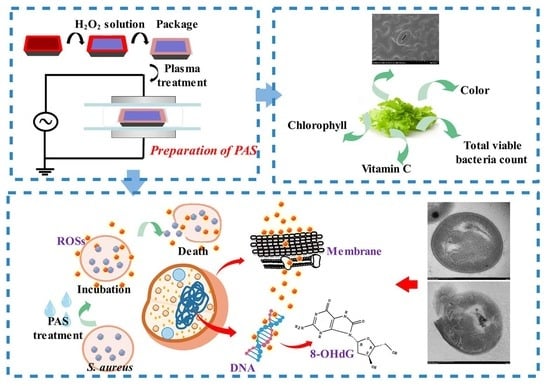
Effect of Plasma-Activated Solution Treatment on Cell Biology of Staphylococcus aureus and Quality of Fresh Lettuces
Abstract
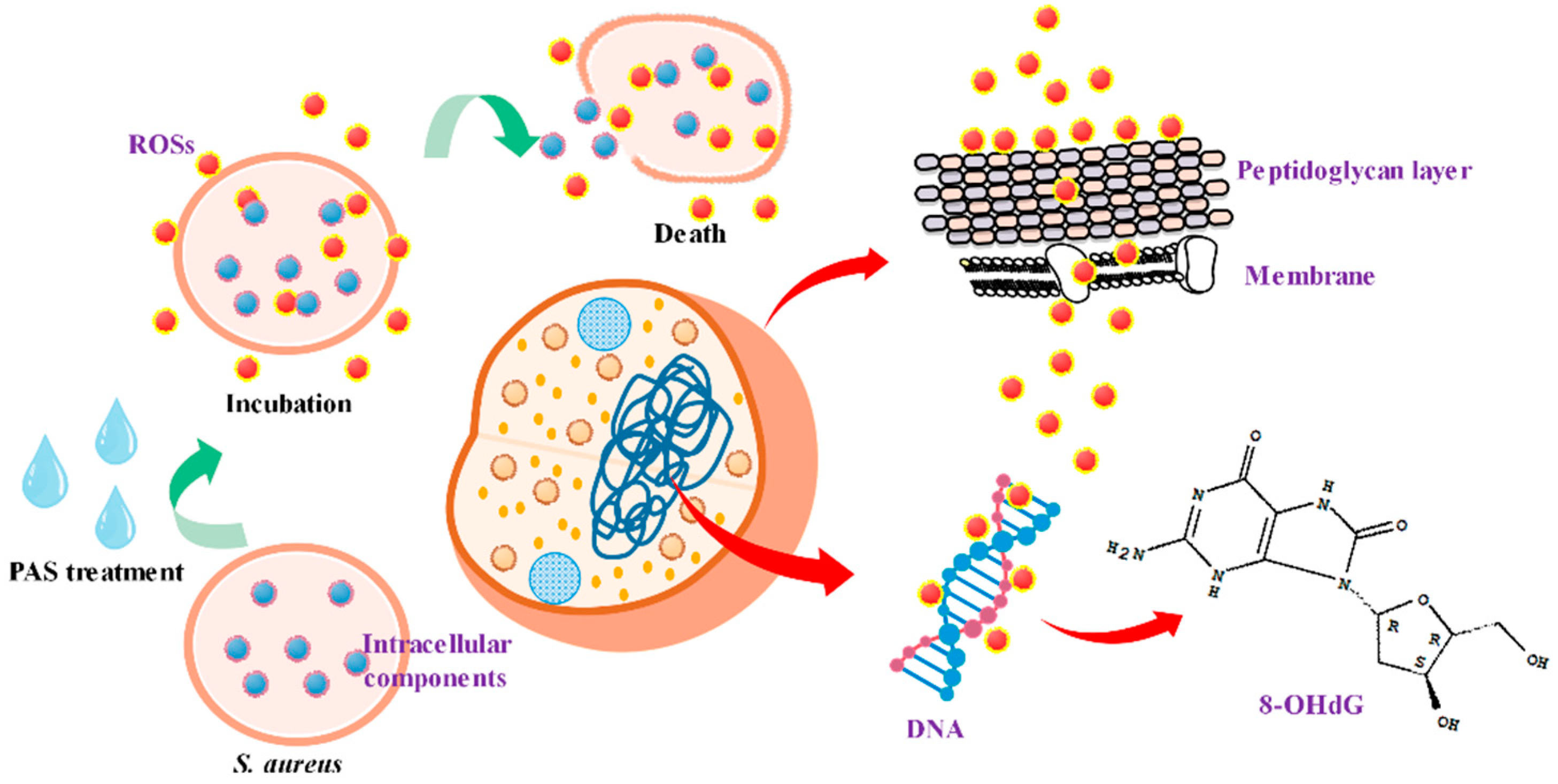
:1. Introduction
2. Materials and Methods
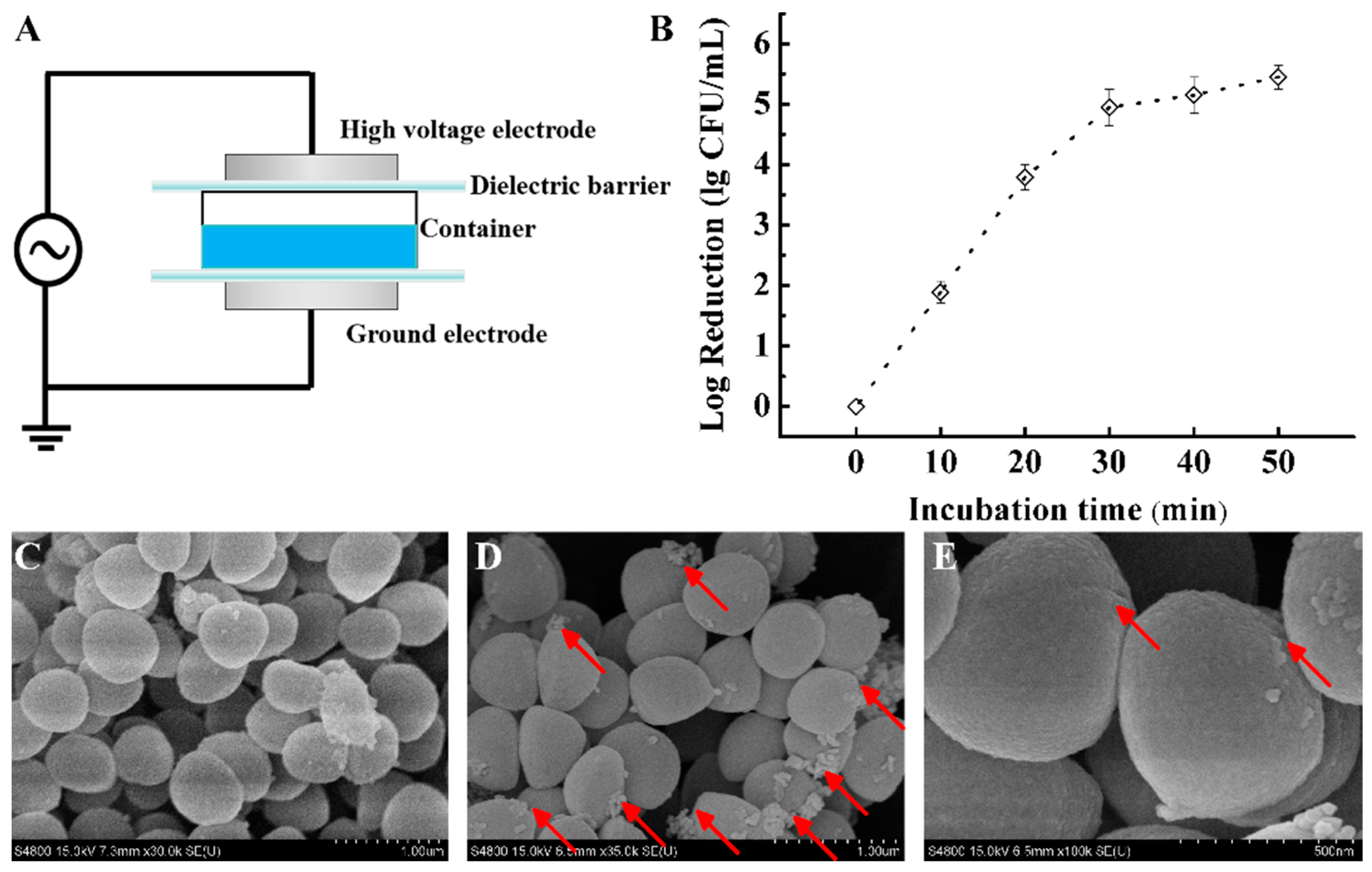
2.1. Preparation of Plasma-Activated Solution (PAS) and Sample Treatments
2.2. Antibacterial Action of PAS
2.2.1. Bacterial Cell Enumeration
2.2.2. Cell Morphology Image
2.2.3. Cell Histology Image
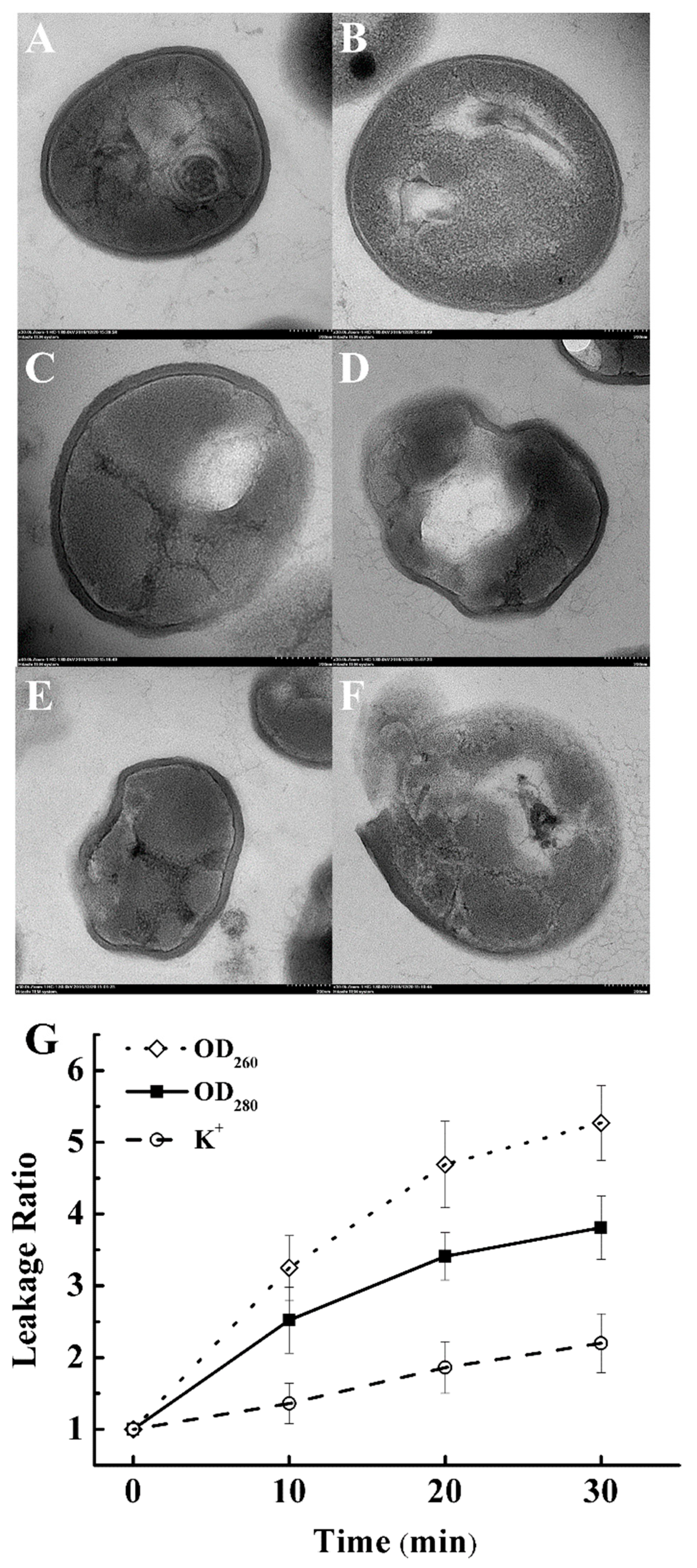
2.2.4. Cell Membrane Integrity
2.3. Oxidative Damages to S. aureus Cells
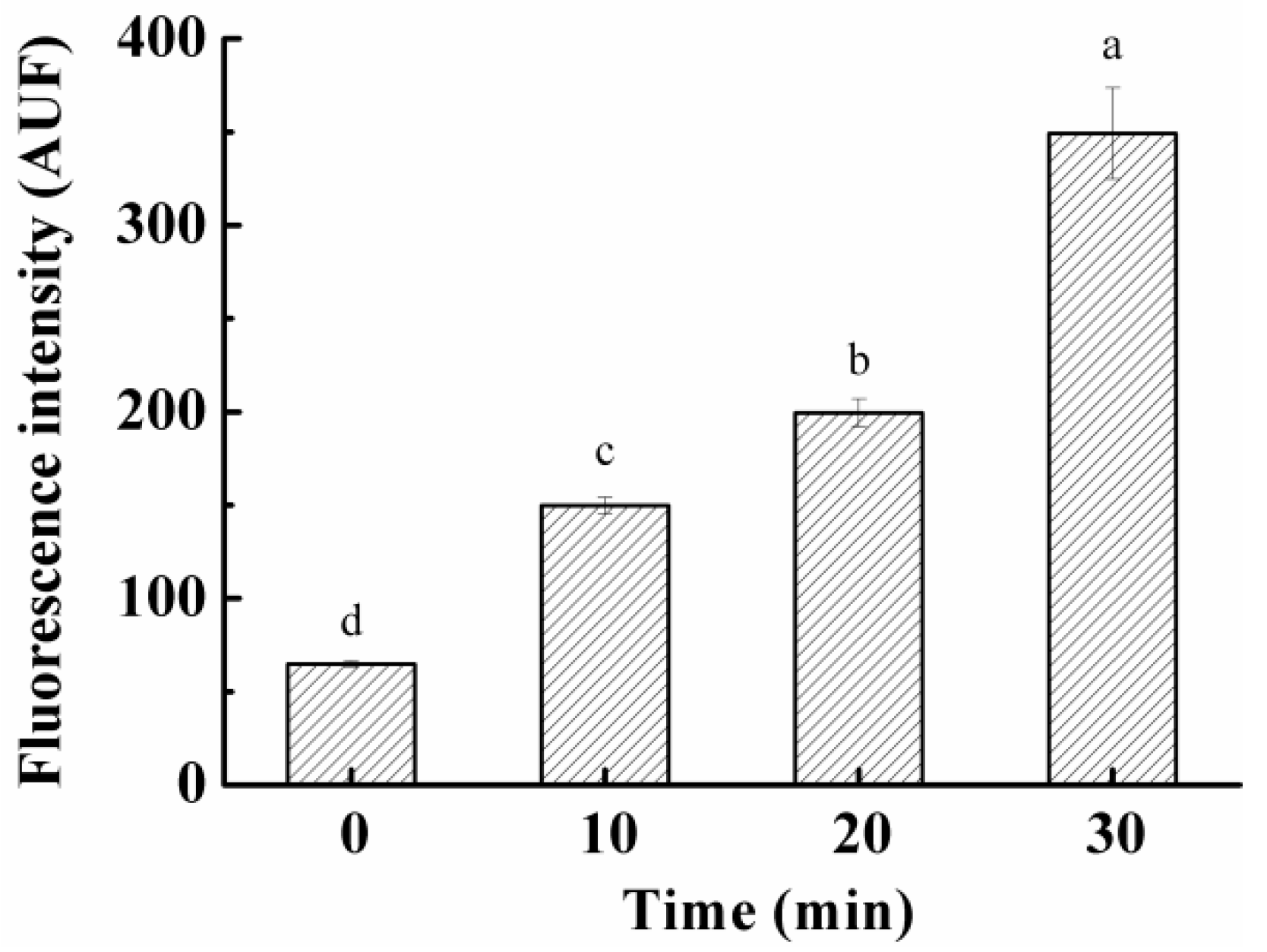
2.3.1. ROS in S. aureus Cells
2.3.2. Peptidoglycan in Cell Wall
2.3.3. Fatty Acids in Cell Membrane
2.3.4. DNA
2.4. Qualities of Fresh Lettuces
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect on S. aureus Survival
3.2. Effect on S. aureus Morphology
3.3. Effect on Cell Histology of S. aureus
3.4. Effect on Cell Membrane Integrity
3.5. Effect on ROS Levels in S. aureus Cells
3.6. Effect on Chemical Bonds of Peptidoglycan Layer
3.7. Effect on Fatty Acids in S. aureus Cell Membrane
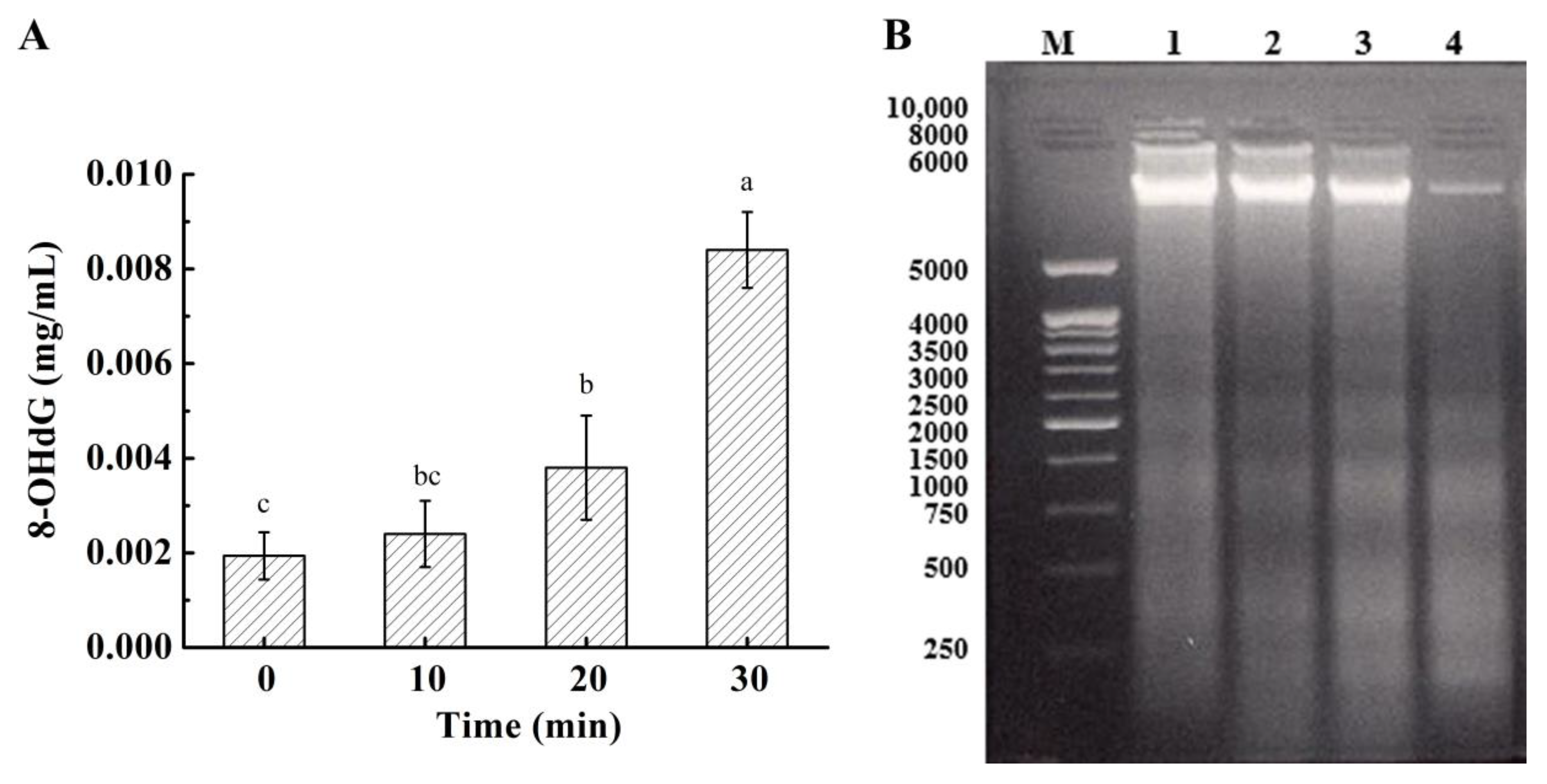
3.8. Effect on DNA in Bacterial Cells
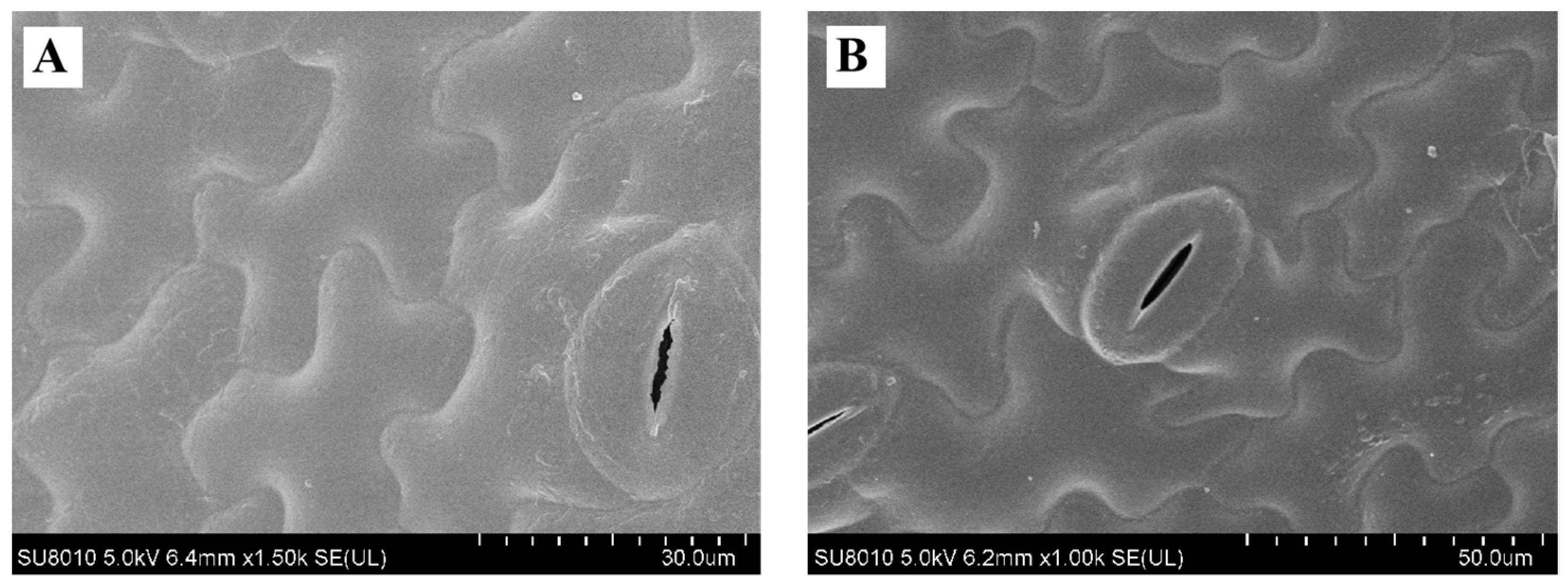
3.9. Effect on Quality of Fresh Lettuces
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Surowsky, B.; Fischer, A.; Schlueter, O.; Knorr, D. Cold plasma effects on enzyme activity in a model food system. Innov. Food Sci. Emerg. Technol. 2013, 19, 146–152. [Google Scholar] [CrossRef]
- Oehmigen, K.; Winter, J.; Hähnel, M.; Wilke, C.; Brandenburg, R.; Weltmann, K.-D.; von Woedtke, T. Estimation of Possible Mechanisms of Escherichia coli Inactivation by Plasma Treated Sodium Chloride Solution. Plasma Process. Polym. 2011, 8, 904–913. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Hashemi, M. Cold atmospheric plasma manipulation of proteins in food systems. Crit. Rev. Food Sci. Nutr. 2018, 58, 2583–2597. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Q.; Kang, C.; Niu, L.; Zhao, D.; Li, K.; Bai, Y. Antibacterial activity and a membrane damage mechanism of plasma-activated water against Pseudomonas deceptionensis CM2. LWT 2018, 96, 395–401. [Google Scholar] [CrossRef]
- Akbar, A.; Anal, A.K. Prevalence and antibiogram study of Salmonella and Staphylococcus aureus in poultry meat. Asian Pac. J. Trop. Biomed. 2013, 3, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Misra, N.; Jo, C. Applications of cold plasma technology for microbiological safety in meat industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Yusupov, M.; Neyts, E.; Khalilov, U.; Snoeckx, R.; Van Duin, A.C.T.; Bogaerts, A. Atomic-scale simulations of reactive oxygen plasma species interacting with bacterial cell walls. New J. Phys. 2012, 14. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Mechanisms of Inactivation by High-Voltage Atmospheric Cold Plasma Differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Tian, Y.; Li, Y.; Ma, R.; Zhang, Q.; Zhang, J.; Fang, J. Bactericidal Effects against S. aureus and Physicochemical Properties of Plasma Activated Water stored at different temperatures. Sci. Rep. 2016, 6, 28505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef]
- Ijabadeniyi, O.; Mbedla, A.; Ajayeoba, T. Microbiological Quality and Antimicrobial Efficacy of Combined Oregano Essential Oil and Acetic Acid on Fresh Lettuce. Ital. J. Food Sci. 2020, 32, 399–409. [Google Scholar]
- Patange, A.; Lu, P.; Boehm, D.; Cullen, P.; Bourke, P. Efficacy of cold plasma functionalised water for improving microbiological safety of fresh produce and wash water recycling. Food Microbiol. 2019, 84, 103226. [Google Scholar] [CrossRef]
- Yang, L.; Yan, W.; Wang, H.; Zhuang, H.; Zhang, J. Shell thickness-dependent antibacterial activity and biocompatibility of gold@silver core–shell nanoparticles. RSC Adv. 2017, 7, 11355–11361. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Pan, J.; Ye, G.; Zhang, Q.; Wang, J.; Zhang, J.; Fang, J. In vitro studies of the antimicrobial effect of non-thermal plasma-activated water as a novel mouthwash. Eur. J. Oral Sci. 2017, 125, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ma, R.; Zhang, Q.; Feng, H.; Liang, Y.; Zhang, J.; Fang, J. Assessment of the Physicochemical Properties and Biological Effects of Water Activated by Non-thermal Plasma Above and Beneath the Water Surface. Plasma Process. Polym. 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Lu, H.; Patil, S.; Keener, K.; Cullen, P.; Bourke, P. Bacterial inactivation by high-voltage atmospheric cold plasma: Influence of process parameters and effects on cell leakage and DNA. J. Appl. Microbiol. 2014, 116, 784–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Zhang, Y.; Cheng, J.-H.; Sun, D.-W. Inactivation of Listeria Monocytogenes at various growth temperatures by ultrasound pretreatment and cold plasma. LWT 2019, 118, 108635. [Google Scholar] [CrossRef]
- Retzko, I.; Friedrich, J.; Lippitz, A.; Unger, W. Chemical analysis of plasma-polymerized films: The application of X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy (NEXAFS) and fourier transform infrared spectroscopy (FTIR). J. Electron. Spectrosc. Relat. Phenom. 2001, 121, 111–129. [Google Scholar] [CrossRef]
- Wang, L.-H.; Wang, M.-S.; Zeng, X.-A.; Liu, Z.-W. Temperature-mediated variations in cellular membrane fatty acid composition of Staphylococcus aureus in resistance to pulsed electric fields. Biochim. Biophys. Acta 2016, 1858, 1791–1800. [Google Scholar] [CrossRef]
- Joshi, S.G.; Cooper, M.; Yost, A.; Paff, M.; Ercan, U.K.; Fridman, G.; Friedman, G.; Fridman, A.; Brooks, A.D. Nonthermal Dielectric-Barrier Discharge Plasma-Induced Inactivation Involves Oxidative DNA Damage and Membrane Lipid Peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 2011, 55, 1053–1062. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Attri, P.; Yadav, D.K.; Choi, J.; Choi, E.H.; Uhm, H.S. Induced apoptosis in melanocytes cancer cell and oxidation in biomolecules through deuterium oxide generated from atmospheric pressure non-thermal plasma jet. Sci. Rep. 2014, 4, 7589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, L.; Huang, M.; Zhang, J.; Yan, W. Effect of dielectric barrier discharge plasma on the degradation of malathion and chlorpyrifos on lettuce. J. Sci. Food Agric. 2020, 101, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.P.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Traylor, M.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. D Appl. Phys. 2011, 44. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Hayashi, R.; Tsuda, T.; Taniguchi, K. High pressure-induced changes of biological membrane—Study on the membrane-bound Na+/K+-ATPase as a model system. Eur. J. Biochem. 2002, 269, 110–118. [Google Scholar] [CrossRef]
- Park, J.H.; Kumar, N.; Park, D.H.; Yusupov, M.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A.; Kang, M.H.; Uhm, H.S.; Choi, E.H.; et al. A comparative study for the inactivation of multidrug resistance bacteria using dielectric barrier discharge and nano-second pulsed plasma. Sci. Rep. 2015, 5, 13849. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liang, Y.; Feng, H.; Ma, R.; Tian, Y.; Zhang, J.; Fang, J. A study of oxidative stress induced by non-thermal plasma-activated water for bacterial damage. Appl. Phys. Lett. 2013, 102, 203701. [Google Scholar] [CrossRef]
- Mandal, R.; Singh, A.; Pratap Singh, A. Recent developments in cold plasma decontamination technology in the food industry. Trends Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Dolezalova, E.; Lukes, P. Membrane damage and active but nonculturable state in liquid cultures of Escherichia coli treated with an atmospheric pressure plasma jet. Bioelectrochemistry 2015, 103, 7–14. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Bayer, A.S.; Prasad, R.; Chandra, J.; Koul, A.; Smriti, M.; Varma, A.; Skurray, R.A.; Firth, N.; Brown, M.; Koo, S.-P.; et al. In Vitro Resistance of Staphylococcus aureus to Thrombin-Induced Platelet Microbicidal Protein Is Associated with Alterations in Cytoplasmic Membrane Fluidity. Infect. Immun. 2000, 68, 3548–3553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becerra, M.C.; Páez, P.L.; Laróvere, L.E.; Albesa, I. Lipids and DNA oxidation in Staphylococcus aureus as a consequence of oxidative stress generated by ciprofloxacin. Mol. Cell. Biochem. 2006, 285, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Devi, Y.; Thirumdas, R.; Sarangapani, C.K.; Deshmukh, R.; Annapure, U. Influence of cold plasma on fungal growth and aflatoxins production on groundnuts. Food Control 2017, 77, 187–191. [Google Scholar] [CrossRef]
- Chauvin, J.; Judée, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017, 7, 4562. [Google Scholar] [CrossRef] [PubMed]

| Orbital | Binding Energy (eV) | Atomic Percentage (%) | |||
|---|---|---|---|---|---|
| Control | 10 min | 20 min | 30 min | ||
| P 2p | 132.6 | 1.64 | 1.79 | 1.87 | 2.27 |
| C 1s | 399.1 | 65.11 | 52.36 | 41.31 | 36.91 |
| O 1s | 530.9 | 23.26 | 36.19 | 48.4 | 55.13 |
| N 1s | 284.4 | 9.99 | 9.66 | 8.42 | 5.69 |
| O/C | 530.9/399.1 | 0.36 | 0.69 | 1.17 | 1.49 |
| Treatment | L* (Brightness) | a* (Greenness) | b* (Yellowness) | Total Viable Bacteria Count (lg CFU/g) | Vitamin C (mg/100 g) | Chlorophyll (mg/g) |
|---|---|---|---|---|---|---|
| Control | 58.37 ± 3.69 | −11.49 ± 1.84 | 36.31 ± 2.97 | 5.18 ± 0.34 | 8.67 ± 0.54 | 1.38 ± 0.18 |
| PAS | 56.14 ± 1.28 | −13.15 ± 2.37 | 35.47 ± 3.24 | 3.95 ± 0.28 | 8.11 ± 0.49 | 1.45 ± 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Qian, J.; Zhuang, H.; Luo, J.; Huang, M.; Yan, W.; Zhang, J. Effect of Plasma-Activated Solution Treatment on Cell Biology of Staphylococcus aureus and Quality of Fresh Lettuces. Foods 2021, 10, 2976. https://doi.org/10.3390/foods10122976
Zhao J, Qian J, Zhuang H, Luo J, Huang M, Yan W, Zhang J. Effect of Plasma-Activated Solution Treatment on Cell Biology of Staphylococcus aureus and Quality of Fresh Lettuces. Foods. 2021; 10(12):2976. https://doi.org/10.3390/foods10122976
Chicago/Turabian StyleZhao, Jianying, Jing Qian, Hong Zhuang, Ji Luo, Mingming Huang, Wenjing Yan, and Jianhao Zhang. 2021. "Effect of Plasma-Activated Solution Treatment on Cell Biology of Staphylococcus aureus and Quality of Fresh Lettuces" Foods 10, no. 12: 2976. https://doi.org/10.3390/foods10122976
APA StyleZhao, J., Qian, J., Zhuang, H., Luo, J., Huang, M., Yan, W., & Zhang, J. (2021). Effect of Plasma-Activated Solution Treatment on Cell Biology of Staphylococcus aureus and Quality of Fresh Lettuces. Foods, 10(12), 2976. https://doi.org/10.3390/foods10122976