Analysis of the Cultured Meat Production System in Function of Its Environmental Footprint: Current Status, Gaps and Recommendations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Methodology
2.2. Selected Studies
3. Results
4. Discussion
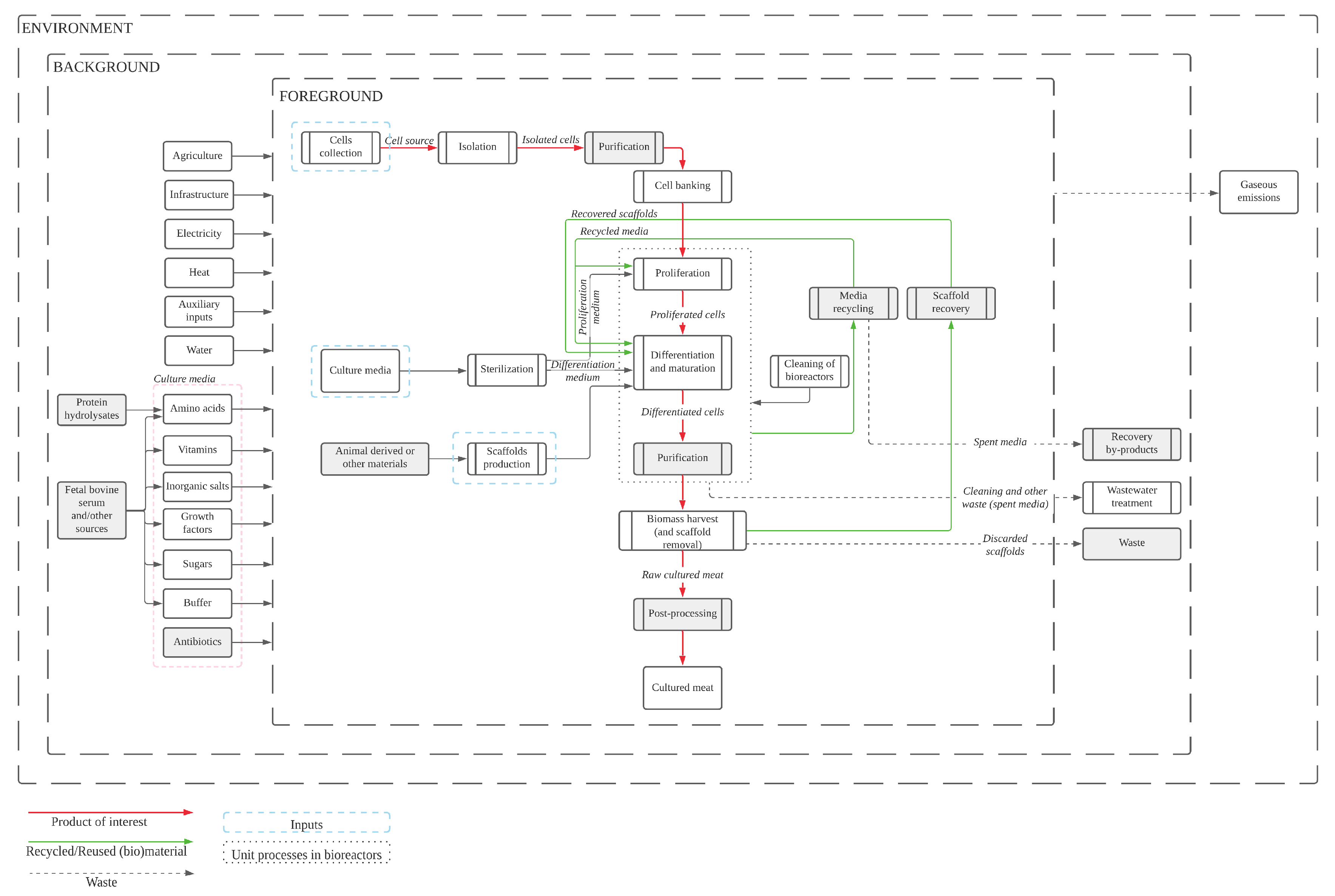
4.1. Bridging the Gaps: Production Process Proposal for Future Assessments
4.2. The Challenge of Life-Cycle Inventories: Supply Chain Analysis
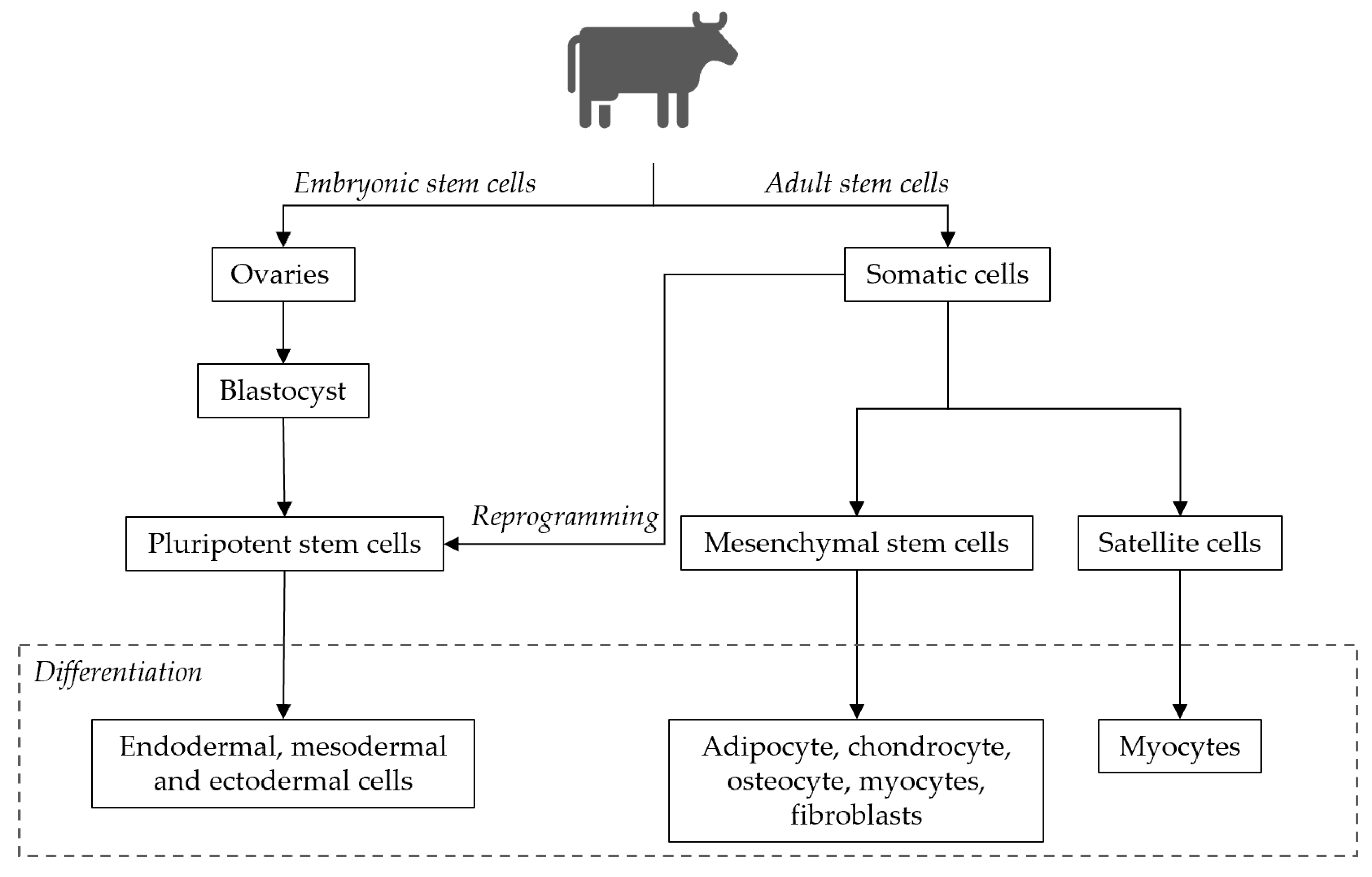
4.2.1. Stem Cells: Collection, Isolation, Purification and Storage
4.2.2. Scaffolds
4.2.3. Culture Media Ingredients
4.2.4. Equipment and Infrastructure
4.2.5. Energy Sources
4.3. Challenges from a Life Cycle Perspective
4.3.1. Goal and Scope
4.3.2. Data Collection
4.3.3. Multifunctionality
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouwman, L.; Goldewijk, K.K.; Van Der Hoek, K.W.; Beusen, A.H.; Van Vuuren, D.P.; Willems, J.; Rufino, M.C.; Stehfest, E. Erratum: Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc. Natl. Acad. Sci. USA 2013, 110, 21196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiking, H. Future protein supply. In Trends in Food Science and Technology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 22, pp. 112–120. [Google Scholar]
- Hagmann, D.; Siegrist, M.; Hartmann, C. Meat avoidance: Motives, alternative proteins and diet quality in a sample of Swiss consumers. Public Health Nutr. 2019, 22, 2448–2459. [Google Scholar] [CrossRef] [PubMed]
- Tukker, A.; Goldbohm, R.; De Koning, A.; Verheijden, M.; Kleijn, R.; Wolf, O.; Pérez-Domínguez, I.; Rueda-Cantuche, J. Environmental impacts of changes to healthier diets in Europe. Ecol. Econ. 2011, 70, 1776–1788. [Google Scholar] [CrossRef]
- Errickson, F.; Kuruc, K.; McFadden, J. Animal-based foods have high social and climate costs. Nat. Food 2021, 2, 274–281. [Google Scholar] [CrossRef]
- Ripple, W.J.; Smith, P.; Haberl, H.; Montzka, S.A.; McAlpine, C.; Boucher, D.H. Ruminants, climate change and climate policy. Nat. Clim. Chang. 2014, 4, 2–5. [Google Scholar] [CrossRef]
- Alvarez, R.A.; Pacala, S.W.; Winebrake, J.J.; Chameides, W.L.; Hamburg, S.P. Greater focus needed on methane leakage from natural gas infrastructure. Proc. Natl. Acad. Sci. USA 2012, 109, 6435–6440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forc-ing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Integovernmental Panel on Climate Change 2021. Available online: https://www.ipcc.ch/report/ar6/wg1/#FullReport (accessed on 11 August 2021).
- Mekonnen, M.M.; Hoekstra, A.Y. The Green, Blue and Gray Water Footprint of Farm Animals and Animal Products; Value of Water Research Report Series No. 48; UNESCO-IHE: Delft, The Netherlands, 2010. [Google Scholar]
- De Backer, C.J.S.; Hudders, L. Meat morals: Relationship between meat consumption consumer attitudes towards human and animal welfare and moral behavior. Meat Sci. 2015, 99, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef] [Green Version]
- Alonso, M.E.; González-Montaña, J.R.; Lomillos, J.M. Consumers’ Concerns and Perceptions of Farm Animal Welfare. Animals 2020, 10, 385. [Google Scholar] [CrossRef] [Green Version]
- Cornish, A.; Raubenheimer, D.; McGreevy, P. What we know about the public’s level of concern for farm animal welfare in food production in developed countries. Animals 2016, 6, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broom, D.M. Animal welfare: An aspect of care, sustainability, and food quality required by the public. J. Vet. Med. Educ. 2010, 37, 83–88. [Google Scholar] [CrossRef]
- Craig, W.J. Health effects of vegan diets. Am. J. Clin. Nutr. 2009, 89, 1627S–1633S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagevos, H. Flexibility in the Frequency of Meat Consumption—Empirical Evidence from The Netherlands. EuroChoices 2014, 13, 40–45. [Google Scholar] [CrossRef]
- Derbyshire, E.J. Flexitarian Diets and Health: A Review of the Evidence-Based Literature. Front. Nutr. 2017, 3, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat Protein Alternatives as Meat Extenders and Meat Analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.; Vecchio, R.; Borrello, M.; Caracciolo, F.; Cembalo, L. Willingness to pay for insect-based food: The role of information and carrier. Food Qual. Prefer. 2019, 72, 177–187. [Google Scholar] [CrossRef]
- Verbeke, W. Profiling consumers who are ready to adopt insects as a meat substitute in a Western society. Food Qual. Prefer. 2015, 39, 147–155. [Google Scholar] [CrossRef]
- Post, M.J.; Levenberg, S.; Kaplan, D.L.; Genovese, N.; Fu, J.; Bryant, C.J.; Negowetti, N.; Verzijden, K.; Moutsatsou, P. Scientific, sustainability and regulatory challenges of cultured meat. Nat. Food 2020, 1, 403–415. [Google Scholar] [CrossRef]
- Hocquette, J.F. Is in vitro meat the solution for the future? Meat Sci. 2016, 120, 167–176. [Google Scholar] [CrossRef]
- Tuomisto, H.L.; Teixeira de Mattos, M.J. Environmental Impacts of Cultured Meat Production. Environ. Sci. Technol. 2011, 45, 6117–6123. [Google Scholar] [CrossRef]
- Lynch, J.; Pierrehumbert, R. Climate Impacts of Cultured Meat and Beef Cattle. Front. Sustain. Food Syst. 2019, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattick, C.S.; Landis, A.E.; Allenby, B.R.; Genovese, N.J. Anticipatory Life Cycle Analysis of In Vitro Biomass Cultivation for Cultured Meat Production in the United States. Environ. Sci. Technol. 2015, 49, 11941–11949. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.C.; Antonioli, F. Exploring consumers’ attitude towards cultured meat in Italy. Meat Sci. 2019, 150, 101–110. [Google Scholar] [CrossRef]
- Chriki, S.; Hocquette, J.-F. The Myth of Cultured Meat: A Review. Front. Nutr. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muralikrishna, I.V.; Manickam, V. Life Cycle Assessment. Environ. Manag. 2017, 57–75. [Google Scholar]
- Wu, Y.; Su, D. Review of Life Cycle Impact Assessment (LCIA) Methods and Inventory Databases. Sustain. Prod. Dev. 2020, 39–55. [Google Scholar] [CrossRef]
- Scharf, A.; Breitmayer, E.; Carus, M. Review and Gap-Analysis of LCA-Studies of Cultured Meat for the Good Food Institute. 2019. Available online: www.nova-institut.eu (accessed on 11 May 2021).
- Smetana, S.; Mathys, A.; Knoch, A.; Heinz, V. Meat alternatives: Life cycle assessment of most known meat substitutes. Int. J. Life Cycle Assess. 2015, 20, 1254–1267. [Google Scholar] [CrossRef]
- Tuomisto, H.; Ellis, M.; Haastrup, P. Environmental impacts of cultured meat: Alternative production scenarios. In Proceedings of the 9th International Conference on Life Cycle Assessment in the Agri-Food Sector, San Francisco, CA, USA, 8–10 October 2014; Schenck, R., Huizenga, D., Eds.; ACLCA: Vashon, WA, USA, 2014; pp. 1360–1366. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC91013 (accessed on 19 August 2021).
- Arvidsson, R.; Tillman, A.-M.; Sandén, B.A.; Janssen, M.; Nordelöf, A.; Kushnir, D.; Molander, S. Environmental Assessment of Emerging Technologies: Recommendations for Prospective LCA. J. Ind. Ecol. 2018, 22, 1286–1294. [Google Scholar] [CrossRef] [Green Version]
- Sinke, P.; Odegard, I. LCA of Cultivated Meat Future Projections for Different Scenarios. 2021. Available online: www.cedelft.eu (accessed on 26 May 2021).
- Williams, A.; Audsley, E.; Sandars, D.L. Determining the Environmental Burdens and Resource Use in the Production of Agricultural and Horticultural Commodities. 2006. Available online: www.silsoe.cranfield.ac.uk (accessed on 11 May 2021).
- European Comission. JRC Publications Repository-International Reference Life Cycle Data System (ILCD) Handbook-General guide for Life Cycle Assessment-Provisions and Action Steps. 2010. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC58190 (accessed on 25 June 2021).
- Fraeye, I.; Kratka, M.; Vandenburgh, H.; Thorrez, L. Sensorial and Nutritional Aspects of Cultured Meat in Comparison to Traditional Meat: Much to Be Inferred. Front. Nutr. 2020, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Good Food Institute. Cultivated Meat Cell Culture Media|Deep Dive|GFI. 2021. Available online: https://gfi.org/science/the-science-of-cultivated-meat/deep-dive-cultivated-meat-cell-culture-media/ (accessed on 21 June 2021).
- D’Este, M.; Alvarado-Morales, M.; Angelidaki, I. Amino acids production focusing on fermentation technologies—A review. Biotechnol. Adv. 2018, 36, 14–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ouyang, F. Recycle of Cytodex-3 in Vero cell culture. Bioprocess Eng. 1999, 21, 207–210. [Google Scholar] [CrossRef]
- Moritz, M.S.M.; Verbruggen, S.E.L.; Post, M.J. Alternatives for large-scale production of cultured beef: A review. J. Integr. Agric. 2015, 14, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Post, M.J. Cultured beef: Medical technology to produce food. J. Sci. Food Agric. 2014, 94, 1039–1041. [Google Scholar] [CrossRef]
- Stephens, N.; Di Silvio, L.; Dunsford, I.; Ellis, M.; Glencross, A.; Sexton, A. Bringing cultured meat to market: Technical, socio-political, and regulatory challenges in cellular agriculture. Trends Food Sci. Technol. 2018, 78, 155–166. [Google Scholar] [CrossRef]
- De Schauwer, C.; Meyer, E.; Van de Walle, G.R.; Van Soom, A. Markers of stemness in equine mesenchymal stem cells: A plea for uniformity. Theriogenology 2011, 75, 1431–1443. [Google Scholar] [CrossRef]
- Ulloa-Montoya, F.; Verfaillie, C.M.; Hu, W.S. Culture systems for pluripotent stem cells. J. Biosci. Bioeng. 2005, 100, 12–27. [Google Scholar] [CrossRef]
- Bogliotti, Y.S.; Wu, J.; Vilariño, M.; Suzuki, K.; Belmonte, J.C.; Ross, P.J. 2 Bovine Embryonic Stem-Like Cells Derived from in Vitro-Produced Blastocysts. Reprod. Fertil. Dev. 2017, 29, 108. [Google Scholar] [CrossRef]
- Kadim, I.T.; Mahgoub, O.; Baqir, S.; Faye, B.; Purchas, R. Cultured meat from muscle stem cells: A review of challenges and prospects. J. Integr. Agric. 2015, 14, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.B.T.; Bressan, F.F.; Murphy, B.D.; Garcia, J.M. Applications of mesenchymal stem cell technology in bovine species. Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ben-Arye, T.; Levenberg, S. Tissue Engineering for Clean Meat Production. Front. Sustain. Food Syst. 2019, 3, 46. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, X.; Li, X.; Du, G.; Zhou, J.; Chen, J. Challenges and possibilities for bio-manufacturing cultured meat. Trends Food Sci. Technol. 2020, 97, 443–450. [Google Scholar] [CrossRef]
- Verbruggen, S.; Luining, D.; van Essen, A.; Post, M.J. Bovine myoblast cell production in a microcarriers-based system. Cytotechnology 2017, 70, 503–512. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, L.; Bai, J. Consumer acceptance of cultured meat in urban areas of three cities in China. Food Control 2020, 118, 107390. [Google Scholar] [CrossRef]
- Wilfart, A.; Gac, A.; Salaün, Y.; Aubin, J.; Espagnol, S. Allocation in the LCA of meat products: Is agreement possible? Clean. Environ. Syst. 2021, 2, 100028. [Google Scholar] [CrossRef]
- de Vries, M.; de Boer, I.J.M. Comparing environmental impacts for livestock products: A review of life cycle assessments. Livest. Sci. 2010, 128, 1–11. [Google Scholar] [CrossRef]
- Marti, D.L.; Johnson, R.J.; Mathews, K.H. Where’s the (not) meat? By products from beef and pork production. J. Curr. Issues Glob. 2012, 5, 397–423. [Google Scholar]
- Romano, E.; Roma, R.; Tidona, F.; Giraffa, G.; Bragaglio, A. Dairy farms and life cycle assessment (LCA): The allocation criterion useful to estimate undesirable products. Sustainability 2021, 13, 4354. [Google Scholar] [CrossRef]
- Yan, M.J.; Humphreys, J.; Holden, N.M. An evaluation of life cycle assessment of European milk production. J. Environ. Manag. 2011, 92, 372–379. [Google Scholar] [CrossRef]
- Yu, C.; Penn, L.D.; Hollembaek, J.; Li, W.; Cohen, L.H. Enzymatic tissue digestion as an alternative sample preparation approach for quantitative analysis using liquid chromatography-tandem mass spectrometry. Anal. Chem. 2004, 76, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kang, H.J.; Lee, D.Y.; Kang, J.H.; Ramani, S.; Park, S.; Hur, S.J. Principal protocols for the processing of cultured meat. J. Anim. Sci. Technol. 2021, 63, 673–680. [Google Scholar] [CrossRef]
- Hendijani, F. Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017, 50, e12334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, S.; Swennen, G.N.M.; Messmer, T.; Gagliardi, M.; Molin, D.G.M.; Li, C.; Zhou, G.; Post, M.J. Maintaining bovine satellite cells stemness through p38 pathway. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Freshney, R.I.; Capes-Davis, A.; Gregory, C.; Przyborski, S. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015; p. 758. [Google Scholar]
- Seah, J.S.H.; Singh, S.; Tan, L.P.; Choudhury, D. Scaffolds for the manufacture of cultured meat. Crit. Rev. Biotechnol. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.J.; De Bank, P.A.; Ellis, M.J. Bioprocess Design Considerations for Cultured Meat Production with a Focus on the Expansion Bioreactor. Front. Sustain. Food Syst. 2019, 3, 44. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]
- Swartz, E. SBE Special Section: Industrial Biotechnology. 2019. Available online: www.aiche.org/cep (accessed on 21 June 2021).
- Eagle, H. Amino acid metabolism in mammalian cell cultures. Science 1959, 130, 432–437. [Google Scholar] [CrossRef]
- van der Valk, J.; Brunner, D.; De Smet, K.; Svenningsen, Å.F.; Honegger, P.; Knudsen, L.E.; Lindl, T.; Noraberg, J.; Price, A.; Scarino, M.L.; et al. Optimization of chemically defined cell culture media-Replacing fetal bovine serum in mammalian in vitro methods. Toxicol. Vitr. 2010, 24, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, E.N.; Cosenza, Z.A.; Baar, K.; Block, D.E. Considerations for the development of cost-effective cell culture media for cultivated meat production. Compr. Rev. Food Sci. Food Saf. 2021, 20, 686–709. [Google Scholar] [CrossRef]
- Schnellbaecher, A.; Binder, D.; Bellmaine, S.; Zimmer, A. Vitamins in cell culture media: Stability and stabilization strategies. Biotechnol. Bioeng. 2019, 116, 1537–1555. [Google Scholar] [CrossRef] [Green Version]
- Yuan, P.; Cui, S.; Liu, Y.; Li, J.; Du, G.; Liu, L. Metabolic engineering for the production of fat-soluble vitamins: Advances and perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 935–951. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Jin, Z.; Zhang, D. Microbial Cell Factories for Green Production of Vitamins. Front. Bioeng. Biotechnol. 2021, 9, 661562. [Google Scholar] [CrossRef]
- Salim, I.; González-García, S.; Feijoo, G.; Moreira, M.T. Assessing the environmental sustainability of glucose from wheat as a fermentation feedstock. J. Environ. Manag. 2019, 247, 323–332. [Google Scholar] [CrossRef]
- Zheng, X.; Baker, H.; Hancock, W.S.; Fawaz, F.; McCaman, M.; Pungor, E. Proteomic analysis for the assessment of different lots of fetal bovine serum as a raw material for cell culture. Part IV. Application of proteomics to the manufacture of biological drugs. Biotechnol. Prog. 2006, 22, 1294–1300. [Google Scholar] [CrossRef]
- Overton, T.W. Recombinant protein production in bacterial hosts. Drug Discov. Today 2014, 19, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, H.-J.; Han, M.; Yoon, N.; Kim, Y.; Ahn, J. Effective production of human growth factors in Escherichia coli by fusing with small protein 6HFh8. Microb. Cell Fact. 2021, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Silva, F.; Quental, M.J.; Ventura, S.; Freire, M.; Coutinho, J.A.P. Good’s buffers as a basis for developing self-buffering and biocompatible ionic liquids for biological research. Green Chem. 2014, 16, 3149–3159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Specht, L. GFI.ORG Creating a healthy, humane, and sustainable food supply. In An Analysis of Culture Medium Costs and Production Volumes for Cultivated Meat; The Good Food Institute: Washington, DC, USA, 2020. [Google Scholar]
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M.N. Antibiotics traces in the aquatic environment: Persistence and adverse environmental impact. Curr. Opin. Environ. Sci. Health 2020, 13, 68–74. [Google Scholar] [CrossRef]
- Kumar, K.; Gupta, S.C.; Chander, Y.; Singh, A.K. Antibiotic Use in Agriculture and Its Impact on the Terrestrial Environment. Adv. Agron. 2005, 87, 1–54. [Google Scholar]
- Stephanopoulos, G.N.; Aristidou, A.A.; Nielsen, J. Examples of Pathway Manipulations: Metabolic Engineering in Practice. Metab. Eng. 1998, 203–283. [Google Scholar] [CrossRef]
- Mohan, M. Perovskite Photovoltaics: Life Cycle Assessment. In Perovskite Photovolt. Basic Adv. Concepts Implement; Academic Press: Cambridge, MA, USA, 2018; Chapter 14; pp. 447–480. [Google Scholar]
- Casey, J.W.; Holden, N.M. Quantification of GHG emissions from sucker-beef production in Ireland. Agric. Syst. 2006, 90, 79–98. [Google Scholar] [CrossRef]
- Kumm, K.I. Sustainability of organic meat production under Swedish conditions. Agric. Ecosyst. Environ. 2002, 88, 95–101. [Google Scholar] [CrossRef]
- Nijdam, D.; Rood, T.; Westhoek, H. The price of protein: Review of land use and carbon footprints from life cycle assessments of animal food products and their substitutes. Food Policy 2012, 37, 760–770. [Google Scholar] [CrossRef]
- Sonesson, U.; Davis, J.; Flysjö, A.; Gustavsson, J.; Witthöft, C. Protein quality as functional unit–A methodological framework for inclusion in life cycle assessment of food. J. Clean. Prod. 2017, 140, 470–478. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Carballo, J.; Cofrades, S. Healthier meat and meat products: Their role as functional foods. Meat Sci. 2001, 59, 5–13. [Google Scholar] [CrossRef]
- Drewnowski, A.; Rehm, C.D.; Martin, A.; Verger, E.O.; Voinnesson, M.; Imbert, P. Energy and nutrient density of foods in relation to their carbon footprint. Am. J. Clin. Nutr. 2015, 101, 184–191. [Google Scholar] [CrossRef]
- Schau, E.M.; Fet, A.M. LCA studies of food products as background for environmental product declarations. Int. J. Life Cycle Assess. 2007, 13, 255–264. [Google Scholar] [CrossRef]
- Heller, M.C.; Keoleian, G.A.; Willett, W.C. Toward a Life Cycle-Based, Diet-level Framework for Food Environmental Impact and Nutritional Quality Assessment: A Critical Review. Environ. Sci. Technol. 2013, 47, 12632–12647. [Google Scholar] [CrossRef]
- Villares, M.; Işıldar, A.; van der Giesen, C.; Guinée, J. Does ex ante application enhance the usefulness of LCA? A case study on an emerging technology for metal recovery from e-waste. Int. J. Life Cycle Assess. 2017, 22, 1618–1633. [Google Scholar] [CrossRef] [Green Version]
- Gavankar, S.; Suh, S.; Keller, A.A. The Role of Scale and Technology Maturity in Life Cycle Assessment of Emerging Technologies: A Case Study on Carbon Nanotubes. J. Ind. Ecol. 2015, 19, 51–60. [Google Scholar] [CrossRef]
- van der Giesen, C.; Cucurachi, S.; Guinée, J.; Kramer, G.J.; Tukker, A. A critical view on the current application of LCA for new technologies and recommendations for improved practice. J. Clean. Prod. 2020, 259, 120904. [Google Scholar] [CrossRef]
- Notarnicola, B.; Sala, S.; Anton, A.; McLaren, S.J.; Saouter, E.; Sonesson, U. The role of life cycle assessment in supporting sustainable agri-food systems: A review of the challenges. J. Clean. Prod. 2017, 140, 399–409. [Google Scholar] [CrossRef]
- Gac, A.; Salou, T.; Espagnol, S.; Ponchant, P.; Dollé, J.-B.; Van Der Werf, H.M.G. An Original Way of Handling Co-Products with a Biophysical Approach in LCAs of Livestock Systems. 2014. Available online: www.ademe.fr/agribalyse-en (accessed on 16 June 2021).
- Sellitto, M.A.; Hermann, F.F. Prioritization of green practices in GSCM: Case study with companies of the peach industry. Gestão Produção 2016, 23, 871–886. [Google Scholar] [CrossRef] [Green Version]
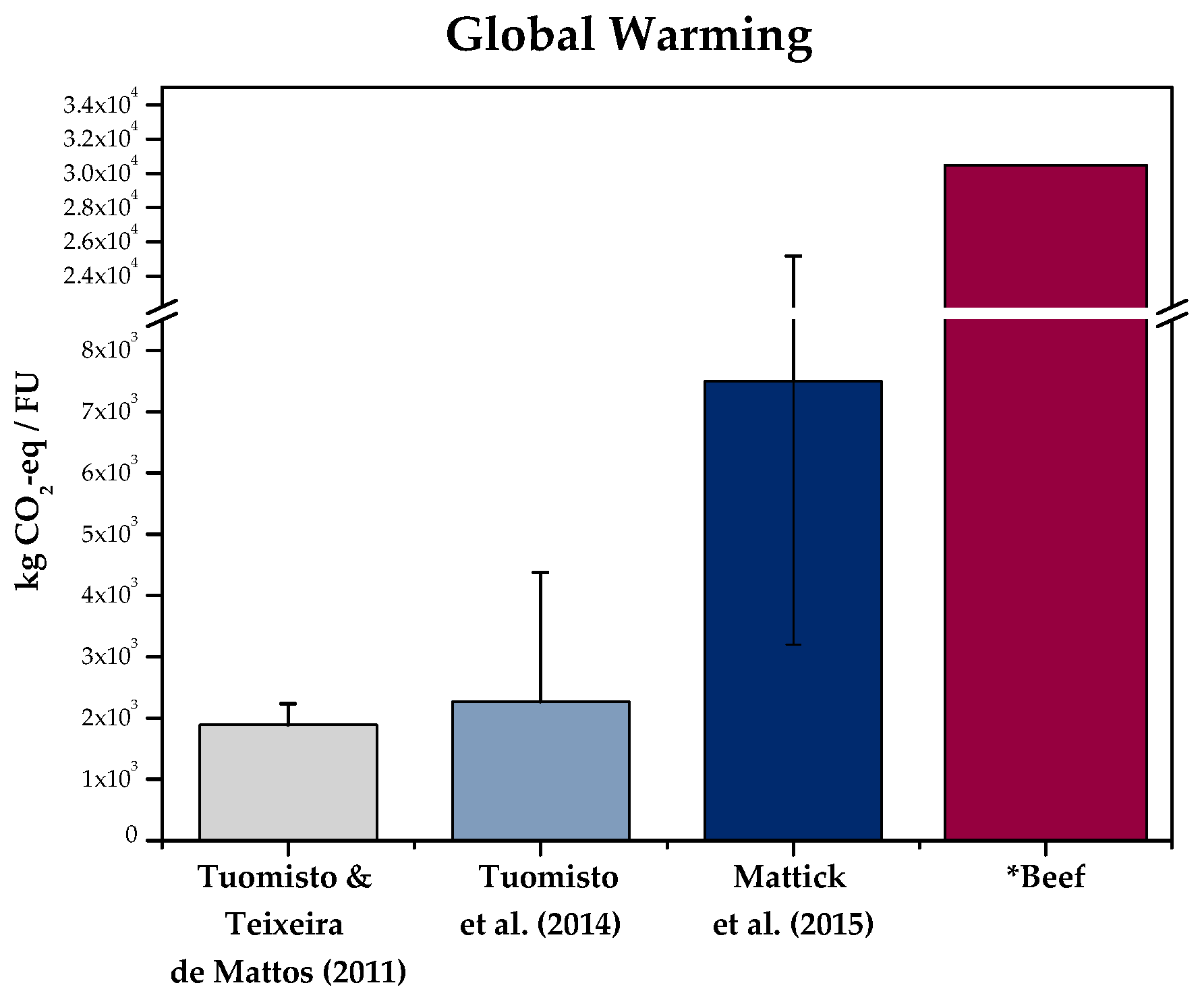
| Tuomisto and Teixeira de Mattos (2011) | Tuomisto et al. (2014) | Smetana et al. (2015) | Mattick et al. (2015) | |
|---|---|---|---|---|
| Functional Unit | 1000 kg cultured meat 1 | 1000 kg cultured meat 2 | Satisfaction of a consumer with 1 kg protein-enriched product ready for consumption 3. | 1 kg of Chinese hamster ovary (CHO) biomass 4 |
| System boundaries | Cradle-to-gate | Cradle-to-gate | Cradle-to-plate | Cradle-to-gate |
| LCI modelling principle | Attributional | Attributional | Attributional | Attributional |
| LCIA method | IPCC 2006 | IPCC 2006 | ReCiPe V1.08 and IMPACT 2002+ | Cumulative energy demand, ecological footprint and CML 2001 |
| Cell type | Stem cells from animal embryo | Stem cells from animal embryo | Stem cells from animal embryo | CHO |
| Feed source | Cyanobacteria hydrolysate | Cyanobacteria hydrolysate, wheat, and corn | Cyanobacteria hydrolysate | Serum-free media supplemented with soy hydrolysate |
| Bioreactor type | Cylinder stirred tank | Hollow fibre | Cylinder stirred tank | Stirred tank |
| Production time | 60 days | 90 days | 60 days | 11 days |
| Tuomisto and Teixeira de Mattos (2011) | Tuomisto et al. (2014) | Smetana et al. (2015) | Mattick et al. (2015) | |
|---|---|---|---|---|
| Cell collection | − | − | − | − |
| Growth factors production | − | − | − | − |
| Scaffold production | − | − | − | + |
| Bioreactor’s production | + | + | + | − |
| Cleaning bioreactor | − | − | − | + |
| Culture media recycling | − | − | − | − |
| Scaffold removal/recovery | − | − | − | − |
| Wastewater treatment | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Escobar, M.I.; Cadena, E.; Nhu, T.T.; Cooreman-Algoed, M.; De Smet, S.; Dewulf, J. Analysis of the Cultured Meat Production System in Function of Its Environmental Footprint: Current Status, Gaps and Recommendations. Foods 2021, 10, 2941. https://doi.org/10.3390/foods10122941
Rodríguez Escobar MI, Cadena E, Nhu TT, Cooreman-Algoed M, De Smet S, Dewulf J. Analysis of the Cultured Meat Production System in Function of Its Environmental Footprint: Current Status, Gaps and Recommendations. Foods. 2021; 10(12):2941. https://doi.org/10.3390/foods10122941
Chicago/Turabian StyleRodríguez Escobar, María Ignacia, Erasmo Cadena, Trang T. Nhu, Margot Cooreman-Algoed, Stefaan De Smet, and Jo Dewulf. 2021. "Analysis of the Cultured Meat Production System in Function of Its Environmental Footprint: Current Status, Gaps and Recommendations" Foods 10, no. 12: 2941. https://doi.org/10.3390/foods10122941
APA StyleRodríguez Escobar, M. I., Cadena, E., Nhu, T. T., Cooreman-Algoed, M., De Smet, S., & Dewulf, J. (2021). Analysis of the Cultured Meat Production System in Function of Its Environmental Footprint: Current Status, Gaps and Recommendations. Foods, 10(12), 2941. https://doi.org/10.3390/foods10122941







