Distribution and Quantification of 1,2-Propylene Glycol Enantiomers in Baijiu
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Sample Preparation
2.4. Qualitation and Quantification of 1,2-PG Enantiomers
2.5. GC-O Analysis and Evaluation
2.6. Aroma Thresholds
2.7. Statistical Analysis
3. Results and Discussion
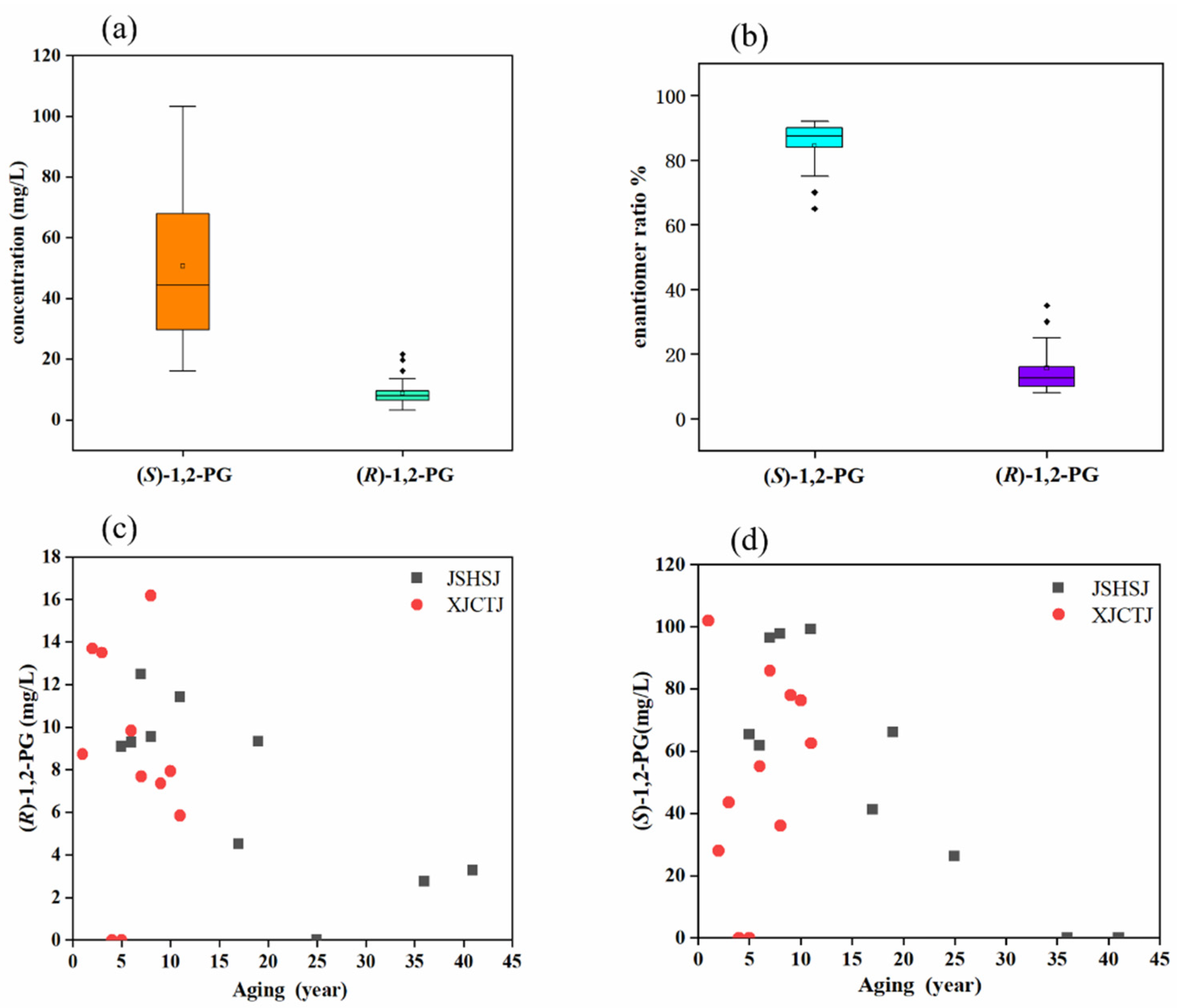
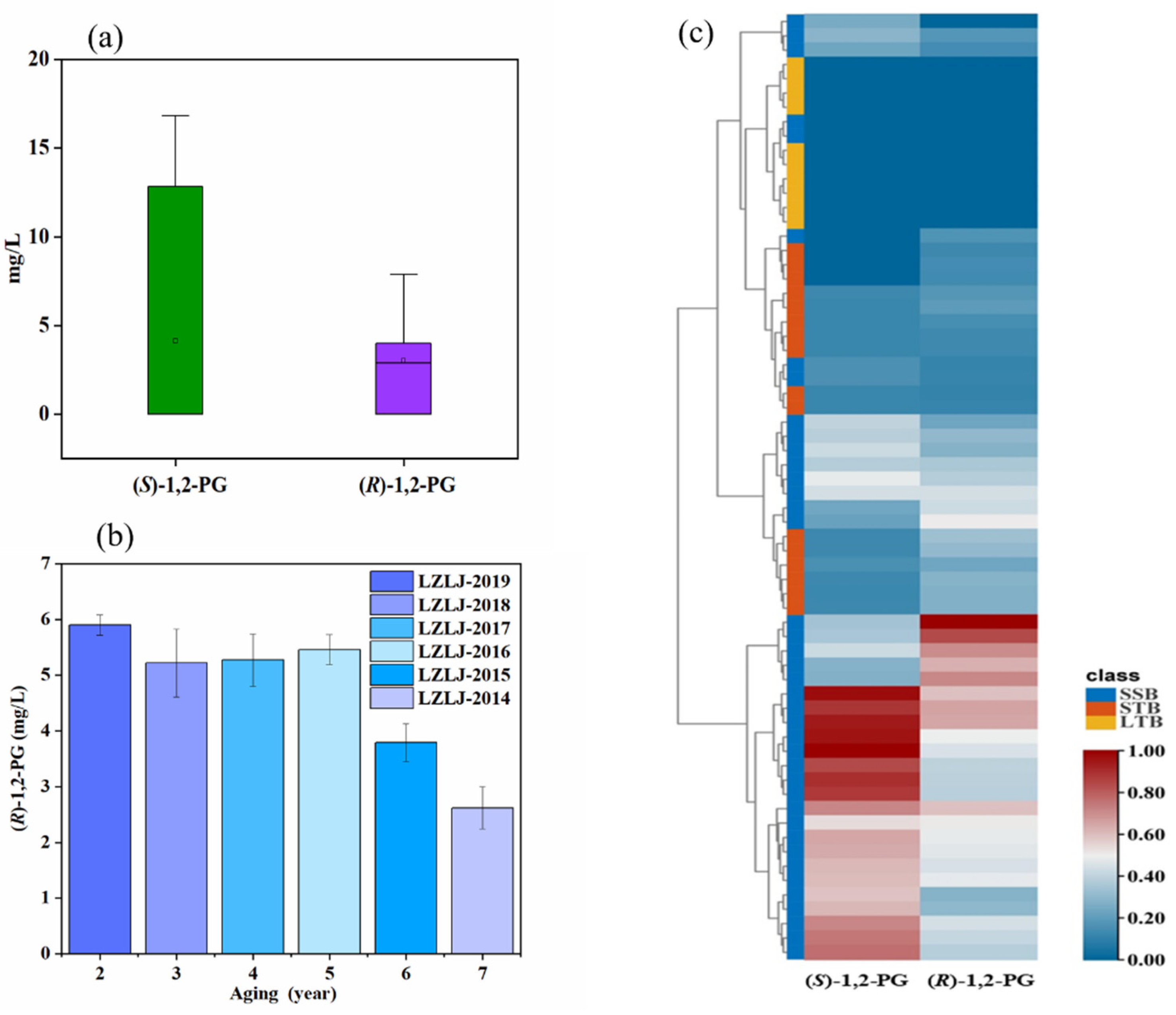
3.1. Enantiomeric Distribution and Concentration of 1,2-PG
3.2. Odor Characteristics of 1,2-PG
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Jia, W.; Fan, Z.B.; Du, A.; Li, Y.L.; Zhang, R.; Shi, Q.Y.; Shi, L.; Chu, X.G. Recent advances in Baijiu analysis by chromatography based technology–A review. Food Chem. 2020, 324, 126899. [Google Scholar] [CrossRef]
- Liu, H.L.; Sun, B.G. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef]
- Han, S.H.; Zhang, W.W.; Li, P.Y.; Li, X.; Liu, J.X.; Xu, B.C.; Luo, D. Characterization of aromatic liquor by gas chromatography and principal component analysis. Anal. Lett. 2017, 50, 777–786. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zhao, J.R.; Liu, X.; Zhang, C.S.; Zhao, Z.G.; Li, X.T.; Sun, B.G. Flavor mystery of Chinese traditional fermented Baijiu. Food Chem. 2021, 369, 130920. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Sun, B.G.; Fan, G.S.; Teng, C.; Xiong, K.; Zhu, Y.P.; Li, J.L.; Li, X.T. The brewing process and microbial diversity of strong flavour Chinese spirits: A review. J. Inst. Brew. 2017, 123, 5–12. [Google Scholar] [CrossRef]
- D’Orazio, G.; Fanali, C.; Asensio-Ramos, M.; Fanali, S. Chiral separations in food analysis. TrAC Trends Anal. Chem. 2017, 96, 151–171. [Google Scholar] [CrossRef]
- Engel, K. Chirality: An important phenomenon regarding biosynthesis, perception, and authenticity of flavor compounds. J. Agric. Food Chem. 2020, 68, 10265–10274. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Cifuentes, A. Chiral analysis in food science. TrAC Trends Anal. Chem. 2020, 123, 115761. [Google Scholar] [CrossRef]
- Dai, Y.F.; Shao, J.Q.; Yang, S.X.; Sun, B.G.; Liu, Y.G.; Ning, T.; Tian, H.Y. Enantioselective Syntheses and Sensory Properties of 2-Methyl-tetrahydrofuran-3-thiol Acetates. J. Agric. Food Chem. 2015, 63, 464–468. [Google Scholar] [CrossRef]
- Tominaga, T.; Niclass, Y.; Frérot, E.; Dubourdieu, D. Stereoisomeric distribution of 3-Mercaptohexan-1-ol and 3-Mercaptohexyl acetate in dry and sweet white wines made from Vitis vinifera (Var. Sauvignon blanc and semillon). J. Agric. Food Chem. 2006, 54, 7251–7255. [Google Scholar] [CrossRef]
- Chen, L.; Capone, D.L.; Jeffery, D.W. Chiral analysis of 3-sulfanylhexan-1-ol and 3-sulfanylhexyl acetate in wine by high-performance liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2017, 998, 83–92. [Google Scholar] [CrossRef]
- Elbashir, A.A.; Aboul-Enein, H.Y. Multidimensional gas chromatography for chiral analysis. Crit. Rev. Anal. Chem. 2018, 48, 416–427. [Google Scholar] [CrossRef]
- Ebeler, S.E. Enantiomeric analysis as a tool for authentication of foods and beverages. ACS Symp. Ser. 2006, 952, 39–49. [Google Scholar]
- Tiritan, M.E.; Fernandes, C.; Maia, A.S.; Pinto, M.; Cass, Q.B. Enantiomeric ratios: Why so many notations? J. Chromatogr. A 2018, 1569, 1–7. [Google Scholar] [CrossRef]
- Marchelli, R.; Dossena, A.; Palla, G. The potential of enantioselective analysis as a quality control tool. Trends Food Sci. Technol. 1996, 7, 113–119. [Google Scholar] [CrossRef]
- Rocco, A.; Aturki, Z.; Fanali, S. Chiral separations in food analysis. TrAC Trends Anal. Chem. 2013, 52, 206–225. [Google Scholar] [CrossRef]
- Ribeiro, C.; Goncalves, R.; Tiritan, M.E. Separation of enantiomers using gas chromatography: Application in forensic toxicology, food and environmental analysis. Crit. Rev. Anal. Chem. 2020, 51, 787–811. [Google Scholar] [CrossRef]
- Langen, J.; Fischer, U.; Cavalar, M.; Coetzee, C.; Wegmann-Herr, P.; Schmarr, H. Enantiodifferentiation of 1,2-propanediol in various wines as phenylboronate ester with multidimensional gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 2425–2439. [Google Scholar] [CrossRef]
- Ebeler, S.E.; Sun, G.M.; Vickers, A.K.; Stremple, P. Gas chromatographic analysis of chiral aroma compounds in wine using modified cyclodextrin stationary phases and solid phase microextraction. ACS Symp. Ser. 2001, 794, 45–56. [Google Scholar]
- Barba, C.; Flores, G.; Herraiz, M. Stereodifferentiation of some chiral aroma compounds in wine using solid phase microextraction and multidimensional gas chromatography. Food Chem. 2010, 123, 846–851. [Google Scholar] [CrossRef]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J. 2-Methylbutyl acetate in wines: Enantiomeric distribution and sensory impact on red wine fruity aroma. Food Chem. 2017, 237, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Matheis, K.; Granvogl, M.; Schieberle, P. Quantitation and enantiomeric ratios of aroma compounds formed by an ehrlich degradation ofl-Isoleucine in fermented foods. J. Agric. Food Chem. 2016, 64, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, O. Enantiomeric differentiation of three key volatile compounds in three different palm wines (Elaeis guineensis, Borassus flabellifer and Nypa fruticans). CYTA J. Food. 2018, 16, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Nonaka, D.; Fujiwara, R.; Hirata, Y.; Tanaka, T.; Kondo, A. Metabolic engineering of 1,2-propanediol production from cellobiose using beta-glucosidase-expressing E. Coli. Bioresour. Technol. 2021, 329, 124858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huo, Z.B.; Ren, D.Z.; Luo, J.; Fu, J.; Li, L.; Jin, F.M. Catalytic conversion of ethyl lactate to 1,2-propanediol over CuO. Chin. J. Chem. Eng. 2016, 24, 126–131. [Google Scholar] [CrossRef]
- Restrepo, J.B.; Bustillo, J.A.; Bula, A.J.; Paternina, C.D. Selection, Sizing, and Modeling of a Trickle Bed Reactor to Produce 1,2 Propanediol from Biodiesel Glycerol Residue. Processes 2021, 9, 479. [Google Scholar] [CrossRef]
- Mochizuki, N.; Kikuchi, K.; Ikeda, M. Determination of enantiomers of 1,2-propanediol in beer by gas chromatography. Seibutsu Kogaku Kaishi 1997, 75, 339–342. [Google Scholar]
- De Revel, G.; Martin, N.; Pripis-Nicolau, L.; Lonvaud-Funel, A.; Bertrand, A. Contribution to the knowledge of malolactic fermentation influence on wine aroma. J. Agric. Food Chem. 1999, 47, 4003–4008. [Google Scholar] [CrossRef]
- Cai, X.; Shen, Y.; Chen, M.; Zhong, M.; Zhou, Y.; Luo, A. Characterisation of volatile compounds in Maotai flavour liquor during fermentation and distillation. J. Inst. Brew. 2019, 125, 453–463. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, J.; Ming, H.; Wang, X.; Du, X.J. The flavor components of strong-flavor Baijiu of mechanization and traditional brewing methods. Food Ferment. Ind. 2021, 1–8. [Google Scholar]
- National Standard of the People’s Republic of China. Guidelines for Determination of Liquor Flavor Substances Threshold; GB/T 33406-2016; China Standards Press: Beijing, China, 2016. [Google Scholar]
- National Standard of the People’s Republic of China. Sensory Analysis-Methodology-General Guidance for Measuring Odour, Flavour and Taste Detection Thresholds by a Three-Alternative Forced-Choice (3-AFC) Procedure; GB/T 22366-2008; China Standards Press: Beijing, China, 2008. [Google Scholar]
- Zheng, X.W.; Han, B.Z. Baijiu.Chinese liquor: History, classification and manufacture. J. Ethn. Foods. 2016, 3, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Jin, G.Y.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Zhao, X.L.; Tang, Y.; Zhang, C.S. The geographical patterns of Chinese liquors during 1995–2004. J. Maps 2017, 13, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.L.; Fan, S.S.; Yan, Y.; Yang, L.; Chen, S.; Xu, Y. Characterization of potent odorants causing a pickle-like Off-Odor in Moutai-Aroma type Baijiu by comparative aroma extract dilution analysis, quantitative measurements, aroma addition, and omission studies. J. Agric. Food Chem. 2020, 68, 1666–1677. [Google Scholar] [CrossRef]
- Niu, W.; Kramer, L.; Mueller, J.; Liu, K.; Guo, J.T. Metabolic engineering of Escherichia coli for the de novo stereospecific biosynthesis of 1,2-propanediol through lactic acid. Metab. Eng. Commun. 2019, 8, e82. [Google Scholar] [CrossRef]
- Suzuki, T.; Onishi, H. Aerobic Dissimilation of l-Rhamnose and the Production of l-Rhamnonic Acid and 1,2-Propanediol by Yeasts. Agric. Biol. Chem. 1968, 32, 888–893. [Google Scholar]
- Schutz, H.; Radler, F. Anaerobic Reduction of Glycerol to Propanediol-1.3 by Lactobacillus brevis and Lactobacillus buchneri. System. Appl. Microbiol. 1984, 5, 169–178. [Google Scholar]
- Pons, A.; Lavigne, V.; Landais, Y.; Darriet, P.; Dubourdieu, D. Distribution and organoleptic impact of sotolon enantiomers in dry white wines. J. Agric. Food Chem. 2008, 56, 1606–1610. [Google Scholar] [CrossRef]
- Cretin, B.N.; Sallembien, Q.; Sindt, L.; Daugey, N.; Buffeteau, T.; Waffo-Teguo, P.; Dubourdieu, D.; Marchal, A. How stereochemistry influences the taste of wine: Isolation, characterization and sensory evaluation of lyoniresinol stereoisomers. Anal. Chim. Acta 2015, 888, 191–198. [Google Scholar] [CrossRef]
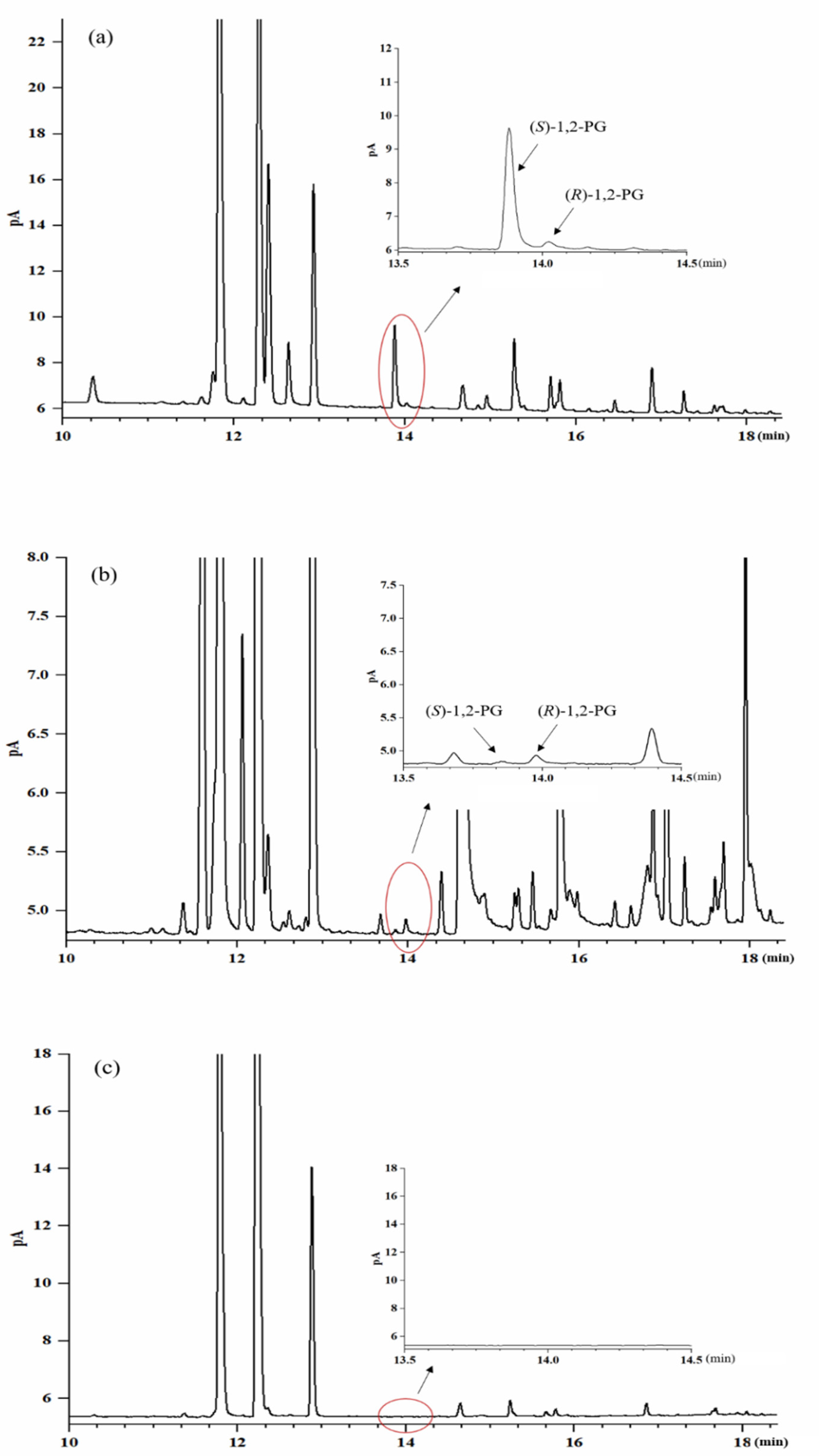
| No. | Compounds | Linearity (mg/L) | R2 | RSD (%) | Recovery Rate (%) | LOD (mg/L) |
|---|---|---|---|---|---|---|
| 1 | (R)-1,2-PG | 0.25–128 | 0.9995 | 0.81–1.54% | 92.40–98.80% | 1.471 |
| 2 | (S)-1,2-PG | 0.25–1024 | 0.9988 | 1.09–5.06% | 98.40–100.85% | 0.791 |
| Mean Concentration ± Standard Deviation (mg/L) * | ||||
|---|---|---|---|---|
| Category | Number of samples | S | R | S/R |
| Baijiu products | 17 | 52.77 ± 23.70 a | 8.72 ± 3.63 a | 87:13 ± 3.17 |
| JSHSJ Vintage Baijiu (1980–2016) | 10 | 64.15 ± 29.63 a | 8.54 ± 3.30 a | 89:11 ± 1.15 |
| XJCTJ Vintage Baijiu (2010–2020) | 11 | 63.01 ± 24.56 a | 10.08 ± 3.53 a | 89:11 ± 3.82 |
| Sample | (S)-1,2-PG (mg/L) | (R)-1,2-PG (mg/L) | ee | S:R |
|---|---|---|---|---|
| SSB (soy sauce aroma-type Baijiu) LM | 34.91 ± 1.16 d | 19.18 ± 2.75 a | 29.09% | 65:35 |
| JSHS | 90.71 ± 2.73 a | 12.61 ± 0.92 cc | 75.59% | 88:12 |
| JSJ | 29.41 ± 3.95 ee | 3.40 ± 0.42 cc | 79.28% | 90:10 |
| ZJ | 73.15 ± 11.05 bb | 11.40 ± 2.36 cc | 73.03% | 87:13 |
| DYT2021 | 62.38 ± 3.29 cc | 8.66 ± 0.35 cc | 75.62% | 88:12 |
| GT | 73.04 ± 4.93 b | 8.55 ± 0.53 cc | 79.04% | 90:10 |
| XJYZ | 24.57 ± 1.65 ee | 8.24 ± 1.15 cc | 49.78% | 75:25 |
| QHL | 49.57 ± 6.30 dd | 7.30 ± 0.61 cc | 74.32% | 87:13 |
| LJ | 91.81 ± 10.34 aa | 7.54 ± 0.42 cc | 84.82% | 92:8 |
| XJ1988 | 38.61 ± 0.35 dd | 6.89 ± 0.36 cc | 69.71% | 85:15 |
| TCSP | 22.33 ± 1.75 e | 9.74 ± 1.62 cc | 39.25% | 70:30 |
| GZJS | 39.44 ± 2.44 dd | 5.85 ± 0.84 cc | 74.15% | 87:13 |
| MTWZ | 45.32 ± 1.81 dd | 8.53 ± 0.38 cc | 68.32% | 84:16 |
| DYT | 89.54 ± 0.48 aa | 7.47 ± 0.05 cc | 84.59% | 92:8 |
| QJ1H | 43.60 ± 1.16 dd | 5.40 ± 0.84 cc | 77.96% | 89:11 |
| MTCX | 28.64 ± 0.92 ee | 12.07 ± 0.57 cc | 40.72% | 70:30 |
| MT43 | 60.02 ± 1.04 cc | 5.47 ± 0.50 cc | 83.29% | 92:8 |
| JSHSJ-1980 | — | 3.26 ± 0.14 cc | — | — |
| JSHS-1985 | 23.60 ± 0.16 ee | 2.74 ± 0.06 c | 79.21% | 90:10 |
| JSHSJ-1996 | 26.17 ± 0.60 ee | — | — | — |
| JSHSJ-2002 | 66.04 ± 3.12 cc | 9.31 ± 2.01 cc | 75.30% | 88:12 |
| JSHSJ-2004 | 41.15 ± 2.09 dd | 4.51 ± 0.24 cc | 80.25% | 90:10 |
| JSHSJ-2010 | 99.24 ± 4.73 aa | 11.40 ± 1.29 cc | 79.39% | 90:10 |
| JSHSJ-2013 | 97.70 ± 1.82 aa | 9.54 ± 2.20 cc | 82.20% | 91:9 |
| JSHSJ-2014 | 96.37 ± 4.27 aa | 12.49 ± 0.89 cc | 77.05% | 89:11 |
| JSHSJ-2015 | 61.72 ± 6.43 cc | 9.28 ± 1.48 cc | 73.87% | 87:13 |
| JSHSJ-2016 | 65.38 ± 1.28 cc | 9.08 ± 2.06 cc | 75.60% | 88:12 |
| XJCTJ-2010 | 62.53 ± 0.69 cc | 5.83 ± 0.48 cc | 82.96% | 91:9 |
| XJCTJ-2011 | 76.27 ± 1.86 bb | 7.93 ± 0.30 cc | 81.17% | 91:9 |
| XJCTJ-2012 | 77.93 ± 1.16 bb | 7.36 ± 0.02 cc | 82.74% | 91:9 |
| XJCTJ-2013 | 36.01 ± 0.28 dd | 16.18 ± 0.52 b | 37.99% | 69:31 |
| XJCTJ-2014 | 85.83 ± 2.63 a | 7.67 ± 0.29 cc | 83.60% | 92:8 |
| XJCTJ-2015 | 55.07 ± 2.14 c | 9.83 ± 0.33 cc | 69.71% | 85:15 |
| XJCTJ-2016 | — | — | — | — |
| XJCTJ-2017 | — | — | — | — |
| XJCTJ-2018 | 43.50 ± 2.79 dd | 13.50 ± 0.15 cc | 52.64% | 76:24 |
| XJCTJ-2019 | 28.05 ± 0.48 ee | 13.69 ± 0.66 cc | 34.39% | 67:33 |
| XJCTJ-2020 | 101.88 ± 2.72 a | 8.74 ± 0.50 cc | 84.20% | 92:8 |
| STB (strong aroma-type Baijiu) | ||||
| LZLJ-TOUQ | 12.95 ± 0.11 a | 3.45 ± 0.14 a | 57.93% | 79:21 |
| LZLJ-TEQ | — | 2.86 ± 0.08 a | — | — |
| LZLJEQ | — | — | — | — |
| MZDQ | — | — | — | — |
| JNC | — | 6.41 ± 2.23 a | — | — |
| WLY | 15.96 ± 0.91 a | 4.55 ± 0.57 a | 55.65% | 78:22 |
| GJ1573 | — | 2.50 ± 0.07 a | — | — |
| SJF | — | — | — | — |
| LZLJ-2012 | — | — | — | — |
| LZLJ-2013 | — | — | — | — |
| LZLJ-2014 | — | 2.62 ± 0.38 a | — | — |
| LZLJ-2015 | — | 3.79 ± 0.34 a | — | — |
| LZLJ-2016 | — | 5.46 ± 0.27 a | — | — |
| LZLJ-2017 | — | 5.27 ± 0.47 a | — | — |
| LZLJ-2018 | — | 5.22 ± 0.61 a | — | — |
| LZLJ-2019 | 13.09 ± 0.22 a | 5.90 ± 0.18 a | 37.85% | 69:31 |
| LTB (light aroma-type Baijiu) | ||||
| LBFJ | — | — | — | — |
| FJQH20 | — | — | — | — |
| FJQXMR | — | — | — | — |
| FJBF | — | — | — | — |
| JXB | — | — | — | — |
| YTX1988 | — | — | — | — |
| FPLJ | — | — | — | — |
| HXEGT | — | — | — | — |
| NLSCN | — | — | — | — |
| NLSEGT | — | — | — | — |
| Compounds | Odor Threshold (in Pure Water) mg/L | Odor Characteristics |
|---|---|---|
| (S)-1,2-PG | 23.92 | Aromas of wood, faint alcoholic |
| (R)-1,2-PG | 4.66 | Sweet aroma, fruity, faint alcoholic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Dai, Y.; Qiu, S.; Sun, B.; Zeng, X. Distribution and Quantification of 1,2-Propylene Glycol Enantiomers in Baijiu. Foods 2021, 10, 3039. https://doi.org/10.3390/foods10123039
Xu H, Dai Y, Qiu S, Sun B, Zeng X. Distribution and Quantification of 1,2-Propylene Glycol Enantiomers in Baijiu. Foods. 2021; 10(12):3039. https://doi.org/10.3390/foods10123039
Chicago/Turabian StyleXu, Hao, Yifeng Dai, Shuyi Qiu, Baoguo Sun, and Xiangyong Zeng. 2021. "Distribution and Quantification of 1,2-Propylene Glycol Enantiomers in Baijiu" Foods 10, no. 12: 3039. https://doi.org/10.3390/foods10123039
APA StyleXu, H., Dai, Y., Qiu, S., Sun, B., & Zeng, X. (2021). Distribution and Quantification of 1,2-Propylene Glycol Enantiomers in Baijiu. Foods, 10(12), 3039. https://doi.org/10.3390/foods10123039





