UV-C Treatment Maintains the Sensory Quality, Antioxidant Activity and Flavor of Pepino Fruit during Postharvest Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Sensory Scores
2.3. Firmness, Respiration Rate, and Ethylene Production
2.4. TSS, Chlorophyll, Vitamin C, Flavonoids, Anthocyanin, and Total Phenolics Content
2.5. POD, APX, and CAT Activity, and MDA Content
2.6. Electronic Nose (E-Nose) Analysis of Volatile Signatures
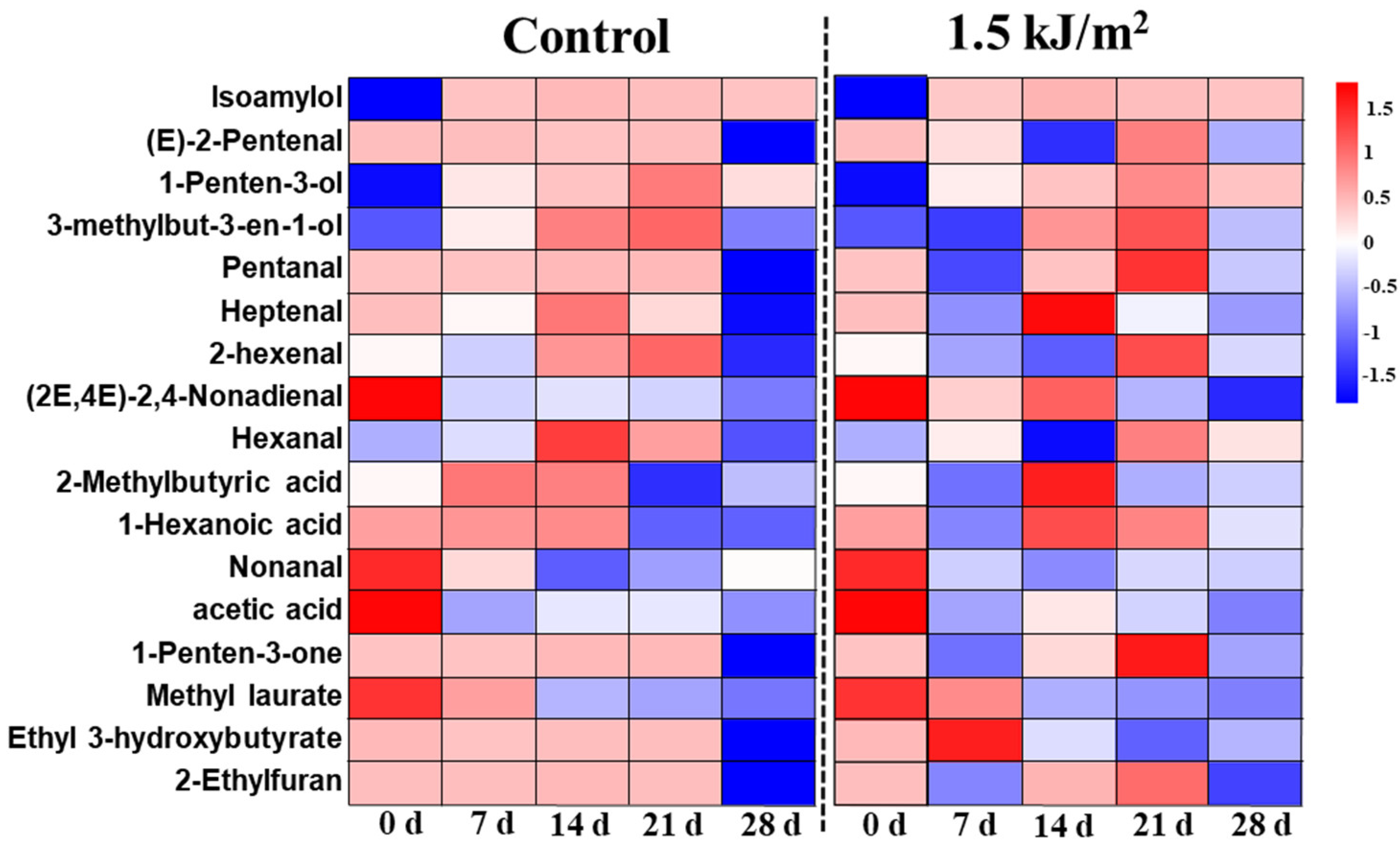
2.7. HS-GC-MS Analysis of Volatiles
2.8. Statistical Analysis
3. Results
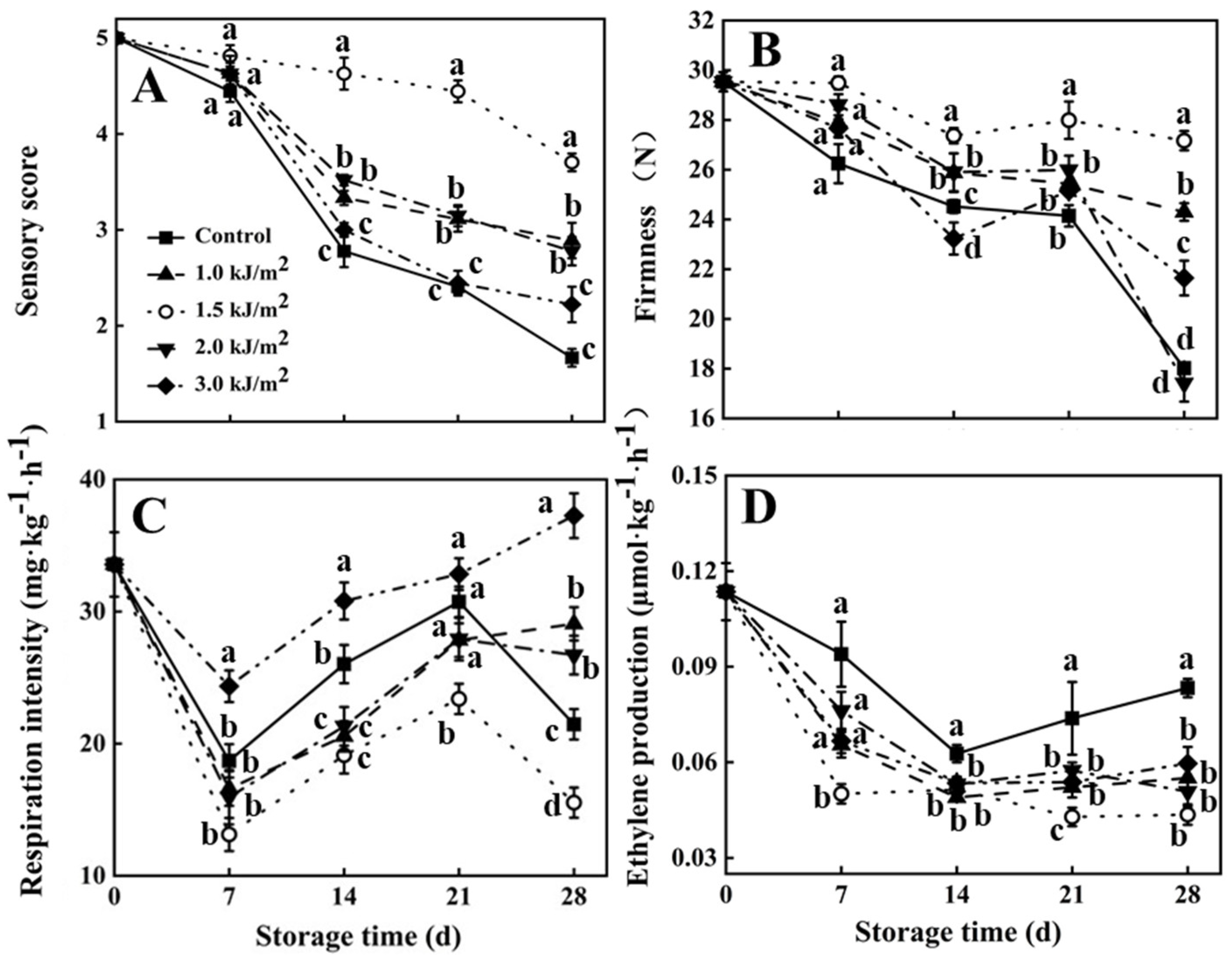
3.1. Sensory Score, Firmness, Respiration Rate, and Ethylene Production
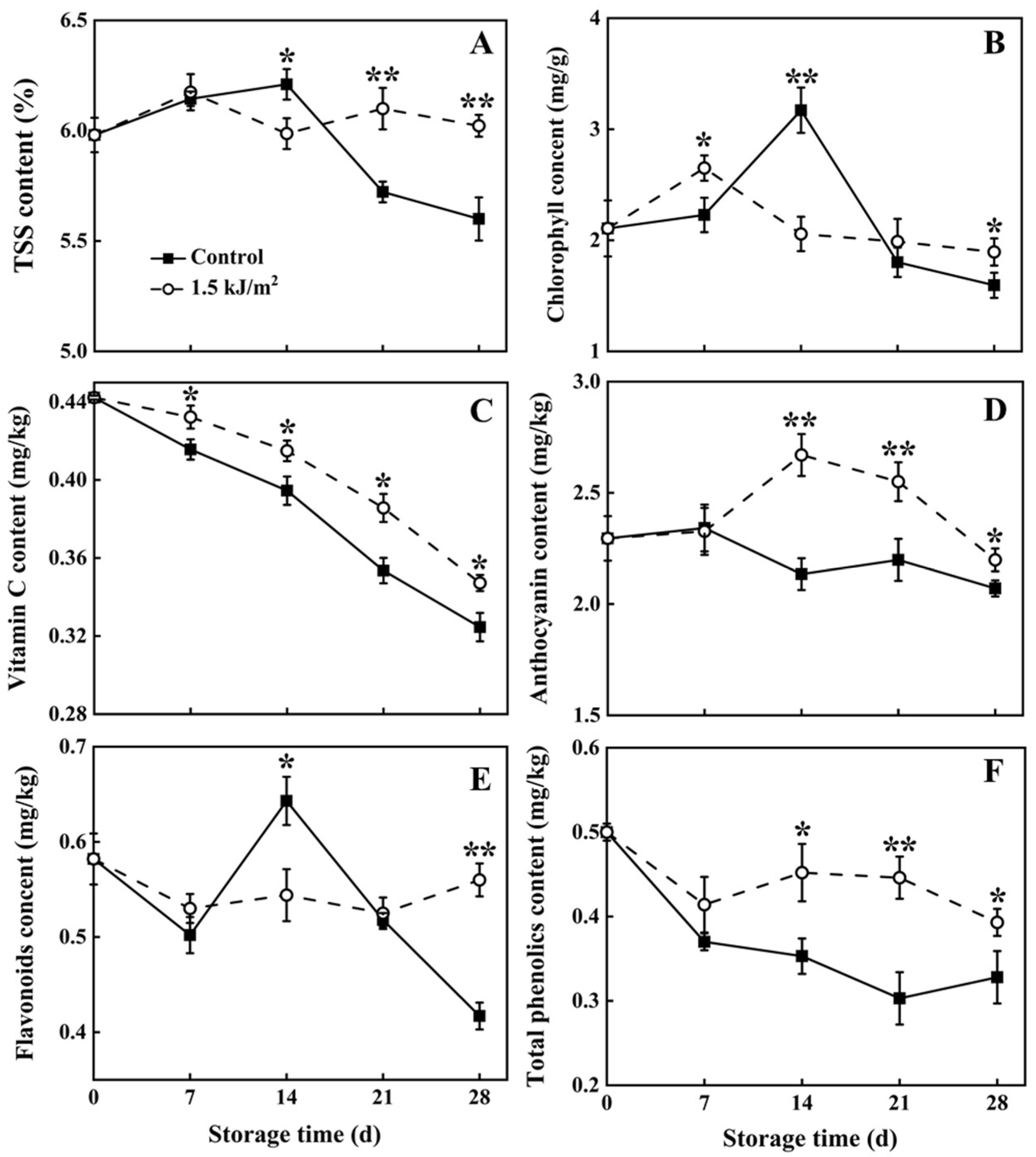
3.2. The Level of TSS, Chlorophyll, Vitamin C, Flavonoids, Anthocyanin, and Total Phenolics
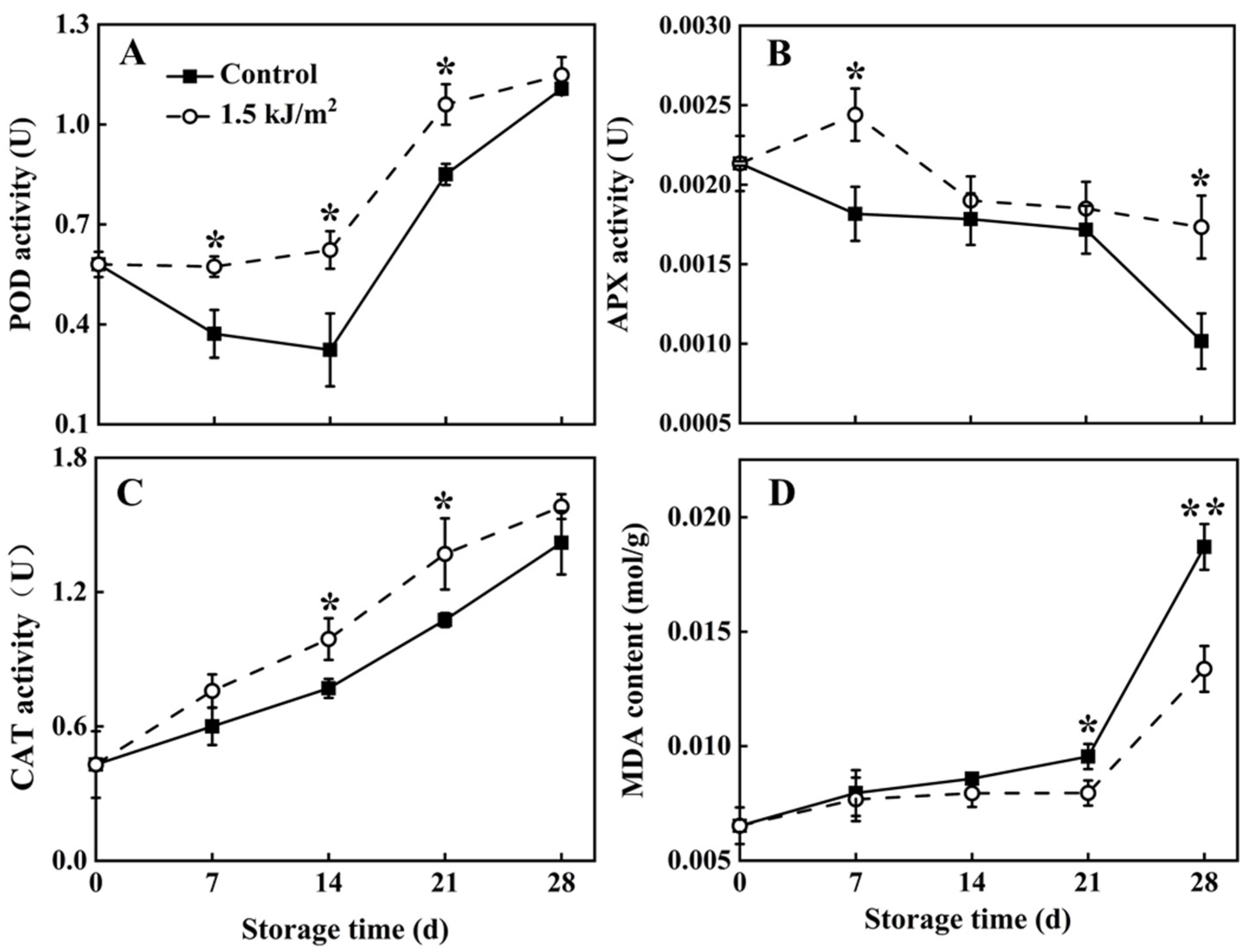
3.3. POD, APX and CAT Activity, and MDA Levels
3.4. Flavor-Related Parameters
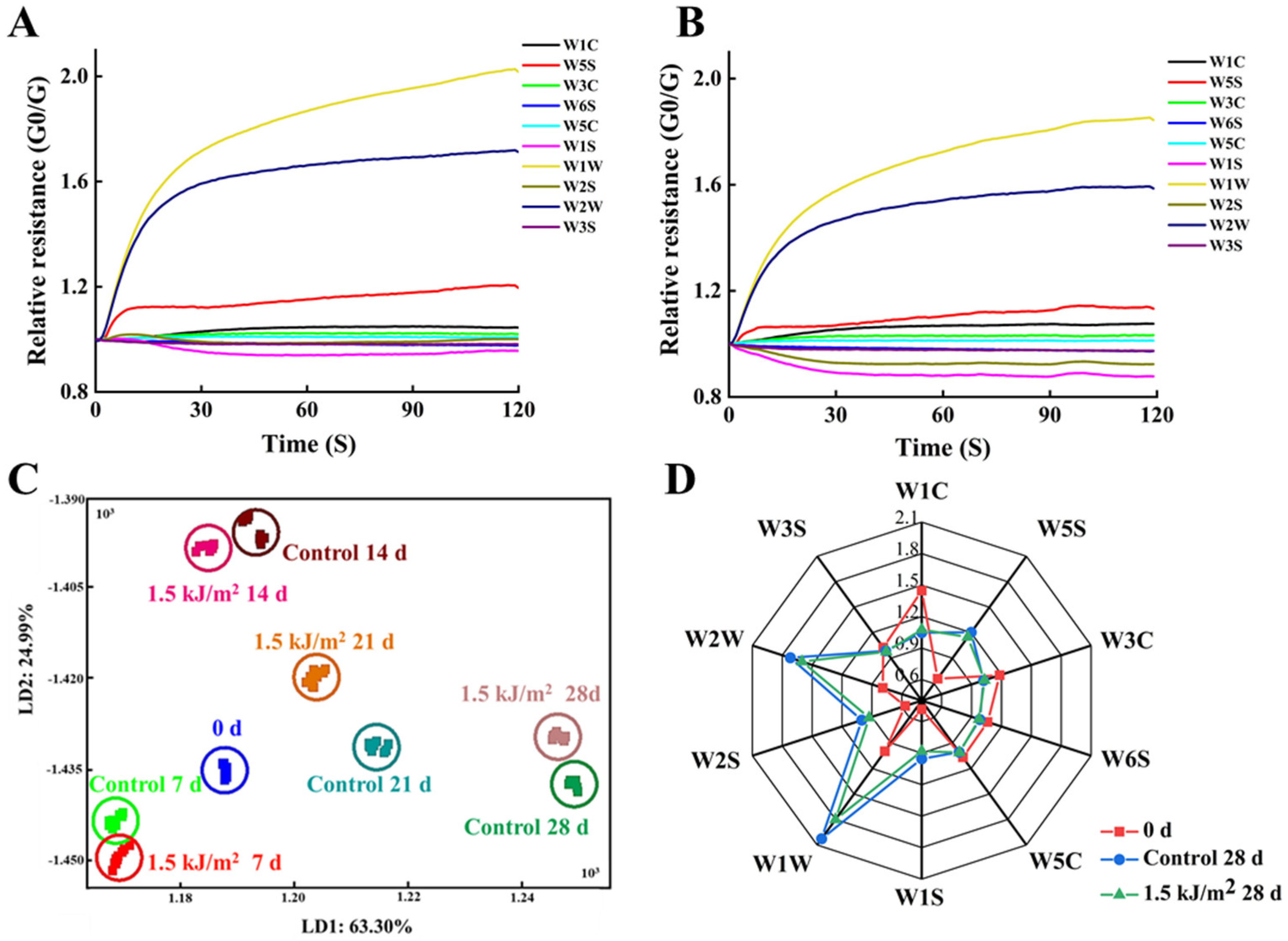
3.4.1. E-Nose Analysis Results
3.4.2. GC-MS Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, G.J.; Jansen, R.K.; Kim, Y.-D. The origin and relationships of the pepino, Solanum muricatum (Solanaceae): Herraiz DNA restriction fragment evidence. Econ. Bot. 1996, 50, 369–380. [Google Scholar] [CrossRef]
- Herraiz, F.; Vilanova, S.; Plazas, M.; Gramazio, P.; Andújar, I.; Rodríguez-Burruezo, A.; Fita, A.; Anderson, G.; Prohens, J. Phenological growth stages of pepino (Solanum muricatum) according to the BBCH scale. Sci. Hortic. 2015, 183, 1–7. [Google Scholar] [CrossRef]
- Contreras, C.; Schwab, W.; Mayershofer, M.; Morales, I.; Gonzalez-Agüero, M.; Defilippi, B.G. Study of physiological and quality parameters during development and ripening of pepino (Solanum muricatum Aiton) fruit. Chil. J. Agric. Res. 2019, 79, 385–395. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Turner, N.A. Pepino (Solanum muricatum): Chemical composition of ripe fruit. J. Sci. Food Agric. 1986, 37, 1217–1222. [Google Scholar] [CrossRef]
- Sanchez, M.; Camara, M.; Prohens, J.; Ruiz, J.J.; Torija, E.; Nuez, F. Variation in carbohydrate content during ripening in two clones of pepino. J. Sci. Food Agric. 2000, 80, 1985–1991. [Google Scholar] [CrossRef]
- Shiota, H.; Young, H.; Paterson, V.J.; Irie, M. Volatile aroma constituents of pepino fruit. J. Sci. Food Agric. 1988, 43, 343–354. [Google Scholar] [CrossRef]
- Sudha, G.; Priya, M.S.; Shree, R.B.I.; Vadivukkarasi, S. Antioxidant activity of ripe and unripe pepino fruit (Solanum muricatum Aiton). J. Food Sci. 2012, 77, C1131–C1135. [Google Scholar] [CrossRef]
- Giovanelli, G.; Buratti, S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009, 112, 903–908. [Google Scholar] [CrossRef]
- Huyskens-Keil, S.; Prono-Widayat, H.; Lüdders, P.; Schreiner, M. Postharvest quality of pepino (Solanum muricatum Ait.) fruit in controlled atmosphere storage. J. Food Eng. 2006, 77, 628–634. [Google Scholar] [CrossRef]
- Rodríguez, B.A.; Prohens, J.; Fita, A.M. Breeding strategies for improving the performance and fruit quality of the pepino (Solanum muricatum): A model for the enhancement of underutilized exotic fruit. Food Res. Int. 2011, 44, 1927–1935. [Google Scholar] [CrossRef]
- Huyskens-Keil, S.; Prono-Widayat, H.; Schreiner, M.; Peters, P. Effect of surface coating and film packaging on the keeping quality of solanaceous crops (Solanum muricatum Ait., Solanum quitoense Lam.). In Proceedings of the IV International Conference on Postharvest Science, Jerusalem, Israel, 26–31 March 2000; pp. 621–625. [Google Scholar] [CrossRef]
- Abdipour, M.; Malekhossini, P.S.; Hosseinifarahi, M.; Radi, M. Integration of UV irradiation and chitosan coating: A powerful treatment for maintaining the postharvest quality of sweet cherry fruit. Sci. Hortic. 2020, 264, 109197. [Google Scholar] [CrossRef]
- Terry, L.A.; Joyce, D.C. Elicitors of induced disease resistance in postharvest horticultural crops: A brief review. Postharvest Biol. Technol. 2004, 32, 1–13. [Google Scholar] [CrossRef]
- Duarte-Sierra, A.; Nadeau, F.; Angers, P.; Michaud, D.; Arul, J. UV-C hormesis in broccoli florets: Preservation, phyto-compounds and gene expression. Postharvest Biol. Technol. 2019, 157, 110965. [Google Scholar] [CrossRef]
- Zhang, K.; Li, W.; Ju, Y.; Wang, X.; Sun, X.; Fang, Y.; Chen, K. Transcriptomic and Metabolomic Basis of Short-and Long-Term Post-Harvest UV-C Application in Regulating Grape Berry Quality Development. Foods 2021, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.; Wilson, C.; Lu, J.; Khan, V.; Chalutz, E.; Droby, S.; Kabwe, M.; Haung, Z.; Adeyeye, O.; Pusey, L.; et al. Plant hormesis induced by ultraviolet light-C for controlling postharvest diseases of tree fruit. Crop Prot. 1996, 15, 129–134. [Google Scholar] [CrossRef]
- Chen, Q.; Song, J.; Bi, J.; Meng, X.; Wu, X. Characterization of volatile profile from ten different varieties of Chinese jujubes by HS-SPME/GC–MS coupled with E-nose. Food Res. Int. 2018, 105, 605–615. [Google Scholar] [CrossRef]
- Raúl, Á.S.; Rhode, N.C.A.; Obdulia, V.L.; Paola, H.C.; Enrique, O.V.C. Ultraviolet Light Stimulation of Bioactive Compounds with Antioxidant Capacity of Fruit and Vegetables. In Plant Secondary Metabolites; Apple Academic Press: New York, NY, USA, 2017; Volume 2, pp. 281–306. [Google Scholar]
- Pérez-Ambrocio, A.; Guerrero-Beltrán, J.; Aparicio-Fernández, X.; Sosa, R.A.; Hernández-Carranza, P.; Cid-Pérez, S.; Ochoa-Velasco, C.E. Effect of blue and ultraviolet-C light irradiation on bioactive compounds and antioxidant capacity of habanero pepper (Capsicum chinense) during refrigeration storage. Postharvest Biol. Technol. 2018, 135, 19–26. [Google Scholar] [CrossRef]
- Zhao, Y.; Ning, M.; Zuo, J.; Shi, J.; Shi, W.; Huang, Y.; Wang, Q.; Feng, B. Effect of UV-C treatment on chilling injury and flavor quality of Solanum muricatum fruit during storage. J. South China Agric. Univ. 2021, 42, 87–96. [Google Scholar] [CrossRef]
- Stevens, C.; Liu, J.; Khan, V.; Lu, J.; Kabwe, M.; Wilson, C.; Igwegbe, E.; Chalutz, E.; Droby, S. The effects of low-dose ultraviolet light-C treatment on polygalacturonase activity, delay ripening and Rhizopus soft rot development of tomatoes. Crop Prot. 2004, 23, 551–554. [Google Scholar] [CrossRef]
- Pluda, D.; Rabinowitch, H.D.; Kafkafi, U. Pepino dulce (Solanum muricatum Ait.) quality characteristics respond to nitrogen nutrition and salinity. J. Am. Soc. Hortic. Sci. 1993, 118, 86–91. [Google Scholar] [CrossRef]
- Sun, B.; Yan, H.; Liu, N.; Wei, J.; Wang, Q. Effect of 1-MCP treatment on postharvest quality characters, antioxidants and glucosinolates of Chinese kale. Food Chem. 2012, 131, 519–526. [Google Scholar] [CrossRef]
- Zhou, F.; Jiang, A.; Feng, K.; Gu, S.; Xu, D.; Hu, W. Effect of methyl jasmonate on wound healing and resistance in fresh-cut potato cubes. Postharvest Biol. Technol. 2019, 157, 110958. [Google Scholar] [CrossRef]
- Pirie, A.; Mullins, M.G. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic acid. Plant Physiol. 1976, 58, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Postharvest UV-C irradiation increased the flavonoids and anthocyanins accumulation, phenylpropanoid pathway gene expression, and antioxidant activity in sweet cherries (Prunus avium L.). Postharvest Biol. Technol. 2021, 175, 111490. [Google Scholar] [CrossRef]
- Esua, O.J.; Chin, N.L.; Yusof, Y.A.; Sukor, R.B. Effects of simultaneous UV-C radiation and ultrasonic energy postharvest treatment on bioactive compounds and antioxidant activity of tomatoes during storage. Food Chem. 2019, 270, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.K.; Jiang, W.B.; Zhao, Y.M. Guidance for Postharvest Physiological and Biochemical Experiments of Fruit and Vegetables; China Light Industry Press: Beijing, China, 2007. [Google Scholar]
- Yan, Z.; Shi, J.; Gao, L.; Wang, Q.; Zuo, J. The combined treatment of broccoli florets with kojic acid and calcium chloride maintains post-harvest quality and inhibits off-odor production. Sci. Hortic. 2020, 262, 109019. [Google Scholar] [CrossRef]
- Aubert, C.; Baumann, S.; Arguel, H. Optimization of the analysis of flavor volatile compounds by liquid−liquid microextraction (LLME). Application to the aroma analysis of melons, peaches, grapes, strawberries, and tomatoes. J. Agric. Food Chem. 2005, 53, 8881–8895. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xie, S.; Xu, M.; Zhang, C.; Wu, N.; Yang, J.; Zhang, L.; Zhang, D.; Jiang, Y.; Wu, C. A novel method for rapid discrimination of bulbus of Fritillaria by using E-nose and electronic tongue technology. Anal. Methods 2015, 7, 943–952. [Google Scholar] [CrossRef]
- Severo, J.; de Oliveira, I.R.; Tiecher, A.; Chaves, F.C.; Rombaldi, C.V. Postharvest UV-C treatment increases bioactive, ester volatile compounds and a putative allergenic protein in strawberry. LWT-Food Sci. Technol. 2015, 64, 685–692. [Google Scholar] [CrossRef]
- Severo, J.; Tiecher, A.; Pirrello, J.; Regad, F.; Latché, A.; Pech, J.-C.; Bouzayen, M.; Rombaldi, C.V. UV-C radiation modifies the ripening and accumulation of ethylene response factor (ERF) transcripts in tomato fruit. Postharvest Biol. Technol. 2015, 102, 9–16. [Google Scholar] [CrossRef]
- Sheng, K.; Zheng, H.; Shui, S.; Yan, L.; Liu, C.; Zheng, L. Comparison of postharvest UV-B and UV-C treatments on table grape: Changes in phenolic compounds and their transcription of biosynthetic genes during storage. Postharvest Biol. Technol. 2018, 138, 74–81. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. UV treatment improved the quality of postharvest fruit and vegetables by inducing resistance. Trends Food Sci. Technol. 2019, 92, 71–80. [Google Scholar] [CrossRef]
- Xu, F.; Wang, S.; Xu, J.; Liu, S.; Li, G. Effects of combined aqueous chlorine dioxide and UV-C on shelf-life quality of blueberries. Postharvest Biol. Technol. 2016, 117, 125–131. [Google Scholar] [CrossRef]
- Bu, J.; Yu, Y.; Aisikaer, G.; Ying, T. Postharvest UV-C irradiation inhibits the production of ethylene and the activity of cell wall-degrading enzymes during softening of tomato (Lycopersicon esculentum L.) fruit. Postharvest Biol. Technol. 2013, 86, 337–345. [Google Scholar] [CrossRef]
- Araque, L.C.O.; Rodoni, L.M.; Darré, M.; Ortiz, C.M.; Civello, P.M.; Vicente, A.R. Cyclic low dose UV-C treatments retain strawberry fruit quality more effectively than conventional pre-storage single high fluence applications. LWT 2018, 92, 304–311. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, S.; Su, X.; Jiang, Y. Respiratory activity and mitochondrial membrane associated with fruit senescence in postharvest peaches in response to UV-C treatment. Food Chem. 2014, 161, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, H.; Cao, J.; Jiang, W. UV-C treatment controls brown rot in postharvest nectarine by regulating ROS metabolism and anthocyanin synthesis. Postharvest Biol. Technol. 2021, 180, 111613. [Google Scholar] [CrossRef]
- Kola, O. Physical and chemical properties of the ripe pepino (Solanum muricatum) fruit grown in Turkey. J. Food Agric. Environ. 2010, 8, 168–171. [Google Scholar] [CrossRef]
- Nguyen, C.T.T.; Kim, J.; Yoo, K.S.; Lim, S.; Lee, E.J. Effect of prestorage UV-A, -B, and-C radiation on fruit quality and anthocyanin of ‘Duke’blueberries during cold storage. J. Agric. Food Chem. 2014, 62, 12144–12151. [Google Scholar] [CrossRef]
- Liao, C.; Liu, X.; Gao, A.; Zhao, A.; Hu, J.; Li, B. Maintaining postharvest qualities of three leaf vegetables to enhance their shelf lives by multiple ultraviolet-C treatment. LWT 2016, 73, 1–5. [Google Scholar] [CrossRef]
- Wu, X.; Guan, W.; Yan, R.; Lei, J.; Xu, L.; Wang, Z. Effects of UV-C on antioxidant activity, total phenolics and main phenolic compounds of the melanin biosynthesis pathway in different tissues of button mushroom. Postharvest Biol. Technol. 2016, 118, 51–58. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, C.; Chen, Y.; Li, H.; Liu, J. Combined effects of ascorbic acid and chitosan on the quality maintenance and shelf life of plums. Sci. Hortic. 2014, 176, 45–53. [Google Scholar] [CrossRef]
- Jiang, Z.; Zheng, Y.; Qiu, R.; Yang, Y.; Xu, M.; Ye, Y.; Xu, M. Short UV-B exposure stimulated enzymatic and nonenzymatic antioxidants and reduced oxidative stress of cold-stored mangoes. J. Agric. Food Chem. 2015, 63, 10965–10972. [Google Scholar] [CrossRef] [PubMed]
- Pongprasert, N.; Sekozawa, Y.; Sugaya, S.; Gemma, H. A novel postharvest UV-C treatment to reduce chilling injury (membrane damage, browning and chlorophyll degradation) in banana peel. Sci. Hortic. 2011, 130, 73–77. [Google Scholar] [CrossRef]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Oshita, S.; Shima, K.; Haruta, T.; Seo, Y.; Kawagoe, Y.; Nakayama, S.; Takahara, H. Discrimination of odors emanating from ‘La France’pear by semi-conducting polymer sensors. Comput. Electron. Agric. 2000, 26, 209–216. [Google Scholar] [CrossRef]
- Manzocco, L.; Da Pieve, S.; Maifreni, M. Impact of UV-C light on safety and quality of fresh-cut melon. Innov. Food Sci. Emerg. Technol. 2011, 12, 13–17. [Google Scholar] [CrossRef]
- Abdipour, M.; Hosseinifarahi, M.; Naseri, N. Combination method of UV-B and UV-C prevents post-harvest decay and improves organoleptic quality of peach fruit. Sci. Hortic. 2019, 256, 108564. [Google Scholar] [CrossRef]
- Contreras, C.; González A., M.; Defilippi, B.G. A review of pepino (Solanum muricatum Aiton) fruit: A quality perspective. HortScience 2016, 51, 1127–1133. [Google Scholar] [CrossRef]
- Galletti, L.; Drouilly, D.; Lizana, L.A.; Berger, H. Atmósfera modificada en frutode pepino dulce. Idesia (Arica) 2006, 24, 35–40. [Google Scholar] [CrossRef]
- Rodríguez-Burruezo, A.; Kollmannsberger, H.; Prohens, J.; Nitz, A.S.; Nuez, F. Analysis of the volatile aroma constituents of parental and hybrid clones of pepino (Solanum muricatum). J. Agric. Food Chem. 2004, 52, 5663–5669. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cai, J.; Zhu, B.Q.; Wu, G.F.; Duan, C.Q.; Chen, G.; Shi, Y. Study of free and glycosidically bound volatile compounds in air-dried raisins from three seedless grape varieties using HS–SPME with GC–MS. Food Chem. 2015, 177, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Lalel, H.J.; Singh, Z.; Tan, S.C. Glycosidically-bound aroma volatile compounds in the skin and pulp of ‘Kensington Pride’mango fruit at different stages of maturity. Postharvest Biol. Technol. 2003, 29, 205–218. [Google Scholar] [CrossRef]
| Category | Number | Formula | Volatile Compounds | Content (mg·kg−1) | ||
|---|---|---|---|---|---|---|
| 0 d | Control 28 d | 1.5 kJ/m2 28 d | ||||
| Alcohols (18) | 1 | C3H8O | 1-Propanol | 1.4002 ± 0.02 | ND | ND |
| 2 | C5H10O | 3-methylbut-3-en-1-ol | 1.54 ± 0.07 | 1.6288 ± 0.03 | 1.3971 ± 0.04 | |
| 3 | C5H12O | Pentanol | 1.8347 ± 0.02 | ND | ND | |
| 4 | C6H14O | Hexanol | 2.1133 ± 0.06 | 2.7704 ± 0.02 | 2.8096 ± 0.03 | |
| 5 | C8H16O | Oct-1-en-3-ol | 1.0308 ± 0.01 | 0.9432 ± 0.01 | ND | |
| 6 | C4H10S2 | 1,4-Butanedithiol | ND | 0.8900 ± 0.04 | ND | |
| 7 | C5H10O | 1-Penten-3-ol | ND | 3.8201 ± 0.13 | 4.2583 ± 0.09 | |
| 8 | C5H12O | Isoamylol | ND | 1.7775 ± 0.07 | 1.7782 ± 0.03 | |
| 9 | C9H18O | cis-6-Nonen-1-ol | ND | 0.9467 ± 0.01 | ND | |
| 10 | C5H12O2 | 2-Isopropoxyethanol | ND | 0.9435 ± 0.01 | 0.9977 ± 0.02 | |
| 11 | C4H10O2 | 1,3-Butanediol | ND | ND | 2.7975 ± 0.08 | |
| 12 | C5H12O | 3-methyl-2-butanol | ND | ND | 4.0145 ± 0.54 | |
| 13 | C6H14O2 | 1,5-Hexanediol | ND | ND | 1.1312 ± 0.03 | |
| 14 | C9H20O | 3-ethylheptan-3-ol | ND | ND | 0.9622 ± 0.01 | |
| 15 | C5H10O | cis-2-Penten-1-ol | ND | ND | 1.4607 ± 0.06 | |
| 16 | C9H18O | cis-2-Nonen-1-ol | ND | ND | 0.9458 ± 0.02 | |
| 17 | C6H14O6 | D-Sorbitol | ND | ND | 0.977 ± 0.01 | |
| 18 | C10H18O | (-)-α-Terpineol | ND | ND | 0.9565 ± 0.01 | |
| Total | 7.919 | 13.7594 | 24.4471 | |||
| Esters (20) | 19 | C7H12O2 | 3-Methyl-3-buten-1-yl acetate | 1.1235 ± 0.04 | 1.0446 ± 0.05 | ND |
| 20 | C10H20O2 | Methyl nonanoate | 0.9666 ± 0.03 | ND | ND | |
| 21 | C11H22O2 | Methyl Caprate | 1.1157 ± 0.01 | ND | ND | |
| 22 | C12H24O2 | Hexyl hexanoate | 1.0269 ± 0.03 | 1.0348 ± 0.04 | 0.9600 ± 0.00 | |
| 23 | C15H30O2 | Methyl myristate | 1.5104 ± 0.05 | ND | 1.0001± 0.02 | |
| 24 | C6H12O3 | Ethyl 3-hydroxybutyrate | 1.1658 ± 0.08 | 0.0018 ± 0.00 | 1.0259± 0.03 | |
| 25 | C9H16O4 | Diethyl dimethylmalonate | 1.2418 ± 0.03 | ND | ND | |
| 26 | C7H14O3 | Methyl 5-methoxypentanoate | 1.1747 ± 0.03 | ND | ND | |
| 27 | C5H10O2 | Butyl formate | ND | 1.0591 ± 0.02 | ND | |
| 28 | C10H20O2 | Butyl Hexanoate | ND | 0.9399 ± 0.03 | ND | |
| 29 | C14H28O2 | Ethyl laurate | ND | 1.5656 ± 0.03 | ND | |
| 30 | C13H26O2 | Methyl laurate | 4.4772 ± 0.34 | 1.2296 ± 0.02 | 1.5939 ± 0.04 | |
| 31 | C18H36O2 | Methyl 15-methylhexadecanoate | ND | 0.9945 ± 0.01 | 0.9530 ± 0.03 | |
| 32 | C8H16O3 | Ethyl 3-hydroxyhexanoate | ND | 0.0018 ± 0.00 | 1.2870 ± 0.03 | |
| 33 | C6H11ClO2 | Methyl 5-chloropentanoate | ND | ND | 1.1431 ± 0.05 | |
| 34 | C8H16O2 | Ethyl 4-methylpentanoate | ND | ND | 1.4664 ± 0.07 | |
| 35 | C12H20O2 | Allyl 3-cyclohexylpropionate | ND | ND | 0.9572 ± 0.04 | |
| 36 | C11H22O2 | Nonyl acetate | ND | ND | 1.2089 ± 0.06 | |
| 37 | C13H24O2 | Ethyl undecylenate | ND | ND | 1.2955 ± 0.05 | |
| 38 | C15H30O2 | Isopropyl dodecanoate | ND | ND | 1.273 ± 0.03 | |
| Total | 9.3254 | 7.8717 | 14.164 | |||
| Aldehydes (16) | 39 | C5H10O | Pentanal | 1.1883 ± 0.04 | 0.6100 ± 0.05 | 1.1409 ± 0.22 |
| 40 | C6H12O | Hexanal | 25.1792 ± 3.93 | 23.4300 ± 2.46 | 15.5164 ± 4.07 | |
| 41 | C5H8O | (E)-2-Pentenal | 1.2655 ± 0.04 | ND | 1.2089 ± 0.03 | |
| 42 | C6H10O | 2-hexenal | 6.8883 ± 1.37 | 5.4300 ± 1.98 | 4.0673 ± 0.85 | |
| 43 | C9H14O | (2E,4E)-2,4-Nonadienal | 3.0414 ± 0.97 | 1.3500 ± 1.02 | 1.9870 ± 0.56 | |
| 44 | C7H12O | Heptenal | 1.2839 ± 0.32 | 0.3200 ± 0.02 | 1.9036 ± 0.36 | |
| 45 | C9H18O | Nonanal | 1.5303 ± 0.39 | 1.2569 ± 0.12 | 0.9751 ± 0.04 | |
| 46 | C8H14O | (2E)-2-Octenal | 1.4123 ± 0.06 | ND | 0.9724 ± 0.01 | |
| 47 | C9H16O | (2E)-2-Nonenal | 1.3177 ± 0.03 | 0.3900 ± 0.01 | 1.8329 ± 0.05 | |
| 48 | C9H14O | (2E,6Z)-nona-2,6-dienal | 1.1767 ± 0.13 | ND | 1.0488 ± 0.09 | |
| 49 | C7H14O | Heptanal | 1.0845 ± 0.04 | ND | ND | |
| 50 | C10H16O | β-Cyclocitral | 0.9903 ± 0.07 | ND | ND | |
| 51 | C10H20O | Decanal | ND | 0.2400 ± 0.01 | ND | |
| 52 | C8H16O | Octanal | ND | ND | 0.9943 ± 0.05 | |
| 53 | C14H30O2 | 1,1-Diethoxydecane | ND | ND | 1.5034 ± 0.17 | |
| 54 | C5H10O | Isovaleraldehyde | ND | ND | 1.8470 | |
| Total | 46.3584 | 33.0269 | 34.998 | |||
| Hydrocarbons (3) | 55 | C6H12 | cyclohexane | 1.7055 ± 0.28 | ND | ND |
| 56 | C5H10 | Cyclopentane | 1.1283 ± 0.17 | ND | ND | |
| 57 | C10H16 | limonene | ND | 0.9522 ± 0.01 | ND | |
| Total | 2.8338 | 0.9522 | 0 | |||
| Acids (10) | 58 | C2H4O2 | acetic acid | 3.0367 ± 0.53 | 1.2403 ± 0.02 | 1.0919 ± 0.01 |
| 59 | C5H10O2 | Pentanoic acid | 0.9601 ± 0.01 | ND | ND | |
| 60 | CH2O2 | Formic Acid | 0.9937 ± 0.02 | ND | ND | |
| 61 | C5H10O2 | 2-Methylbutyric acid | 1.1698 ± 0.30 | 1.1022 ± 0.05 | 1.0498 ± 0.02 | |
| 62 | C6H12O2 | 1-Hexanoic acid | 2.2315 ± 0.27 | ND | 3.1323 ± 0.51 | |
| 63 | C9H18O2 | Nonanoic acid | 1.2055 ± 0.23 | ND | ND | |
| 64 | C9H16O2 | 2-nonenoic acid | 0.9751 ± 0.02 | ND | ND | |
| 65 | C4H6O4 | Succinic acid | ND | 1.0831 ± 0.04 | 0.9740 ± 0.09 | |
| 66 | C12H24O2 | Lauric acid | ND | 1.0005 ± 0.03 | ND | |
| 67 | C6H10O2 | trans-Hex-2-enoic acid | ND | 1.0670 ± 0.01 | 1.8462 ± 0.17 | |
| Total | 10.5724 | 5.4931 | 8.0942 | |||
| Others (6) | 68 | C6H4Cl2 | 1,3-Dichlorobenzene | 1.1040 ± 0.23 | 0.9509 ± 0.16 | ND |
| 69 | C6H8O | 2-Ethylfuran | 1.1188 ± 0.15 | 0.0010 ± 0.00 | 0.9898 ± 0.02 | |
| 70 | C5H8O | 1-Penten-3-one | 1.8548 ± 0.07 | 0.0020 ± 0.00 | 1.6495 ± 0.08 | |
| 71 | C13H20O | β-ionone | 0.9661 ± 0.01 | ND | ND | |
| 72 | C6H10S | Diallyl sulfide | 1.5546 ± 0.13 | ND | ND | |
| 73 | C4H6O4S | Thiomalic acid | ND | 2.7241 ± 0.14 | ND | |
| Total | 6.5983 | 3.678 | 2.6393 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zuo, J.; Yuan, S.; Shi, W.; Shi, J.; Feng, B.; Wang, Q. UV-C Treatment Maintains the Sensory Quality, Antioxidant Activity and Flavor of Pepino Fruit during Postharvest Storage. Foods 2021, 10, 2964. https://doi.org/10.3390/foods10122964
Zhao Y, Zuo J, Yuan S, Shi W, Shi J, Feng B, Wang Q. UV-C Treatment Maintains the Sensory Quality, Antioxidant Activity and Flavor of Pepino Fruit during Postharvest Storage. Foods. 2021; 10(12):2964. https://doi.org/10.3390/foods10122964
Chicago/Turabian StyleZhao, Yaqi, Jinhua Zuo, Shuzhi Yuan, Wenlin Shi, Junyan Shi, Bihong Feng, and Qing Wang. 2021. "UV-C Treatment Maintains the Sensory Quality, Antioxidant Activity and Flavor of Pepino Fruit during Postharvest Storage" Foods 10, no. 12: 2964. https://doi.org/10.3390/foods10122964
APA StyleZhao, Y., Zuo, J., Yuan, S., Shi, W., Shi, J., Feng, B., & Wang, Q. (2021). UV-C Treatment Maintains the Sensory Quality, Antioxidant Activity and Flavor of Pepino Fruit during Postharvest Storage. Foods, 10(12), 2964. https://doi.org/10.3390/foods10122964







