86 a | 1.49 a | 1.81 a | --- |
| Solventy | 1.71 a | 1.66 a | --- | 1.57 b | 1.81 a | * | 1.74 a | 1.69 a,b | 1.40 b | 1.93 a | ** |
| Sweet | 2.30 a | 2.31 a | --- | 2.40 a | 2.22 a | --- | 2.29 a,b | 2.31 a,b | 2.50 a | 2.13 b | --- |
| Oily finish | 1.71 a | 1.76 a | --- | 2.15 a | 1.31 b | *** | 1.97 a | 1.46 b | 1.97 a | 1.17 b | * |
| Sour | 0.64 a | 0.68 a | --- | 0.77 a | 0.56 a | --- | 0.75 a | 0.54 a | 0.79 a | 0.58 a | --- |
| Sulphury | 0.94 a | 1.02 a | --- | 1.37 a | 0.59 b | *** | 1.33 a | 0.55 b | 1.42 a | 0.63 b | * |
| Stale/Mouldy | 0.36 a | 0.28 a | --- | 0.30 a | 0.34 a | --- | 0.22 a | 0.50 a | 0.38 a | 0.18 a | --- |
| Aroma Active Compounds | Sensory Attributes | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pungent | Feinty/Earthy | Cereal/Grainy | Malty/Biscuity | Green/Grassy | Floral | Fresh Fruit | Dried Fruit | Soapy | Solventy | Sweet | Oily Finish | Sour | Sulphury | Stale/Mouldy | ||
| 1 | 3-Methylbutanal (Isovaleraldehyde) (butter/cheese/chocolate) | - | - | - | - | 0.726 * | - | - | - | - | - | - | - | - | - | - |
| 2 | Propyl acetate (pear/fruity) | - | - | - | - | - | −0.759 * | - | - | - | - | - | - | - | - | - |
| 3 | 1,1-Diethoxyethane (apple/fruity) | - | - | - | - | - | - | - | - | - | 0.734 * | - | - | - | - | −0.775 * |
| 4 | Ethyl isobutyrate (fruity/sweet/cherry) | - | −0.798 * | - | - | - | - | - | - | −0.819 * | - | - | −0.795 * | -0.750 * | - | - |
| 5 | 3-Methyl-1-butanol (Isoamyl alcohol) (fermented/yeast) | - | - | - | - | - | - | −0.719 * | - | - | - | - | - | - | - | - |
| 6 | Unknown 1 (soil) | - | - | - | - | - | - | - | - | −0.708 * | - | - | - | - | - | - |
| 7 | Ethyl butyrate (fruity/apple) | - | - | - | - | - | - | - | - | - | 0.8138 * | −0.722 * | −0.753* | −0.761 * | - | - |
| 8 | Unknown 2 (coffee) | −0.714* | - | - | - | - | - | - | - | - | - | - | - | -0.708 * | - | - |
| 9 | Ethyl 3-methylbutyrate (Ethyl Isovalerate) (pineapple/apple) | - | - | 0.823 * | - | - | - | - | - | −0.891 ** | - | - | - | - | - | - |
| 10 | Furfural (baked/toasted almond) | - | - | - | 0.779 * | - | - | - | - | - | - | - | - | 0.726 * | 0.724 * | - |
| 11 | 2-Butylfuran (stable) | - | 0.881 ** | - | - | - | - | - | - | 0.782 * | −0.823 * | 0.770 * | 0.870 ** | 0.796 * | - | 0.077 * |
| 12 | Methional (boiled potato) | - | - | 0.847 ** | - | −0.730 * | - | −0.722 * | - | −0.767 * | - | - | - | - | - | - |
| 13 | Unknown 3 (fruity/butter) | - | - | 0.721 * | - | - | - | - | - | - | - | - | - | - | - | - |
| 14 | Unknown 4 (floral/fruity) | - | - | 0.724 * | - | - | - | - | - | −0.868 ** | - | - | −0.733 * | −0.730 * | - | - |
| 15 | 2-Pentylfuran (gas/bad smell) | - | - | 0.801 * | - | - | - | −0.837 ** | - | - | - | - | - | - | - | - |
| 16 | 1-Octen-3-one (metallic/mineral/mushroom) | - | 0.886 ** | - | 0.743 * | - | - | - | - | - | −0.865 ** | 0.8139 * | 0.884** | 0.915 *** | 0.888 ** | - |
| 17 | Unknown 5 (soil/herbal) | −0.715 * | -0.930 ** | - | −0.782 * | - | - | - | - | −0.741 * | 0.781 * | −0.746 * | −0.966 *** | −0.95 *** | −0.966 *** | - |
| 18 | Unknown 7 (coffee) | - | - | - | - | −0.716 * | - | - | - | - | - | - | - | - | - | - |
| 19 | Unknown 8 (lemon/cleaning fluid) | - | 0.798 * | - | - | - | - | - | - | 0.815 * | - | - | 0.808 * | 0.784 ** | - | - |
| 20 | (E)-2-Nonenal (fried/toasted/fatty) | - | - | - | - | - | - | - | - | - | - | 0.737 * | - | - | - | - |
| 21 | Unknown 9 (grass) | - | - | - | - | - | −0.736 * | −0.761 * | −0.810 * | - | - | - | - | - | - | - |
| 22 | β-Damascenone honey/tea/plum | - | −0.735 * | - | −0.857 ** | - | - | - | 0.866 ** | - | 0.727 * | - | - | −0.740 * | −0.709 * | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyraleou, M.; Herb, D.; O’Reilly, G.; Conway, N.; Bryan, T.; Kilcawley, K.N. The Impact of Terroir on the Flavour of Single Malt Whisk(e)y New Make Spirit. Foods 2021, 10, 443. https://doi.org/10.3390/foods10020443
Kyraleou M, Herb D, O’Reilly G, Conway N, Bryan T, Kilcawley KN. The Impact of Terroir on the Flavour of Single Malt Whisk(e)y New Make Spirit. Foods. 2021; 10(2):443. https://doi.org/10.3390/foods10020443
Chicago/Turabian StyleKyraleou, Maria, Dustin Herb, Grace O’Reilly, Neil Conway, Tom Bryan, and Kieran N. Kilcawley. 2021. "The Impact of Terroir on the Flavour of Single Malt Whisk(e)y New Make Spirit" Foods 10, no. 2: 443. https://doi.org/10.3390/foods10020443
APA StyleKyraleou, M., Herb, D., O’Reilly, G., Conway, N., Bryan, T., & Kilcawley, K. N. (2021). The Impact of Terroir on the Flavour of Single Malt Whisk(e)y New Make Spirit. Foods, 10(2), 443. https://doi.org/10.3390/foods10020443