Exploring the DPP-IV Inhibitory, Antioxidant and Antibacterial Potential of Ovine “Scotta” Hydrolysates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
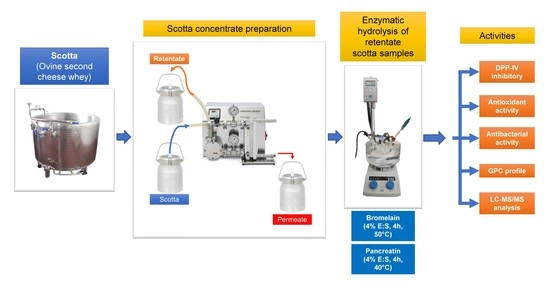
2.2. Scotta Concentrate Preparation
2.3. Enzymatic Hydrolysis of Retentate Scotta Samples
2.4. DPP-IV Inhibitory Activity
2.5. ABTS Radical Scavenging Activity
2.6. Antibacterial Assays
2.7. Gel Permeation Chromatography
2.8. LC-MS/MS Analysis
3. Results and Discussion
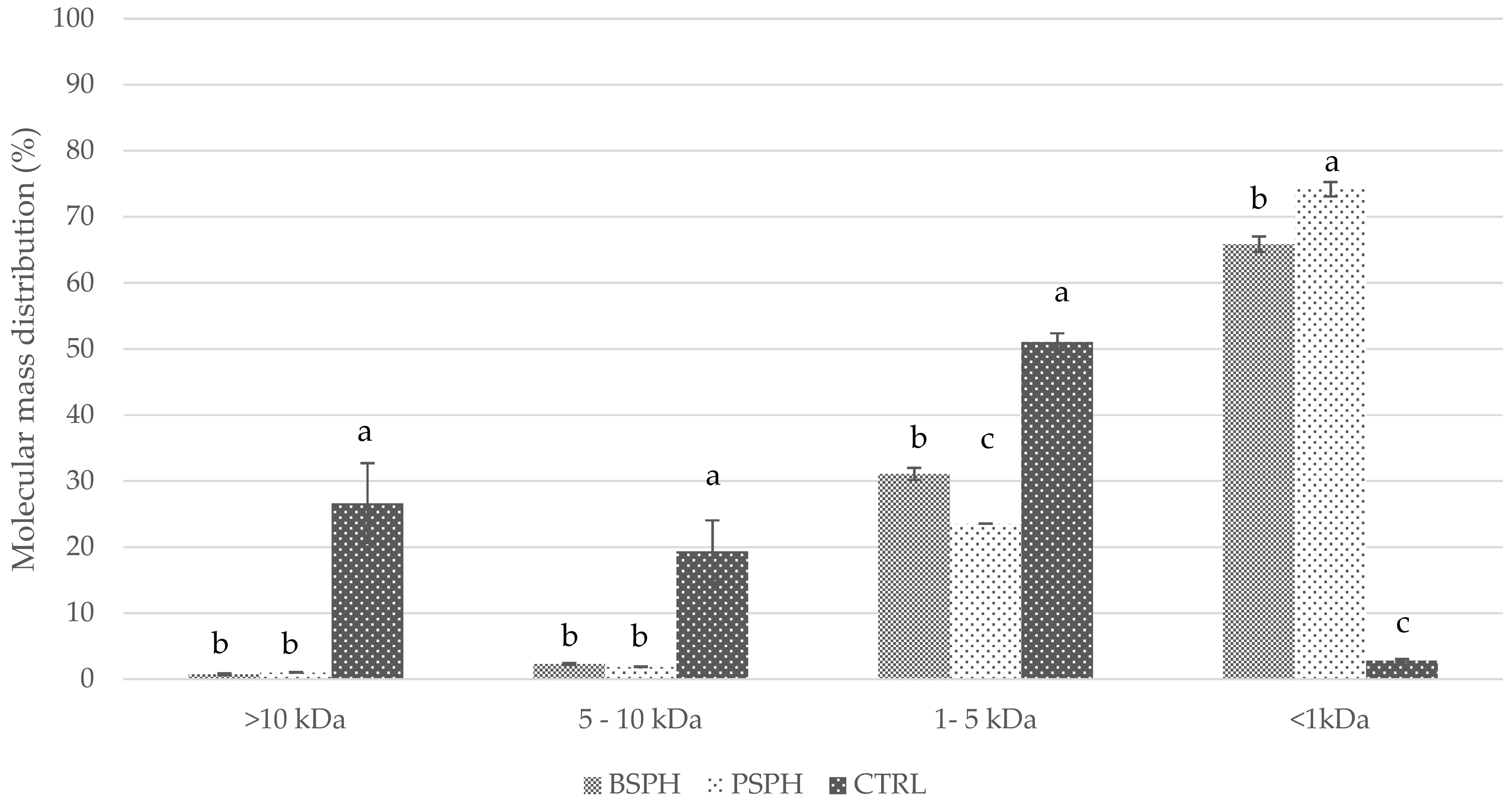
3.1. DPP-IV Inhibitory, Antioxidant Activity and GPC Profile of the Selected Hydrolysates
3.2. Antibacterial Activities of Hydrolysates
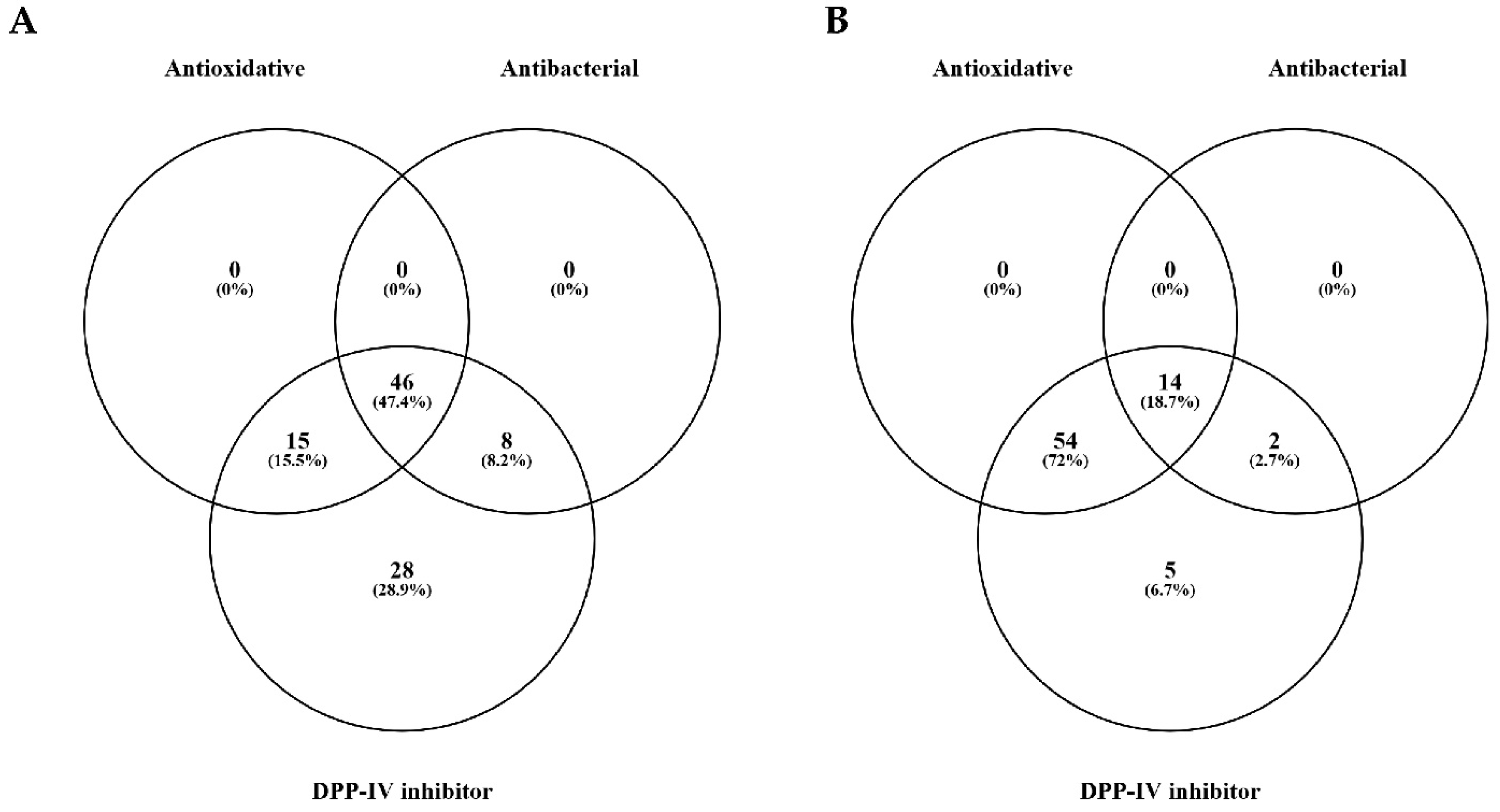
3.3. LC-MS/MS Analysis of Scotta Hydrolysates
| ID Protein | Identified Peptide | Log2 BSPH vs. CTRL | Activity | Reference |
|---|---|---|---|---|
| β-casein | GPIPNSLPQNILPLT79–94 GPIPNSLPQNILPLTQ79–95 | 3.82 3.78 | Antioxidative; DPP-IV inhibitory; | [55,56] |
| β-casein | YQEPVLGPVR206–215 | 2.1 | Antioxidative; DPP-IV inhibitory; | [57,58,59,60,61,62,63,64,65] |
| β-lactoglobulin | VLVLDTDYK110–118 VLVLDTDYKK110–119 VLVLDTDYKKY110–120 VLVLDTDYKKYL110–121 VLVLDTDYKKYLL110–122 KVLVLDTDYKKY109–120 ENKVLVLDTDYKK107–119 ENKVLVLDTDYKKY107–120 ENKVLVLDTDYKKYL107–121 DALENKVLVLDTDYKK104–119 DALENKVLVLDTDYKKY104–120 IDALENKVLVLDTDYKK103–119 IDALENKVLVLDTDYKKY103–120 | 2.21 3.05 3.42 2.72 2.17 1.67 2.48 3.45 2.18 1.90 2.43 2.33 3.05 | Antioxidative; Antibacterial; DPP-IV inhibitory; | [66,67,68,69,70,71,72,73] |
| β-lactoglobulin | IPAVFKIDALNENK96–109 TKIPAVFKIDALNENK94–109 | 2.43 1.83 | Antioxidative; Antibacterial; DPP-IV inhibitory; | [26,67,68,71,74,75,76] |
| k-casein | DQDKTEIPAINTIASAEPTVHS134–155 AIPPKKDQDKTEIPAINT128–145 EIPAINTIASAEPTVHS139–155 IPPKKDQDKTEIPAINTIA129–147 IPAINTIASAEPTVHS140–155 IPPKKDQDKTEIPAIN129–144 IPPKKDQDKTEIPAINT129–145 PPKKDQDKTEIPAINTIAS130–148 AIPPKKDQDKTEIPAINTIA128–147 AIPPKKDQDKTEIPAIN128–144 KDQDKTEIPAINT132–145 KDQDKTEIPAINTIA132–147 EIPAINTIASAEPTVH139–154 DQDKTEIPAINTIAS134–148 IPAINTIASAEPTVH140–154 DQDKTEIPAINTIASAEPTVH144–154 TEIPAINTIASAEPTVHS138–155 PPKKDQDKTEIPAInTIASAEP130–151 AIPPKKDQDKTEIPAINTIASAEPTVHS128–155 DQDKTEIPAINTI134–146 KDQDKTEIPAINTIASAEPTVHS133–155 PPKKDQDKTEIPAINTIA130–147 KDQDKTEIPAIN133–144 DQDKTEIPAINTIA134–147 | 5.40 5.39 5.28 4.69 4.62 4.49 4.46 3.96 3.90 3.84 3.59 3.46 3.37 3.29 3.22 3.14 2.99 2.87 2.78 2.59 2.33 2.31 1.84 1.50 | DPP-IV inhibitory; | [26,41,75,77,78] |
| β-lactoglobulin | VYVEELKPTPEG59–70 ELKPTPEGNLEILLQ63–77 EELKPTPEGNL62–72 VYVEELKPTPEGNL59–72 VYVEELKPTPEGNLE59–73 EELKPTPEGNLEILL62–76 | 2.10 2.03 1.99 1.74 1.69 1.67 | Antioxidative; DPP-IV inhibitory; | [31] |
| β-casein | EMPFPKYPVEPFT123–135 MPFPKYPVEPFTE124–136 EMPFPKYPVEPFTE123–136 MPFPKYPVEPFT124–135 MPFPKYPVEPFTES124–137 EMPFPKYPVEPFTES123–137 MPFPKYPVEPF124–134 EMPFPKYPVEPF123–134 | 4.23 4.01 3.96 3.90 3.39 3.15 1.93 1.90 | Antibacterial; DPP-IV inhibitory; | [27,65,79,80,81] |
| β-casein | YQEPVLGPVR208–212 | 2.10 | DPP-IV inhibitory; Antioxidative; | [58,60,62,64] |
| β-lactoglobulin | VYVEELKPTPEG59–70 VYVEELKPTPEGNL59–72 VYVEELKPTPEGNLE59–73 | 2.10 1.74 1.69 | DPP-IV inhibitory; Antioxidative; Antibacterial | [24,82,83] |
| β-lactoglobulin | ENKVLVLDTDYKKY107–120 VLVLDTDYKKY111–120 LDTDYKKYL113–121 VLVLDTDYKK112–120 IDALNENKVLVLDTDYKKY102–120 LVLDTDYKKY111–120 VLVLDTDYKKYL112–121 VLDTDYKKY112–120 LVLDTDYKKYL111–121 LDTDYKKYLL113–122 ENKVLVLDTDYKK107–119 DALNENKVLVLDTDYKKY103–120 LDTDYKKY113–120 IDALNENKVLVLDTDYKK102–119 LVLDTDYKK111–119 ENKVLVLDTDYKKYL107–121 VLVLDTDYKKYLL112–122 DALNENKVLVLDTDYKK103–119 KVLVLDTDYKKY109–120 | 3.45 3.42 3.18 3.05 3.05 2.94 2.72 2.62 2.52 2.48 2.48 2.43 2.38 2.33 2.23 2.18 2.17 1.90 1.67 | Antioxidative; Antibacterial; DPP-IV inhibitory; | [44,84] |
| β-lactoglobulin | IPAVFKIDALNENK96–109 IDALNENKVLVLDTDYKKY102–120 IDALNENKVL102–111 TKIPAVFKIDALNENK94–109 IDALNENKVLVLDTDYKK102–119 KIPAVFKIDALNENK95–109 KIDALNENK101–109 VFKIDALNENK99–109 IDALNENKV102–110 | 3.09 3.05 2.60 2.46 2.33 2.30 2.21 1.99 1.96 | DPP-IV inhibitory; Antioxidative; Antibacterial; | [44,85] |
| β-lactoglobulin | LDIQKVAGTWHS27–39 | 1.92 | DPP-IV inhibitory; Antioxidative; | [86] |
| β-lactoglobulin | AASDISLLDAQSAPLR43–59 LAMAASDISLLDAQSAPLR39–59 MAASDISLLDAQSAPLR42–59 AASDISLLDAQSAPLRV43–59 | 2.57 2.37 1.88 1.53 | DPP-IV inhibitory; | [68,71] |
| β-lactoglobulin | TPEVDNEALEKFDKALK143–159 TPEVDNEALEKFDKALKA142–160 DNEALEKFDKALK147–159 EVDNEALEKFDKALK145–159 | 4.03 2.92 2.38 2.01 | DPP-IV inhibitory; Antioxidative; | [76] |
| ID Protein | Identified Peptide | Log2 PSPH vs. CTRL | Activity | Reference |
|---|---|---|---|---|
| β-casein | TGPIPNSLPQNILPL78–92 | 2.53 | DPP-IV inhibitory; Antioxidative; | [55,56] |
| β-casein | QEPVLGPVRGPFPI207–220 QEPVLGPVRGPFP207–219 YQEPVLGPVRGPFPI206–220 LYQEPVLGPVRGPFPI205–220 EPVLGPVRGPFPI208–220 | 4.06 3.44 2.45 1.91 1.85 | DPP-IV inhibitory; | [57,58,59,60,61,62,63,64,65] |
| β-lactoglobulin | KIDALNENKVLVLDTDYK101–118 KIDALNENKVLVLDTDYKK101–119 VLVLDTDYKKY112–120 IDALNENKVLVLDTDYKK102–119 VLVLDTDYKKYL112–121 KIDALNENKVLVLDTDYKKY101–120 | 2.83 2.50 2.20 2.04 1.93 1.57 | DPP-IV inhibitory; Antioxidative; Antibacterial; | [66,67,68,69,70,71,72,73] |
| β-lactoglobulin | IPAVFKIDALNENK96–109 | 1.76 | DPP-IV inhibitory; Antioxidative; Antibacterial; | [26,67,68,71,74,75,76] |
| k-casein | KDQDKTEIPAINTIASAEPT133–152 KDQDKTEIPAINT133–144 TEIPAINTIASAEPTVH138–154 KDQDKTEIPAINTIA133–146 KDQDKTEIPAINTIASAEPTVH133–154 DQDKTEIPAINTIASAEPT134–152 DQDKTEIPAINTIASAEPTVH134–154 KDQDKTEIPAIN133–143 KDQDKTEIPAINTIAS133–147 KDQDKTEIPAI133–142 EIPAINTIASAEPTVH139–154 KDQDKTEIPAINTI133–145 DQDKTEIPAINTIAS134–147 DQDKTEIPAINTIA134–146 DQDKTEIPAINTI134–145 MAIPPKKDQDKTEIPA127–142 AIPPKKDQDKTEIPAIN128–144 AIPPKKDQDKTEIPAINTIA128–147 PPKKDQDKTEIPAIN130–144 MAIPPKKDQDKTEIPAINTIA127–147 AIPPKKDQDKTEIPA128–142 | 5.99 5.85 5.17 5.13 5.07 5.05 5.04 4.39 3.96 3.92 3.91 3.90 3.65 3.42 3.32 2.95 2.70 2.64 2.32 2.09 1.68 | DPP-IV inhibitory; Antioxidative; | [26,41,75,77,78] |
| β-lactoglobulin | VEELKPTPEGNLE61–73 VEELKPTPEGNLEI61–74 VEELKPTPEGNLEILLQK61–78 VEELKPTPEGNLEIL61–76 YVEELKPTPEGNLE60–73 VEELKPTPEGDLE VYVEELKPTPEGN59–71 VYVEELKPTPEGNLE59–73 YVEELKPTPEGN60–70 YVEELKPTPEGNLEI59–74 YVEELKPTPEGNLEILLQK59–78 YVEELKPTPEGNLEIL59–75 VYVEELKPTPEGNLEILLQK58–78 VEELKPTPEGNL60–72 RVYVEELKPTPEGNLEILLQK58–78 VYVEELKPTPEGNL58–72 | 3.51 3.35 2.97 2.96 2.89 2.64 2.64 2.63 2.61 2.45 2.28 2.27 2.02 1.93 1.87 1.76 | DPP-IV inhibitory; Antioxidative; | [31] |
| β-casein | EMPFPKYPVEPF129–134 | 1.93 | DPP-IV inhibitory; Antibacterial; | [27,65,79,80,81] |
| β-casein | YQEPVLGPVRGPFPI208–217 LYQEPVLGPVRGPFPI206–215 | 2.45 1.91 | DPP-IV inhibitory; Antioxidative; | [58,60,62,64] |
| β-lactoglobulin | VYVEELKPTPEGN59–71 VYVEELKPTPEGNLE59–73 VYVEELKPTPEGNLEILLQK59–78 RVYVEELKPTPEGNLEILLQK58–78 VYVEELKPTPEGNL59–72 | 2.64 2.63 2.02 1.87 1.76 | DPP-IV inhibitory; Antioxidative; | [24,82,83] |
| β-lactoglobulin | KIDALNENKVLVLDTDYKK101–119 VLVLDTDYKKY112–120 VLDTDYKKYL112–121 IDALNENKVLVLDTDYKK102–119 VLVLDTDYKKYL112–121 KIDALNENKVLVLDTDYKKY101–120 | 2.50 2.20 2.12 2.04 1.93 1.57 | DPP-IV inhibitory; Antibacterial; Antioxidative; | [44,84] |
| β-lactoglobulin | KIDALNENKV101–110 KIDALNENK101–109 KIDALNENKVLVLDTDYK101–118 KIDALNENKVLVLDTDYKK101–119 IDALNENKVLVLDTDYKK102–119 IPAVFKIDALNENK96–109 KIDALNENKVLVLDTDYKKY100–120 | 3.39 2.84 2.83 2.50 2.04 1.76 1.57 | DPP-IV inhibitory; Antioxidative; | [44,85] |
| β-lactoglobulin | GLDIQKVAGTWH27–38 | 1.73 | DPP-IV inhibitory; Antioxidative; | [86] |
| β-lactoglobulin | SLAMAASDISLLDAQSAPLRV39–59 SLAMAASDISLLDAQSAPLR39–58 | 2.56 2.21 | DPP-IV inhibitory; Antibacterial; | [68,71] |
| β-lactoglobulin | ALKALPMHI157–165 | 2.03 | DPP-IV inhibitory; Antioxidative; | [76] |
| ID Protein | Identified Peptide | Log2 BSPH vs. PSPH | Activity |
|---|---|---|---|
| β-casein | GPIPNSLPQNILPLT79–93 GPIPNSLPQNILPLTQ79–94 LVYPFTGPIPNSLPQNILPLTQTPVVVPPFLQPEIMGVPK73–112 SLPQNILPLTQTPVVVPPFLQPEIMGVPKVKET72–116 TGPIPNSLPQNILPLTQTPVVVPPFLQPEIMGVPKVKETMVPKH78–121 SLPQNILPLTQTPVVVPPFLQPEIMGVPKVKETMVPKH72–121 SLPQNILPLTQTPVVVPPFLQPEIMGVPKVK72–114 SLPQNILPLTQTPVVVPPFLQPEIMGVPK72–120 FTGPIPNSLPQNILPLTQTPVVVPPFLQPEIMGVPKVKETMVPKH77–121 FTGPIPNSLPQNILPLTQTPVVVPPFLQPEIMGVPKVKETMVPK77–120 SLPQNILPLTQTPVVVPPFLQPEIMGVPKVKETMVPK72–120 TGPIPNSLPQNILPLTQTPVVVPPFLQPEIMGVPKVKETMVPK78–120 | 3.82 3.78 −1.54 −1.62 −1.69 −1.91 −1.92 −1.98 −2.44 −3.44 −3.47 −3.53 | DPP-IV inhibitory; Antioxidative; |
| β-casein | YQEPVLGPVR206–215 YQEPVLGPVRGPFP206–219 VLPVPQKAVPQRDMPIQAFLLYQEPVLGPVRGPFP185–219 LSLSQPKVLPVPQKAVPQRDMPIQAFLLYQEPVLGPV178–214 AVPQRDMPIQAFLLYQEPVLGPVRGPFPI192–220 SLSQPKVLPVPQKAVPQRDMPIQAFLLYQEPVLGPVRGPFPILV179–222 AVPQRDMPIQAFLLYQEPVLGPVRGPFP192–219 EPVLGPVRGPFPIIV208–222 EPVLGPVRGPFPILV208–222 EPVLGPVRGPFPI208–220 FLLYQEPVLGPVRGPFP203–219 VLPVPQKAVPQRDMPIQAFLLYQEPVLGPVRGPFPILV185–222 VLPVPQKAVPQRDMPIQAFLLYQEPVLGPVRGPFPI185–220 YQEPVLGPVRGPFPIIV206–222 YQEPVLGPVRGPFPILV206–222 VLPVPQKAVPQRDMPIQAFLLYQEPVLGPVRGPFPIL185–221 EPVLGPVRGPFPII208–221 EPVLGPVRGPFPIL208–221 EPVLGPVRGPFP208–219 | 2.10 −1.61 −1.64 −1.71 −1.75 −1.77 −1.80 −1.89 −1.89 −2.01 −2.10 −2.14 −2.24 −2.31 −2.31 −2.33 −2.59 −2.59 −2.98 | DPP-IV inhibitory; |
| β-lactoglobulin | ENKVLVLDTDYKKY107–118 VLVLDTDYKKY110–120 VLVLDTDYKK112–119 IDALNENKVLVLDTDYKKY102–120 VLVLDTDYKKYL112–120 ENKVLVLDTDYKK107–119 DALNENKVLVLDTDYKKY103–120 IDALNENKVLVLDTDYKK102–119 VLVLDTDYK112–118 ENKVLVLDTDYKKYL107–121 VLVLDTDYKKYLL112–120 DALNENKVLVLDTDYKK103–119 KVLVLDTDYKKY109–120 | 3.45 3.42 3.05 3.05 2.72 2.48 2.43 2.33 2.21 2.18 2.17 1.90 1.67 | DPP-IV inhibitory; Antioxidative; Antibacterial; |
| β-lactoglobulin | IPAVFKIDALNENK106–109 TKIPAVFKIDALNENK104–109 KIPAVFKIDALNENK105–109 | 3.09 2.46 2.30 | DPP-IV inhibitory; Antioxidative; Antibacterial; |
| k-casein | DQDKTEIPAINTIASAEPTVHS134–155 AIPPKKDQDKTEIPAINT128–145 EIPAINTIASAEPTVHS139–155 IPPKKDQDKTEIPAINTIA129–147 IPAINTIASAEPTVHS140–155 IPPKKDQDKTEIPAIN129–144 IPPKKDQDKTEIPAINT129–145 PPKKDQDKTEIPAINTIAS130–148 AIPPKKDQDKTEIPAINTIA128–147 AIPPKKDQDKTEIPAIN128–144 KDQDKTEIPAINT133–145 KDQDKTEIPAINTIA133–147 EIPAINTIASAEPTVH139–154 DQDKTEIPAINTIAS134–148 IPAINTIASAEPTVH140–154 DQDKTEIPAINTIASAEPTVH134–154 TEIPAINTIASAEPTVHS138–155 PPKKDQDKTEIPAINTIASAEP130–151 AIPPKKDQDKTEIPAINTIASAEPTVHS128–155 DQDKTEIPAINTI134–146 KDQDKTEIPAINTIASAEPTVHS133–155 PPKKDQDKTEIPAINTIA130–147 KDQDKTEIPAIN133–144 DQDKTEIPAINTIA134–147 FMAIPPKKDQDKTEIPAINTIASAEPTVH126–154 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTEAVVNAVDNP127–169 KTEIPAINTIASAEPTVH137–154 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTEAVV127–163 IPPKKDQDKTEIPAINTIASAEPTVH129–154 MAIPPKKDQDKTEIPAINTIASAEPTVHSTP127–157 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTEAVVNAV127–166 MAIPPKKDQDKTEIPAINTIASAEPTV127–153 MAIPPKKDQDKTEIPAINTIASAEP127–151 MAIPPKKDQDKTEIPAINT127–144 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTEAVVNA127–165 AIPPKKDQDKTEIPAINTIASAEPTVH128–154 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTEAVVNAVDNPE127–170 PPKKDQDKTEIPAINTIASAEPTVHSTPTTEAVVNAVDNPEA129–169 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTEAVVNAVDNPEA127–169 PPKKDQDKTEIPAINTIASAEPTV129–153 MAIPPKKDQDKTEIPAINTIASAEPTVHST127–156 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTEA127–161 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTT127–159 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTEAVVN127–164 MAIPPKKDQDKTEIPAINTIASAEPT127–152 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTEAV127–162 MAIPPKKDQDKTEIPAIN127–144 MAIPPKKDQDKTEIPAINTIAS127–148 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTEAVVNAVDN127–168 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTEAVVNAVDNPEASS127–173 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTEAVVNAVDNPEAS127–172 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTE127–160 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPTTEAVVNAVD127–167 MAIPPKKDQDKTEIPAINTIASA127–149 MAIPPKKDQDKTEIPAINTIASAEPTVHSTPT127–158 MAIPPKKDQDKTEIPAINTIASAE127–150 MAIPPKKDQDKTEIPAINTIASAEPTVHS127–155 MAIPPKKDQDKTEIPAINTIASAEPTVH127–154 | 5.40 5.39 5.28 4.69 4.62 4.49 4.46 3.96 3.90 3.84 3.59 3.46 3.37 3.29 3.22 3.14 2.99 2.87 2.78 2.59 2.33 2.31 1.84 1.50 −1.53 −1.71 −1.94 −2.01 −2.18 −2.36 −2.37 −2.39 −2.42 −2.66 −2.77 −2.98 −3.04 −3.16 −3.19 −3.42 −3.52 −3.69 −3.69 −3.73 −3.90 −3.95 −3.96 −4.01 −4.04 −4.11 −4.28 −4.40 −4.64 −4.80 −4.81 −4.82 −5.60 −6.28 | DPP-IV inhibitory; Antioxidative; |
| β-lactoglobulin | VYVEELKPTPEG59–70 ELKPTPEGNLEILLQ63–77 EELKPTPEGNL62–72 VYVEELKPTPEGNL59–72 VYVEELKPTPEGNLE59–73 EELKPTPEGNLEILL62–76 | 2.10 2.03 1.99 1.74 1.69 1.67 | DPP-IV inhibitory; Antioxidative; |
| β-casein | EMPFPKYPVEPFT122–135 MPFPKYPVEPFTE123–136 EMPFPKYPVEPFTE122–136 MPFPKYPVEPFT123–135 MPFPKYPVEPFTES123–137 EMPFPKYPVEPFTES122–137 MPFPKYPVEPF123–134 EMPFPKYPVEPF122–134 VKETMVPKHKEMPFPKYPVEPFTESQSLTLTDVE113–156 HKEMPFPKYPVEPFTESQ121–138 HKEMPFPKYPVEPFTESQSLTLTDVEKLH121–149 HKEMPFPKYPVEPFTESQSLT121–141 HKEMPFPKYPVEPFTESQSLTLTDVE121–146 HKEMPFPKYPVEPFTESQSLTLTDVEKLHLPLPLVQ121–156 HKEMPFPKYPVEPFTESQS121–138 HKEMPFPKYPVEPFTESQSL121–139 VKETMVPKHKEMPFPKYPVEPFTESQSL113–140 HKEMPFPKYPVEPFTESQSLTLTDVEK121–147 VKETMVPKHKEMPFPKYPVEPFTESQS113–139 EMPFPKYPVEPFTESQSLTLTDVEKLHLPLP122–153 HKEMPFPKYPVEPFTESQSLTLTDVEKLHLPLP121–153 | 4.23 4.01 3.96 3.90 3.39 3.15 1.93 1.90 −1.50 −1.57 −1.59 −1.64 −1.73 −1.74 −1.75 −1.90 −2.55 −2.66 −2.67 −2.75 −3.66 | DPP-IV inhibitory; Antibacterial; |
| β-casein | YQEPVLGPVR206–215 YQEPVLGPVRGPFP206–219 VLPVPQKAVPQRDMPIQAFLLYQEPVLGPVRGPFP185–219 AVPQRDMPIQAFLLYQEPVLGPVRGPFPI192–220 SLSQPKVLPVPQKAVPQRDMPIQAFLLYQEPVLGPVRGPFPILV178–222 AVPQRDMPIQAFLLYQEPVLGPVRGPFP192–219 FLLYQEPVLGPVRGPFP203–219 VLPVPQKAVPQRDMPIQAFLLYQEPVLGPVRGPFPILV185–222 VLPVPQKAVPQRDMPIQAFLLYQEPVLGPVRGPFPI185–220 YQEPVLGPVRGPFPIIV206–222 YQEPVLGPVRGPFPILV206–222 VLPVPQKAVPQRDMPIQAFLLYQEPVLGPVRGPFPIL185–220 | 2.10 −1.61 −1.64 −1.75 −1.77 −1.80 −2.10 −2.14 −2.24 −2.31 −2.31 −2.33 | DPP-IV inhibitory; Antioxidative; |
| β-lactoglobulin | VYVEELKPTPEG59–70 VYVEELKPTPEGNL59–72 VYVEELKPTPEGNLE59–73 | 2.10 1.74 1.69 | DPP-IV inhibitory; Antibacterial; Antioxidative; |
| β-lactoglobulin | ENKVLVLDTDYKKY107–120 VLVLDTDYKKY110–120 LDTDYKKYL113–121 VLVLDTDYKK110–119 IDALNENKVLVLDTDYKKY102–120 LVLDTDYKKY111–120 VLVLDTDYKKYL110–121 VLDTDYKKY112–120 LVLDTDYKKYL111–121 LDTDYKKYLL113–122 ENKVLVLDTDYKK107–119 DALNENKVLVLDTDYKKY103–120 LDTDYKKY113–120 IDALNENKVLVLDTDYKK102–119 LVLDTDYKK111–119 ENKVLVLDTDYKKYL107–121 VLVLDTDYKKYLL109–122 DALNENKVLVLDTDYKK103–119 KVLVLDTDYKKY109–120 | 3.45 3.42 3.18 3.05 3.05 2.94 2.72 2.62 2.52 2.48 2.48 2.43 2.38 2.33 2.23 2.18 2.17 1.90 1.67 | DPP-IV inhibitory; Antibacterial; Antioxidative; |
| β-lactoglobulin | IPAVFKIDALNENK106–109 IDALNENKVLVLDTDYKKY102–120 IDALNENKVL102–111 TKIPAVFKIDALNENK94–109 IDALNENKVLVLDTDYKK102–119 KIPAVFKIDALNENK95–109 KIDALNENK101–109 VFKIDALNENK99–109 IDALNENKV102–110 | 3.09 3.05 2.60 2.46 2.33 2.30 2.21 1.99 1.96 | DPP-IV inhibitory; Antioxidative; |
| β-lactoglobulin | IPAVFKIDALNENK106–109 IDALNENKVLVLDTDYKKY102–120 IDALNENKVL102–111 TKIPAVFKIDALNENK94–109 IDALNENKVLVLDTDYKK102–119 KIPAVFKIDALNENK95–109 KIDALNENK101–109 VFKIDALNENK99–109 IDALNENKV102–110 | 3.09 3.05 2.60 2.46 2.33 2.30 2.21 1.99 1.96 | DPP-IV inhibitory; Antioxidative; |
| β-lactoglobulin | IPAVFKIDALNENK106–109 IDALNENKVLVLDTDYKKY102–120 IDALNENKVL102–111 TKIPAVFKIDALNENK94–109 IDALNENKVLVLDTDYKK102–119 KIPAVFKIDALNENK95–109 KIDALNENK101–109 VFKIDALNENK99–109 IDALNENKV102–110 | 3.09 3.05 2.60 2.46 2.33 2.30 2.21 1.99 1.96 | DPP-IV inhibitory; Antioxidative; |
| β-lactoglobulin | LDIQKVAGTWHS28–39 IIVTQTMKGLDIQKVAGTWH19–38 | 1.92 −2.06 | DPP-IV inhibitory; Antioxidative; |
| β-lactoglobulin | AASDISLLDAQSAPLR43–58 LAMAASDISLLDAQSAPLR40–58 MAASDISLLDAQSAPLR42–58 AASDISLLDAQSAPLRV43–59 | 2.57 2.37 1.88 1.53 | DPP-IV inhibitory; Antibacterial; |
| β-lactoglobulin | TPEVDNEALEKFDKALK143–159 TPEVDNEALEKFDKALKA143–160 DNEALEKFDKALK147–159 EVDNEALEKFDKALK145–159 NEALEKFDKALK148–159 EALEKFDKALKALPMH149–164 NEALEKFDKALKALPMH148–164 NEALEKFDKALKALPMHIR148–166 EALEKFDKALKALPMHIR149–166 | 4.03 2.92 2.38 2.01 −1.62 −1.77 −2.37 −2.65 −2.96 | DPP-IV inhibitory; Antioxidative; |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salvatore, E.; Pes, M.; Falchi, G.; Pagnozzi, D.; Furesi, S.; Fiori, M.; Roggio, T.; Addis, M.F.; Pirisi, A. Effect of whey concentration on protein recovery in fresh ovine ricotta cheese. J. Dairy Sci. 2014, 97, 4686–4694. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy by-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- ISTAT Latte e Prodotti Lattiero Caseari: Prodotti per Tipo di Unità Produttiva. Available online: http://dati.istat.it/Index.aspx?QueryId=25267 (accessed on 19 September 2021).
- Pulina, G.; Milán, M.J.; Lavín, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Invited review: Current production trends, farm structures, and economics of the dairy sheep and goat sectors. J. Dairy Sci. 2018, 101, 6715–6729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISTAT Latte e Prodotti Lattiero Caseari: Prodotti-Reg. Available online: http://dati.istat.it/Index.aspx?QueryId=25520 (accessed on 19 September 2021).
- Sansonetti, S.; Curcio, S.; Calabrò, V.; Iorio, G. Optimization of ricotta cheese whey (RCW) fermentation by response surface methodology. Bioresour. Technol. 2010, 101, 9156–9162. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445-446, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.A. Treatment of cheese processing wastewater by physicochemical and biological methods. Int. J. Microbiol. Res. 2013, 4, 321–332. [Google Scholar]
- Monti, L.; Donati, E.; Zambrini, A.V.; Contarini, G. Application of membrane technologies to bovine Ricotta cheese exhausted whey (scotta). Int. Dairy J. 2018, 85, 121–128. [Google Scholar] [CrossRef]
- Pintado, M.E.; Macedo, A.C.; Malcata, F.X. Review: Technology, Chemistry and Microbiology of Whey Cheeses. Food Sci. Technol. Int. 2001, 7, 105–116. [Google Scholar] [CrossRef]
- Sommella, E.; Pepe, G.; Ventre, G.; Pagano, F.; Conte, G.M.; Ostacolo, C.; Manfra, M.; Tenore, G.C.; Russo, M.; Novellino, E.; et al. Detailed peptide profiling of “Scotta”: From a dairy waste to a source of potential health-promoting compounds. Dairy Sci. Technol. 2016, 96, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Secchi, N.; Giunta, D.; Pretti, L.; García, M.R.; Roggio, T.; Mannazzu, I.; Catzeddu, P. Bioconversion of ovine scotta into lactic acid with pure and mixed cultures of lactic acid bacteria. J. Ind. Microbiol. Biotechnol. 2012, 39, 175–181. [Google Scholar] [CrossRef]
- Ribeiro, J.E.; Martini, M.; Altomonte, I.; Salari, F.; Nardoni, S.; Sorce, C.; Silva, F.L.D.; Andreucci, A. Production of Chlorella protothecoides biomass, chlorophyll and carotenoids using the dairy industry by-product scotta as a substrate. Biocatal. Agric. Biotechnol. 2017, 11, 207–213. [Google Scholar] [CrossRef]
- Vincenzi, A.; Maciel, M.J.; Burlani, É.L.; Oliveira, E.C.; Volpato, G.; Lehn, D.N.; de Souza, C.F.V. Ethanol Bio-Production from ricotta cheese whey by several strains of the yeast Kluyveromyces. Am. J. Food Technol. 2014, 9, 281–291. [Google Scholar] [CrossRef]
- Monari, S.; Ferri, M.; Russo, C.; Prandi, B.; Tedeschi, T.; Bellucci, P.; Zambrini, A.V.; Donati, E.; Tassoni, A. Enzymatic production of bioactive peptides from scotta, an exhausted by-product of ricotta cheese processing. PLoS ONE 2019, 14, e0226834. [Google Scholar] [CrossRef] [Green Version]
- Pontonio, E.; Montemurro, M.; De Gennaro, G.V.; Miceli, V.; Rizzello, C.G. Anthypertensive Peptides from Ultrafiltration and Fermentation of the Ricotta Cheese Exhausted Whey: Design and Characterization of a Functional Ricotta Cheese. Foods 2021, 10, 2573. [Google Scholar] [CrossRef]
- Raho, S.; Carofiglio, V.E.; Montemurro, M.; Miceli, V.; Centrone, D.; Stufano, P.; Schioppa, M.; Pontonio, E.; Rizzello, C.G. Production of the polyhydroxyalkanoate PHBV from ricotta cheese exhausted whey by haloferax mediterranei fermentation. Foods 2020, 9, 1459. [Google Scholar] [CrossRef]
- Maragkoudakis, P.; Vendramin, V.; Bovo, B.; Treu, L.; Corich, V.; Giacomini, A. Potential use of scotta, the by-product of the ricotta cheese manufacturing process, for the production of fermented drinks. J. Dairy Res. 2016, 83, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Vasmara, C.; Marchetti, R. Initial pH influences in-batch hydrogen production from scotta permeate. Int. J. Hydrogen Energy 2017, 42, 14400–14408. [Google Scholar] [CrossRef]
- Vasmara, C.; Pindo, M.; Micheletti, D.; Marchetti, R. Initial pH influences microbial communities composition in dark fermentation of scotta permeate. Int. J. Hydrogen Energy 2018, 43, 8707–8717. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Ramírez-Suarez, J.C.; Yada, R.Y. Plant proteases for bioactive peptides release: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2147–2163. [Google Scholar] [CrossRef] [PubMed]
- Dinika, I.; Verma, D.K.; Balia, R.; Utama, G.L.; Patel, A.R. Potential of cheese whey bioactive proteins and peptides in the development of antimicrobial edible film composite: A review of recent trends. Trends Food Sci. Technol. 2020, 103, 57–67. [Google Scholar] [CrossRef]
- Andler, S.M.; Goddard, J.M. Transforming food waste: How immobilized enzymes can valorize waste streams into revenue streams. NPJ Sci. Food 2018, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Bello-Pérez, E.; Márquez-Hernández, R.I.; Hernández-Castellano, L.E. Bioactive peptides from milk: Animal determinants and their implications in human health. J. Dairy Res. 2019, 86, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minj, S.; Anand, S. Whey Proteins and Its Derivatives: Bioactivity, Functionality, and Current Applications. Dairy 2020, 1, 16. [Google Scholar] [CrossRef]
- Tulipano, G.; Sibilia, V.; Caroli, A.M.; Cocchi, D. Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides 2011, 32, 835–838. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Martini, S.; Shamsia, S.; Helal, A.; Conte, A. Biological activities and peptidomic profile of in vitro-digested cow, camel, goat and sheep milk. Int. Dairy J. 2018, 81, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Dipeptidyl peptidase-IV inhibitory activity of dairy protein hydrolysates. Int. Dairy J. 2012, 25, 97–102. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Fitzgerald, R.J. Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides 2013, 39, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Ministero della Salute Diabete Mellito Tipo 2. Available online: https://www.salute.gov.it/portale/nutrizione/dettaglioContenutiNutrizione.jsp?lingua=italiano&id=5511&area=nutrizione&menu=croniche (accessed on 12 October 2021).
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited review: Whey proteins as antioxidants and promoters of cellular antioxidant pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef] [Green Version]
- El-Zahar, K.; Sitohy, M.; Dalgalarrondo, M.; Choiset, Y.; Métro, F.; Haertlé, T.; Chobert, J.M. Purification and physicochemical characterization of ovine β-lactoglobulin and α-lactalbumin. Food/Nahrung 2004, 48, 177–183. [Google Scholar] [CrossRef]
- López-Expósito, I.; Gómez-Ruiz, J.A.; Amigo, L.; Recio, I. Identification of antibacterial peptides from ovine αs2-casein. Int. Dairy J. 2006, 16, 1072–1080. [Google Scholar] [CrossRef]
- Atanasova, J.; Ivanova, I. Antibacterial peptides from goat and sheep milk proteins. Biotechnol. Biotechnol. Equip. 2010, 24, 1799–1803. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Food-derived dipeptidyl-peptidase IV inhibitors as a potential approach for glycemic regulation—Current knowledge and future research considerations. Trends Food Sci. Technol. 2016, 54, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lestari, P. Suyata Antibacterial activity of hydrolysate protein from Etawa goat milk hydrolysed by crude extract bromelain. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012111. [Google Scholar] [CrossRef]
- Cabizza, R.; Rubattu, N.; Salis, S.; Pes, M.; Comunian, R.; Paba, A.; Addis, M.; Testa, M.C.; Urgeghe, P.P. Transfer of oxytetracycline from ovine spiked milk to whey and cheese. Int. Dairy J. 2017, 70, 12–17. [Google Scholar] [CrossRef]
- Petretto, G.L.; Maldini, M.; Addis, R.; Chessa, M.; Foddai, M.; Rourke, J.P.; Pintore, G. Variability of chemical composition and antioxidant activity of essential oils between Myrtus communis var. Leucocarpa DC and var. Melanocarpa DC. Food Chem. 2016, 197, 124–131. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- ComBase DMFit for Excel. Available online: https://www.combase.cc/index.php/en/8-category-en-gb/21-tools (accessed on 19 June 2021).
- Le Maux, S.; Nongonierma, A.B.; FitzGerald, R.J. Peptide composition and dipeptidyl peptidase IV inhibitory properties of β-lactoglobulin hydrolysates having similar extents of hydrolysis while generated using different enzyme-to-substrate ratios. Food Res. Int. 2017, 99, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Old, W.M.; Meyer-Arendt, K.; Aveline-Wolf, L.; Pierce, K.G.; Mendoza, A.; Sevinsky, J.R.; Resing, K.A.; Ahn, N.G. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol. Cell. Proteom. 2005, 4, 1487–1502. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, S.; Cacciotto, C.; Pagnozzi, D.; Puggioni, G.M.G.; Uzzau, S.; Ciaramella, P.; Guccione, J.; Penati, M.; Pollera, C.; Moroni, P.; et al. Proteomic changes in the milk of water buffaloes (Bubalus bubalis) with subclinical mastitis due to intramammary infection by Staphylococcus aureus and by non-aureus staphylococci. Sci. Rep. 2019, 9, 15850. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Cheng, J.; Wu, H. Discovery of food-derived dipeptidyl peptidase IV inhibitory peptides: A review. Int. J. Mol. Sci. 2019, 20, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nongonierma, A.B.; FitzGerald, R.J. Dipeptidyl peptidase IV inhibitory properties of a whey protein hydrolysate: Influence of fractionation, stability to simulated gastrointestinal digestion and food-drug interaction. Int. Dairy J. 2013, 32, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Song, J.J.; Wang, Q.; Du, M.; Ji, X.M.; Mao, X.Y. Identification of dipeptidyl peptidase-IV inhibitory peptides from mare whey protein hydrolysates. J. Dairy Sci. 2017, 100, 6885–6894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konrad, B.; Anna, D.; Marek, S.; Marta, P.; Aleksandra, Z.; Józefa, C. The Evaluation of Dipeptidyl Peptidase (DPP)-IV, α-Glucosidase and Angiotensin Converting Enzyme (ACE) Inhibitory Activities of Whey Proteins Hydrolyzed with Serine Protease Isolated from Asian Pumpkin (Cucurbita ficifolia). Int. J. Pept. Res. Ther. 2014, 20, 483–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Inhibition of dipeptidyl peptidase (DPP)-IV and α-glucosidase activities by pepsin-treated whey proteins. J. Agric. Food Chem. 2013, 61, 7500–7506. [Google Scholar] [CrossRef]
- Power, O.; Nongonierma, A.B.; Jakeman, P.; FitzGerald, R.J. Food protein hydrolysates as a source of dipeptidyl peptidase IV inhibitory peptides for the management of type 2 diabetes. Proc. Nutr. Soc. 2014, 73, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, A.P.F.; Daroit, D.J.; Fontoura, R.; Meira, S.M.M.; Segalin, J.; Brandelli, A. Hydrolysates of sheep cheese whey as a source of bioactive peptides with antioxidant and angiotensin-converting enzyme inhibitory activities. Peptides 2014, 61, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; FitzGerald, R.J. Enzymes Exogenous to Milk in Dairy Technology: Proteinases. Encycl. Dairy Sci. Second Ed. 2011, 2, 289–296. [Google Scholar] [CrossRef]
- Biziulevičius, G.A.; Kislukhina, O.V.; Kazlauskaite, J.; Žukaite, V. Food-protein enzymatic hydrolysates possess both antimicrobial and immunostimulatory activities: A “cause and effect” theory of bifunctionality. FEMS Immunol. Med. Microbiol. 2006, 46, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Tulipano, G.; Faggi, L.; Nardone, A.; Cocchi, D.; Caroli, A.M. Characterisation of the potential of β-lactoglobulin and α-lactalbumin as sources of bioactive peptides affecting incretin function: In silico and in vitro comparative studies. Int. Dairy J. 2015, 48, 66–72. [Google Scholar] [CrossRef]
- Uenishi, H.; Kabuki, T.; Seto, Y.; Serizawa, A.; Nakajima, H. Isolation and identification of casein-derived dipeptidyl-peptidase 4 (DPP-4)-inhibitory peptide LPQNIPPL from gouda-type cheese and its effect on plasma glucose in rats. Int. Dairy J. 2012, 22, 24–30. [Google Scholar] [CrossRef]
- Otte, J.; Shalaby, S.M.; Zakora, M.; Pripp, A.H.; El-Shabrawy, S.A. Angiotensin-converting enzyme inhibitory activity of milk protein hydrolysates: Effect of substrate, enzyme and time of hydrolysis. Int. Dairy J. 2007, 17, 488–503. [Google Scholar] [CrossRef]
- Sandré, C.; Gleizes, A.; Forestier, F.; Gorges-Kergot, R.; Chilmonczyk, S.; Léonil, J.; Moreau, M.-C.; Labarre, C. A peptide derived from bovine β-casein modulates functional properties of bone marrow-derived macrophages from germfree and human flora-associated mice. J. Nutr. 2001, 131, 2936–2942. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.V.; Malcata, F.X. Caseins as source of bioactive peptides. Int. Dairy J. 2005, 15, 1–15. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Pimentel, T.C.; Ferrão, L.L.; Almada, C.N.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.M.; Nascimento, J.S.; Silva, M.C.; et al. Sheep Milk: Physicochemical Characteristics and Relevance for Functional Food Development. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. [Google Scholar] [CrossRef]
- Sowmya, K.; Bhat, M.I.; Bajaj, R.K.; Kapila, S.; Kapila, R. Buffalo Milk Casein Derived Decapeptide (YQEPVLGPVR) Having Bifunctional Anti-inflammatory and Antioxidative Features under Cellular Milieu. Int. J. Pept. Res. Ther. 2019, 25, 623–633. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Underwood, M.A.; Dallas, D.C. Release of functional peptides from mother’s milk and fortifier proteins in the premature infant stomach. PLoS ONE 2018, 13, e0208204. [Google Scholar] [CrossRef]
- Liu, H.; Tu, M.; Cheng, S.; Chen, H.; Wang, Z.; Du, M. An anticoagulant peptide from beta-casein: Identification, structure and molecular mechanism. Food Funct. 2019, 10, 886–892. [Google Scholar] [CrossRef]
- Hao, X.; Yang, W.; Zhu, Q.; Zhang, G.; Zhang, X.; Liu, L.; Li, X.; Hussain, M.; Ni, C.; Jiang, X. Proteolysis and ACE-inhibitory peptide profile of Cheddar cheese: Effect of digestion treatment and different probiotics. LWT 2021, 145, 111295. [Google Scholar] [CrossRef]
- Murray, N.M.; O’Riordan, D.; Jacquier, J.C.; O’Sullivan, M.; Holton, T.A.; Wynne, K.; Robinson, R.C.; Barile, D.; Nielsen, S.D.; Dallas, D.C. Peptidomic screening of bitter and nonbitter casein hydrolysate fractions for insulinogenic peptides. J. Dairy Sci. 2018, 101, 2826–2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nongonierma, A.B.; Fitzgerald, R.J. Structure activity relationship modelling of milk protein-derived peptides with dipeptidyl peptidase IV (DPP-IV) inhibitory activity. Peptides 2016, 79, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Zhang, X.; Yuan, Y.; Qu, Y.; Feng, Z. Separation of antioxidant peptides from pepsin hydrolysate of whey protein isolate by ATPS of EOPO Co-polymer (UCON)/Phosphate. Sci. Rep. 2017, 7, 13320. [Google Scholar] [CrossRef] [Green Version]
- Mann, B.; Kumari, A.; Kumar, R.; Sharma, R.; Prajapati, K.; Mahboob, S.; Athira, S. Antioxidant activity of whey protein hydrolysates in milk beverage system. J. Food Sci. Technol. 2015, 52, 3235–3241. [Google Scholar] [CrossRef]
- Pellegrini, A.; Dettling, C.; Thomas, U.; Hunziker, P. Isolation and characterization of four bactericidal domains in the bovine β-lactoglobulin. Biochim. Biophys. Acta Gen. Subj. 2001, 1526, 131–140. [Google Scholar] [CrossRef]
- Elbarbary, H.A.; Ejima, A.; Sato, K. Generation of antibacterial peptides from crude cheese whey using pepsin and rennet enzymes at various pH conditions. J. Sci. Food Agric. 2019, 99, 555–563. [Google Scholar] [CrossRef]
- Worsztynowicz, P.; Białas, W.; Grajek, W. Integrated approach for obtaining bioactive peptides from whey proteins hydrolysed using a new proteolytic lactic acid bacteria. Food Chem. 2020, 312, 126035. [Google Scholar] [CrossRef]
- Chatterjee, A.; Kanawjia, S.K.; Khetra, Y.; Saini, P. Discordance between in silico & in vitro analyses of ACE inhibitory & antioxidative peptides from mixed milk tryptic whey protein hydrolysate. J. Food Sci. Technol. 2015, 52, 5621–5630. [Google Scholar] [CrossRef]
- Conway, V.; Gauthier, S.F.; Pouliot, Y. Antioxidant activities of buttermilk proteins, whey proteins, and their enzymatic hydrolysates. J. Agric. Food Chem. 2013, 61, 364–372. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Strategies for the discovery and identification of food protein-derived biologically active peptides. Trends Food Sci. Technol. 2017, 69, 289–305. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Isolation and characterization of peptides with dipeptidyl peptidase-IV inhibitory activity from pepsin-treated bovine whey proteins. Peptides 2014, 54, 39–48. [Google Scholar] [CrossRef]
- Silveira, S.T.; Martínez-Maqueda, D.; Recio, I.; Hernández-Ledesma, B. Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem. 2013, 141, 1072–1077. [Google Scholar] [CrossRef]
- Power, O.; Fernández, A.; Norris, R.; Riera, F.A.; FitzGerald, R.J. Selective enrichment of bioactive properties during ultrafiltration of a tryptic digest of β-lactoglobulin. J. Funct. Foods 2014, 9, 38–47. [Google Scholar] [CrossRef]
- Bella, A.M.; Erickson, R.H.; Kim, Y.S. Rat intestinal brush border membrane dipeptidyl-aminopeptidase IV: Kinetic properties and substrate specificities of the purified enzyme. Arch. Biochem. Biophys. 1982, 218, 156–162. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, C.; Ji, H. Purification, identification and molecular mechanism of two dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from Antarctic krill (Euphausia superba) protein hydrolysate. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1064, 56–61. [Google Scholar] [CrossRef]
- Ouertani, A.; Chaabouni, I.; Mosbah, A.; Long, J.; Barakat, M.; Mansuelle, P.; Mghirbi, O.; Najjari, A.; Ouzari, H.I.; Masmoudi, A.S.; et al. Two new secreted proteases generate a casein-derived antimicrobial peptide in Bacillus cereus food born isolate leading to bacterial competition in milk. Front. Microbiol. 2018, 9, 1148. [Google Scholar] [CrossRef]
- Bounouala, F.Z.; Roudj, S.; Karam, N.E.; Recio, I.; Miralles, B. Casein Hydrolysates by Lactobacillus brevis and Lactococcus lactis Proteases: Peptide Profile Discriminates Strain-Dependent Enzyme Specificity. J. Agric. Food Chem. 2017, 65, 9324–9332. [Google Scholar] [CrossRef] [Green Version]
- FitzGerald, R.J.; Cermeño, M.; Khalesi, M.; Kleekayai, T.; Amigo-Benavent, M. Application of in silico approaches for the generation of milk protein-derived bioactive peptides. J. Funct. Foods 2020, 64, 103636. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Dávalos, A.; Bartolomé, B.; Amigo, L. Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulln. Identification of active peptides by HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 588–593. [Google Scholar] [CrossRef]
- Almaas, H.; Eriksen, E.; Sekse, C.; Comi, I.; Flengsrud, R.; Holm, H.; Jensen, E.; Jacobsen, M.; Langsrud, T.; Vegarud, G.E. Antibacterial peptides derived from caprine whey proteins, by digestion with human gastrointestinal juice. Br. J. Nutr. 2011, 106, 896–905. [Google Scholar] [CrossRef] [Green Version]
- Contreras, M. del M.; Hernández-Ledesma, B.; Amigo, L.; Martín-Álvarez, P.J.; Recio, I. Production of antioxidant hydrolyzates from a whey protein concentrate with thermolysin: Optimization by response surface methodology. LWT Food Sci. Technol. 2011, 44, 9–15. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Gauthier, S.F.; Britten, M.; Fliss, I.; Robitaille, G.; Jean, J. Antibacterial activity of peptides extracted from tryptic hydrolyzate of whey protein by nanofiltration. Int. Dairy J. 2013, 28, 94–101. [Google Scholar] [CrossRef]
- Théolier, J.; Hammami, R.; Labelle, P.; Fliss, I.; Jean, J. Isolation and identification of antimicrobial peptides derived by peptic cleavage of whey protein isolate. J. Funct. Foods 2013, 5, 706–714. [Google Scholar] [CrossRef]


| Tested Organisms | Source | Medium | Temperature and Time of Incubation |
|---|---|---|---|
| Staphylococcus aureus 20,231 DSMZ | DSMZ | BHI | 37 °C × 24 h |
| Listeria monocytogenes B Listeria monocytogenes C | DAFS | BHI | 37 °C × 24 h |
| Listeria monocytogenes E | DAFS | BHI | 37 °C × 24 h |
| Listeria monocytogenes 20,600 DSMZ | DSMZ | BHI | 37 °C × 24 h |
| Salmonella bongori 13,772 DSMZ | DSMZ | BHI | 37 °C × 24 h |
| Run | BSPH | PSPH | CTRL |
|---|---|---|---|
| DPP-IV IC50 (mg mL−1) | 8.5 b ± 0.2 | 13 a ± 1 | n.d. |
| ABTS IC50 (mg mL−1) | 0.79 b ± 0.03 | 0.87 ab ± 0.01 | 1.06 a ± 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabizza, R.; Fancello, F.; Petretto, G.L.; Addis, R.; Pisanu, S.; Pagnozzi, D.; Piga, A.; Urgeghe, P.P. Exploring the DPP-IV Inhibitory, Antioxidant and Antibacterial Potential of Ovine “Scotta” Hydrolysates. Foods 2021, 10, 3137. https://doi.org/10.3390/foods10123137
Cabizza R, Fancello F, Petretto GL, Addis R, Pisanu S, Pagnozzi D, Piga A, Urgeghe PP. Exploring the DPP-IV Inhibitory, Antioxidant and Antibacterial Potential of Ovine “Scotta” Hydrolysates. Foods. 2021; 10(12):3137. https://doi.org/10.3390/foods10123137
Chicago/Turabian StyleCabizza, Roberto, Francesco Fancello, Giacomo Luigi Petretto, Roberta Addis, Salvatore Pisanu, Daniela Pagnozzi, Antonio Piga, and Pietro Paolo Urgeghe. 2021. "Exploring the DPP-IV Inhibitory, Antioxidant and Antibacterial Potential of Ovine “Scotta” Hydrolysates" Foods 10, no. 12: 3137. https://doi.org/10.3390/foods10123137
APA StyleCabizza, R., Fancello, F., Petretto, G. L., Addis, R., Pisanu, S., Pagnozzi, D., Piga, A., & Urgeghe, P. P. (2021). Exploring the DPP-IV Inhibitory, Antioxidant and Antibacterial Potential of Ovine “Scotta” Hydrolysates. Foods, 10(12), 3137. https://doi.org/10.3390/foods10123137