Tea and Chicory Extract Characterization, Classification and Authentication by Non-Targeted HPLC-UV-FLD Fingerprinting and Chemometrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Instrumentation
2.3. Samples and Sample Extraction Procedure
2.4. Data Analysis
3. Results and Discussion
3.1. HPLC-UV and HPLC-FLD Fingerprints
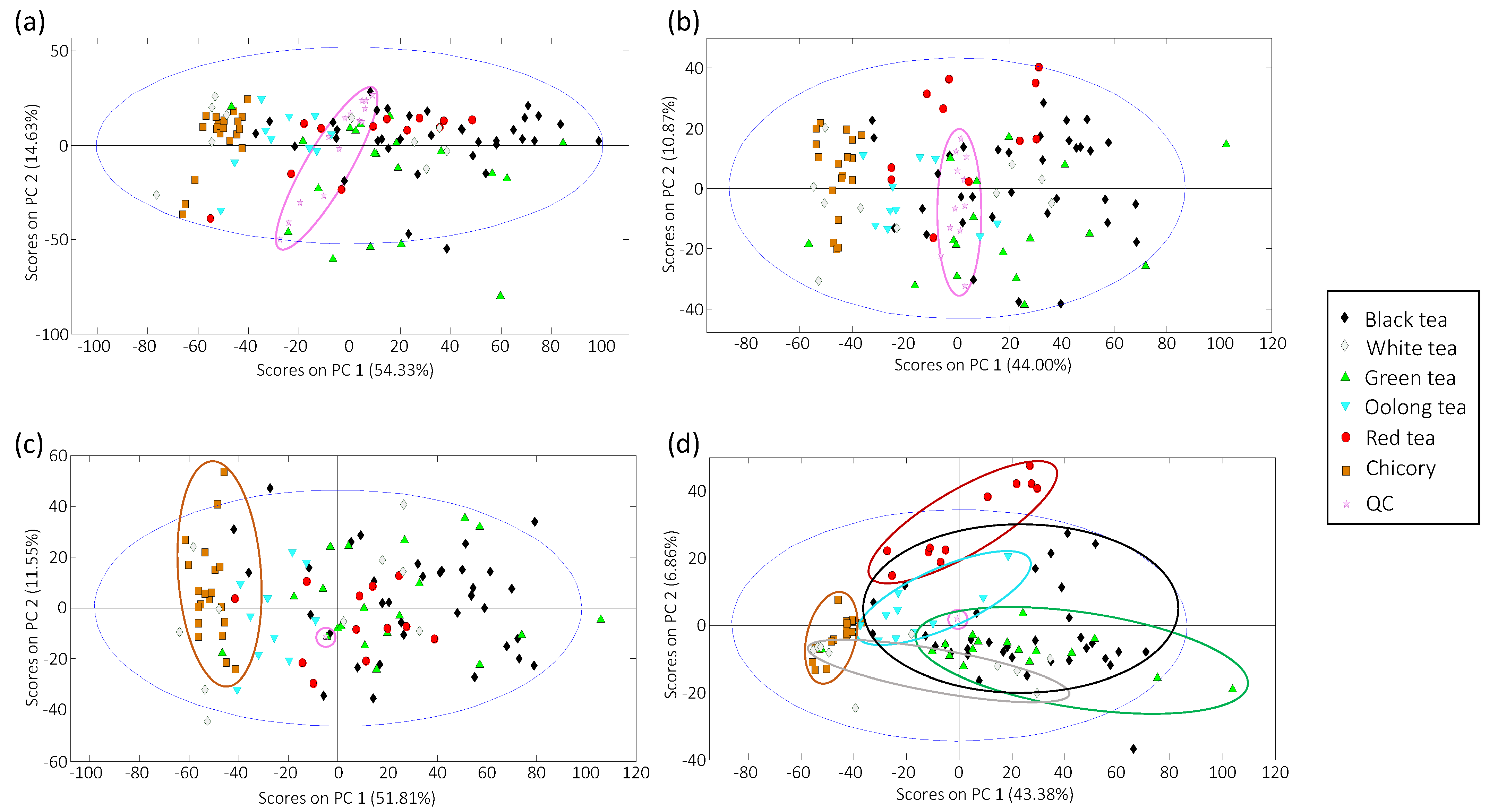
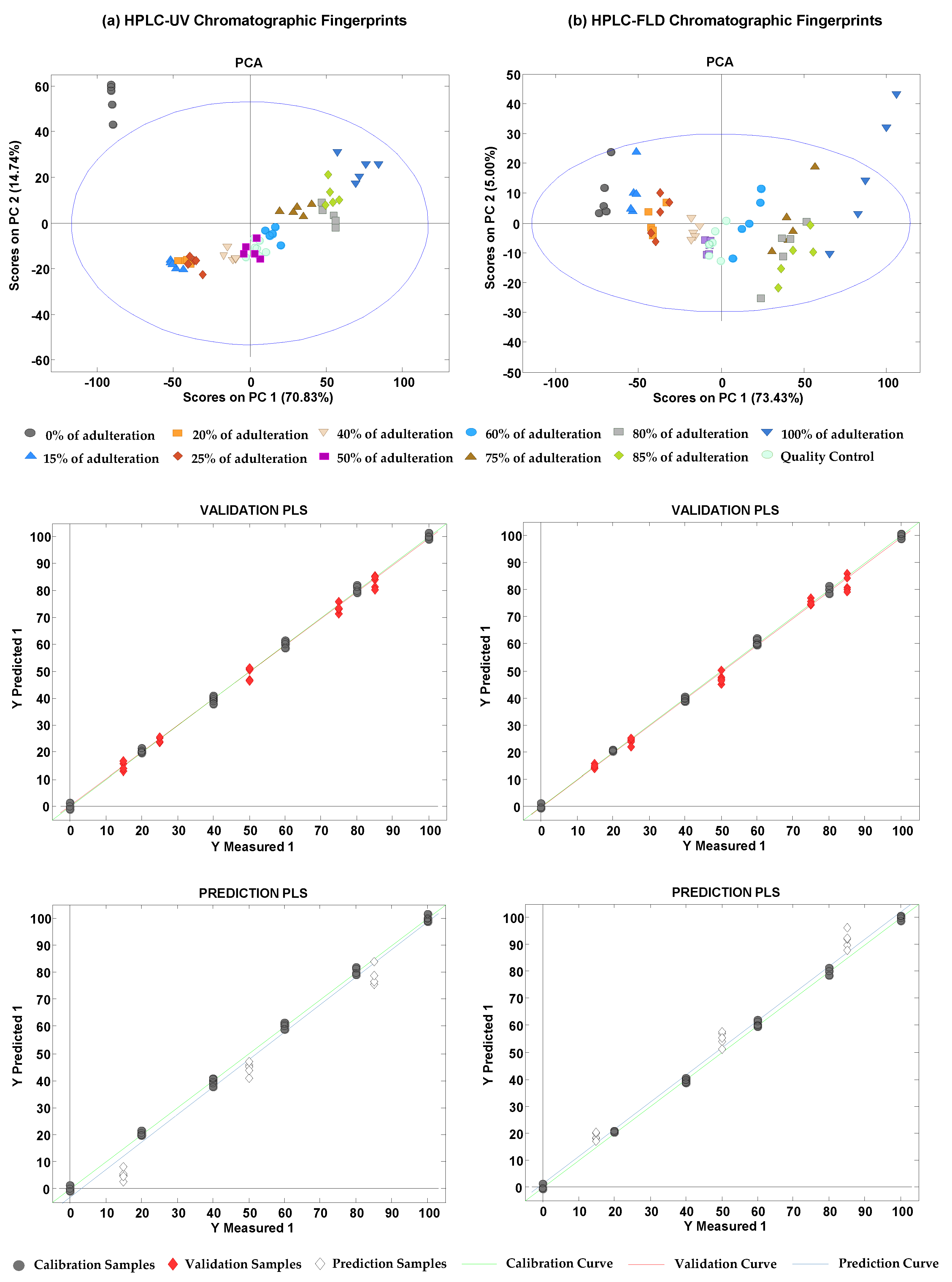
3.2. Sample Exploration by PCA
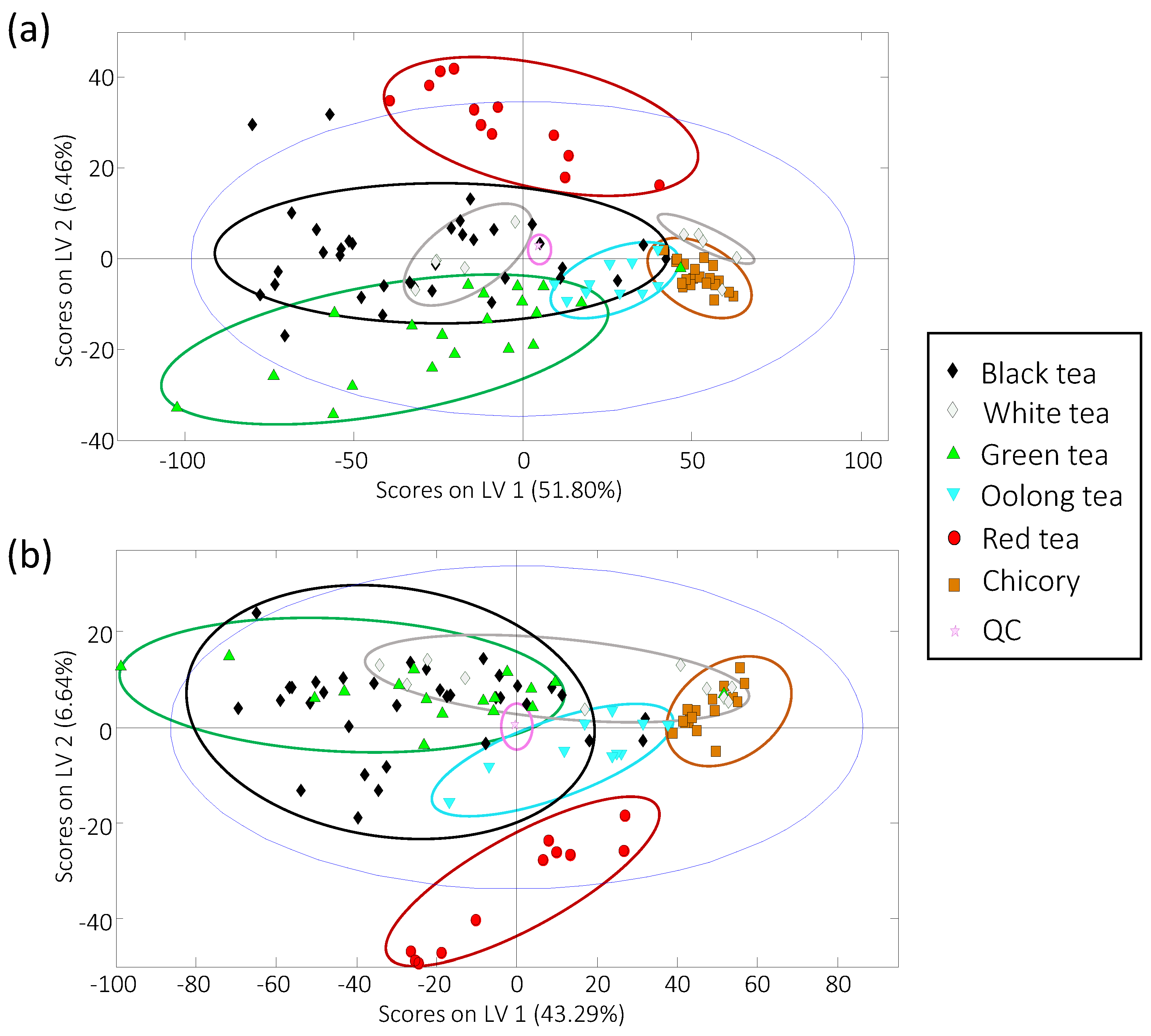
3.3. Sample Classification by Partial Least Squares-Discriminant Analysis
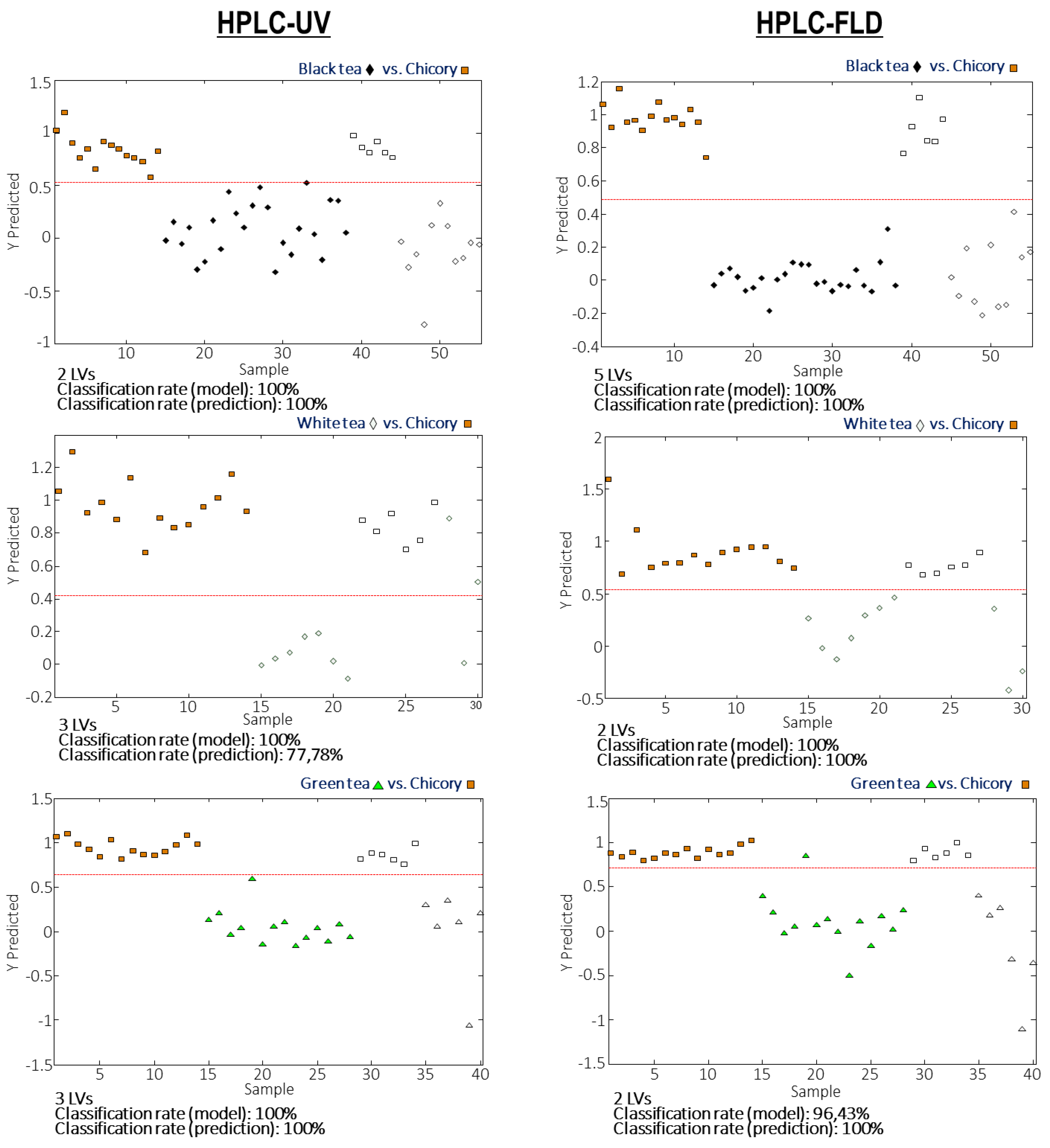
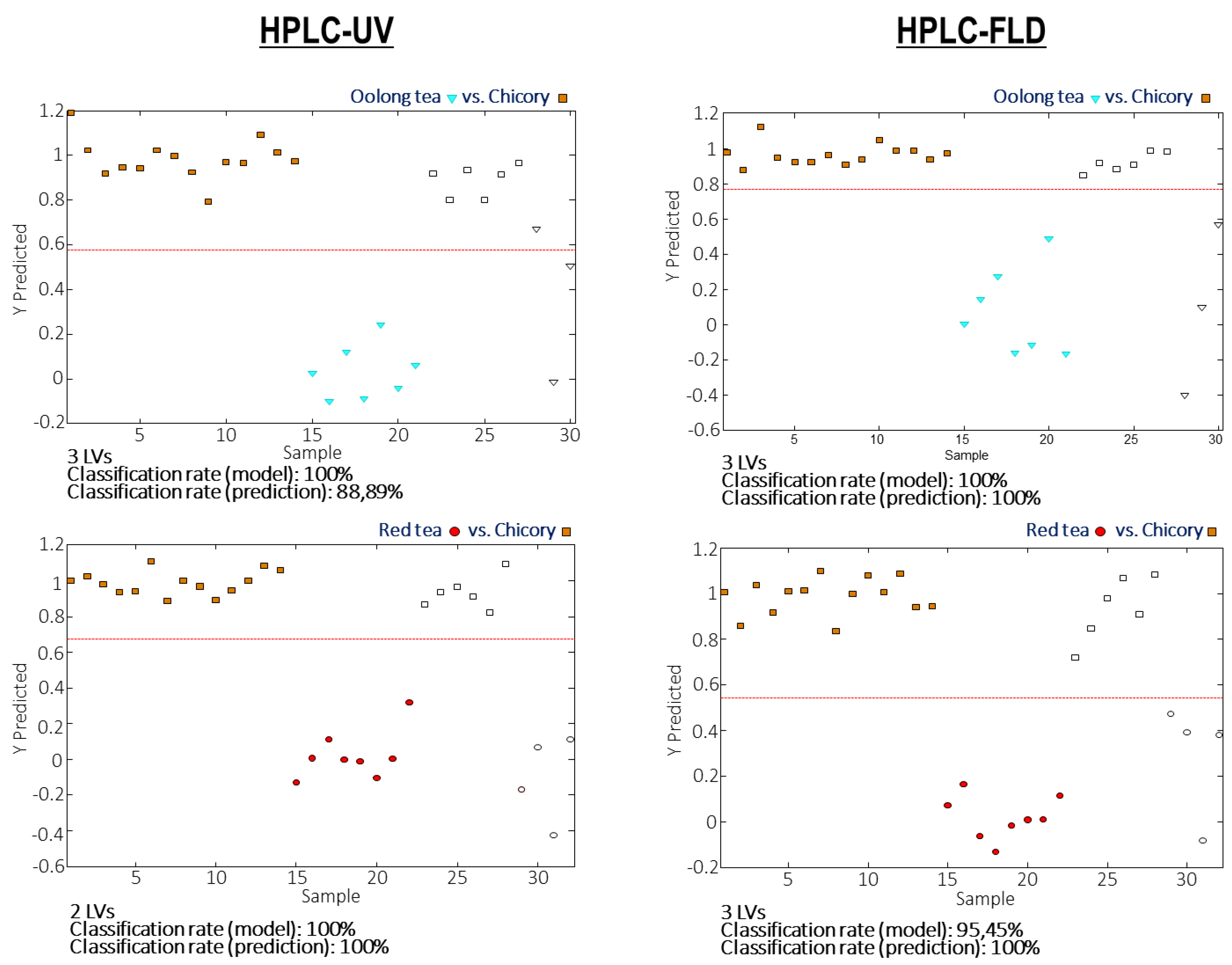
3.4. Detection and Quantitation of Tea Adulterations with Chicory by PLS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Núñez, O. Current Legal Context in Food Integrity and Authenticity. In Chromatographic and Related Separation Techniques in Food Integrity and Authenticity. Volume A: Advances in Chromatographic Techniques; Núñez, O., Campmajó, G., Eds.; World Scientific Publishing: London, UK, 2021; pp. 1–24. ISBN 978-1-78634-994-1. [Google Scholar]
- Spink, J.; Moyer, D.C. Defining the Public Health Threat of Food Fraud. J. Food Sci. 2011, 76, R157–R163. [Google Scholar] [CrossRef]
- Kennedy, S.P.; Gonzales, P.; Roungchun, J. Coffee and tea fraud. In Food Fraud. A Global Threat with Public Health and Economic Consequences; Hellberg, R.S., Everstine, K., Sklare, S.A., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2021; pp. 139–150. ISBN 978-0-12-817242-1. [Google Scholar]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial Effects of Green Tea—A Review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- McKenzie, J.S.; Jurado, J.M.; de Pablos, F. Characterisation of tea leaves according to their total mineral content by means of probabilistic neural networks. Food Chem. 2010, 123, 859–864. [Google Scholar] [CrossRef]
- Palacios-Morillo, A.; Alcázar, Á.; De Pablos, F.; Jurado, J.M. Differentiation of tea varieties using UV-Vis spectra and pattern recognition techniques. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2013, 103, 79–83. [Google Scholar] [CrossRef]
- Jie, G.; Lin, Z.; Zhang, L.; Lv, H.; He, P.; Zhao, B. Free radical scavenging effect of Pu-erh tea extracts and their protective effect on oxidative damage in human fibroblast cells. J. Agric. Food Chem. 2006, 54, 8058–8064. [Google Scholar] [CrossRef]
- Jang, M.H.; Piao, X.L.; Kim, J.M.; Kwon, S.W.; Park, J.H. Antilipogenic Effect of Green Tea Extract in C57BL/6J-Lepob/ob Mice. Phyther. Res. 2009, 23, 467–471. [Google Scholar] [CrossRef]
- Okello, E.J.; Savelev, S.U.; Perry, E.K. In vitro anti-β-secretase and dual anti-cholinesterase activities of Camellia sinensis L. (tea) relevant to treatment of dementia. Phyther. Res. 2004, 18, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Singh, S.; Tanwar, K. Recognition and Evaluation of Authenticity of Tea and Coffee. Int. J. Adv. Res. Sci. Commun. Technol. 2020, 11, 19–26. [Google Scholar]
- Pal, A.D.; Das, T. Analysis of adulteration in black tea. Int. J. Biol. Res. 2018, 3, 253–257. [Google Scholar]
- Ma, G.; Zhang, Y.; Zhang, J.; Wang, G.; Chen, L.; Zhang, M.; Liu, T.; Liu, X.; Lu, C. Determining the geographical origin of Chinese green tea by linear discriminant analysis of trace metals and rare earth elements: Taking Dongting Biluochun as an example. Food Control 2016, 59, 714–720. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Liao, R.; Zhao, J.; Yang, H.; Wang, F. Authentication of the geographical origin of Guizhou green tea using stable isotope and mineral element signatures combined with chemometric analysis. Food Control 2021, 125, 107954. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, Y.; Yan, J.; Huang, R.; Zhao, X.; Zheng, X.; Mo, M.; Tan, S.; Tong, H. CNHO and mineral element stable isotope ratio analysis for authentication in tea. J. Food Compos. Anal. 2020, 91, 103513. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, Y.; Zhang, Y.; Shi, Y.; Hu, G.; Zhu, J.; Rogers, K.M. Geographical traceability of Chinese green tea using stable isotope and multi-element chemometrics. Rapid Commun. Mass Spectrom. 2019, 33, 778–788. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Li, Y.C.; Wen, X.; Peng, Y.; Ni, X. Use of mineral multi-elemental analysis to authenticate geographical origin of different cultivars of tea in Guizhou, China. J. Sci. Food Agric. 2020, 100, 3046–3055. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.S. A minireview of analytical methods for the geographical origin analysis of teas (Camellia sinensis). Crit. Rev. Food Sci. Nutr. 2012, 52, 775–780. [Google Scholar] [CrossRef]
- Xia, W.; Li, Z.; Yu, C.C.; Liu, Z.; Nie, J.; Li, C.; Shao, S.; Zhang, Y.; Rogers, K.M.; Yuan, Y. Understanding processing, maturity and harvest period effects to authenticate early-spring Longjing tea using stable isotopes and chemometric analyses. Food Control 2021, 124, 107907. [Google Scholar] [CrossRef]
- Inarejos-García, A.M.; Helbig, I.; Klette, P.; Weber, S.; Maeder, J.; Morlock, G.E. Authentication of Commercial Powdered Tea Extracts (Camellia sinensis L.) by Gas Chromatography. ACS Food Sci. Technol. 2021, 1, 596–604. [Google Scholar] [CrossRef]
- Fang, S.; Huang, W.J.; Wei, Y.; Tao, M.; Hu, X.; Li, T.; Kalkhajeh, Y.K.; Deng, W.W.; Ning, J. Geographical origin traceability of Keemun black tea based on its non-volatile composition combined with chemometrics. J. Sci. Food Agric. 2019, 99, 6937–6943. [Google Scholar] [CrossRef]
- Campmajó, G.; Núñez, N.; Núñez, O. The Role of Liquid Chromatography-Mass Spectrometry in Food Integrity and Authenticity. In Mass Spectrometry—Future Perceptions and Applications; Kamble, G.S., Ed.; IntechOpen: London, UK, 2019; pp. 3–24. ISBN 978-1-83962-756-9. [Google Scholar]
- Campmajó, G.; Núñez, O. Chromatographic Fingerprinting Approaches in Food Authentication. In Chromatographic and Related Separation Techniques in Food Integrity and Authenticity. Volume A: Advances in Chromatographic Techniques; Núñez, O., Campmajó, G., Eds.; World Scientific Publishing: London, UK, 2021; pp. 137–165. ISBN 978-1-78634-994-1. [Google Scholar]
- Budínová, G.; Vláčil, D.; Mestek, O.; Volka, K. Application of infrared spectroscopy to the assessment of authenticity of tea. Talanta 1998, 47, 255–260. [Google Scholar] [CrossRef]
- He, W.; Zhou, J.; Cheng, H.; Wang, L.; Wei, K.; Wang, W.; Li, X. Validation of origins of tea samples using partial least squares analysis and Euclidean distance method with near-infrared spectroscopy data. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2012, 86, 399–404. [Google Scholar] [CrossRef]
- Yan, S.M.; Liu, J.P.; Xu, L.; Fu, X.S.; Cui, H.F.; Yun, Z.Y.; Yu, X.P.; Ye, Z.H. Rapid discrimination of the geographical origins of an oolong tea (Anxi-Tieguanyin) by near-infrared spectroscopy and partial least squares discriminant analysis. J. Anal. Methods Chem. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Firmani, P.; De Luca, S.; Bucci, R.; Marini, F.; Biancolillo, A. Near infrared (NIR) spectroscopy-based classification for the authentication of Darjeeling black tea. Food Control 2019, 100, 292–299. [Google Scholar] [CrossRef]
- Liu, P.; Wen, Y.; Huang, J.; Xiong, A.; Wen, J.; Li, H.; Huang, Y.; Zhu, X.; Ai, S.; Wu, R. A novel strategy of near-infrared spectroscopy dimensionality reduction for discrimination of grades, varieties and origins of green tea. Vib. Spectrosc. 2019, 105, 102984. [Google Scholar] [CrossRef]
- Mishra, P.; Nordon, A.; Tschannerl, J.; Lian, G.; Redfern, S.; Marshall, S. Near-infrared hyperspectral imaging for non-destructive classification of commercial tea products. J. Food Eng. 2018, 238, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Aboulwafa, M.M.; Youssef, F.S.; Gad, H.A.; Sarker, S.D.; Nahar, L.; Al-Azizi, M.M.; Ashour, M.L. Authentication and discrimination of green tea samples using UV–vis, FTIR and HPLC techniques coupled with chemometrics analysis. J. Pharm. Biomed. Anal. 2019, 164, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Diniz, P.H.G.D.; Barbosa, M.F.; De Melo Milanez, K.D.T.; Pistonesi, M.F.; De Araújo, M.C.U. Using UV-Vis spectroscopy for simultaneous geographical and varietal classification of tea infusions simulating a home-made tea cup. Food Chem. 2016, 192, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.Q.; Yin, X.L.; Gu, H.W.; Sun, W.; Ding, B.; Hu, X.C.; Ma, L.A.; Wei, S.D.; Liu, Z.; Ye, S.Y. HPLC-DAD fingerprints combined with chemometric techniques for the authentication of plucking seasons of Laoshan green tea. Food Chem. 2021, 347, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Wei, K.; Cheng, H.; He, W.; Li, X.; Gong, W. Geographical tracing of Xihu Longjing tea using high performance liquid chromatography. Food Chem. 2014, 146, 98–103. [Google Scholar] [CrossRef]
- Navratilova, K.; Hrbek, V.; Kratky, F.; Hurkova, K.; Tomaniova, M.; Pulkrabova, J.; Hajslova, J. Green tea: Authentication of geographic origin based on UHPLC-HRMS fingerprints. J. Food Compos. Anal. 2019, 78, 121–128. [Google Scholar] [CrossRef]
- Sentellas, S.; Saurina, J. The Role of Chemometrics in Food Integrity and Authenticity. In Chromatographic and Related Separation Techniques in Food Integrity and Authenticity. Volume A: Advances in Chromatographic Techniques; Núñez, O., Campmajó, G., Eds.; World Scientific Publishing: London, UK, 2021; pp. 167–200. ISBN 978-1-78634-994-1. [Google Scholar]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; de Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]

| Time (min) | Elution | % Methanol | Flow Rate (µL/min) |
|---|---|---|---|
| 0–15 | Linear gradient | 20–75% | 400 |
| 15–17 | Linear gradient | 75–95% | 400 |
| 17–19 | Isocratic | 95% | 400 |
| 19–19.2 | Linear gradient | 95–20% | 400 |
| 19.2–25 | Isocratic | 20% | 400 |
| Sample Class | Sample Type | Number of Samples |
|---|---|---|
| Tea | Black tea | 35 |
| Green tea | 20 | |
| Oolong tea | 10 | |
| Red tea | 12 | |
| White tea | 10 | |
| Chicory | Chicory | 20 |
| Tea (%) | Chicory (% Adulterant) | |
|---|---|---|
| Calibration set | 100 | 0 |
| 80 | 20 | |
| 60 | 40 | |
| 40 | 60 | |
| 20 | 80 | |
| 0 | 100 | |
| Validation set | 85 | 15 |
| 75 | 25 | |
| 50 | 50 | |
| 25 | 75 | |
| 15 | 85 |
| HPLC-UV Fingerprints | ||||||
| LVs | Linearity (R2) | Calibration Error (%) | Cross-Validation Error (%) | External Validation Error (%) | Prediction Error (%) | |
| Red tea | 4 | 0.999 | 1.0 | 1.5 | 2.0 | 8.0 |
| Green tea | 5 | 0.999 | 0.5 | 1.3 | 1.0 | 15.7 |
| Black tea | 4 | 0.999 | 1.0 | 3.6 | 2.1 | 21.6 |
| Oolong tea | 2 | 0.998 | 1.4 | 6.4 | 1.8 | 15.0 |
| White tea | 3 | 0.999 | 1.3 | 2.3 | 2.2 | 80.7 |
| HPLC-FLD Fingerprints | ||||||
| LVs | Linearity (R2) | Calibration Error (%) | Cross-Validation Error (%) | External Validation Error (%) | Prediction Error (%) | |
| Red tea | 4 | 0.999 | 0.9 | 2.4 | 2,5 | 5.7 |
| Green tea | 5 | 0.999 | 0.3 | 1.1 | 1.4 | 18.3 |
| Black tea | 3 | 0.999 | 1.1 | 1.6 | 1.6 | 2.6 |
| Oolong tea | 2 | 0.998 | 1.4 | 1.9 | 1.4 | 13.0 |
| White tea | 3 | 0.999 | 0.8 | 2.3 | 3.7 | 132.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pons, J.; Bedmar, À.; Núñez, N.; Saurina, J.; Núñez, O. Tea and Chicory Extract Characterization, Classification and Authentication by Non-Targeted HPLC-UV-FLD Fingerprinting and Chemometrics. Foods 2021, 10, 2935. https://doi.org/10.3390/foods10122935
Pons J, Bedmar À, Núñez N, Saurina J, Núñez O. Tea and Chicory Extract Characterization, Classification and Authentication by Non-Targeted HPLC-UV-FLD Fingerprinting and Chemometrics. Foods. 2021; 10(12):2935. https://doi.org/10.3390/foods10122935
Chicago/Turabian StylePons, Josep, Àlex Bedmar, Nerea Núñez, Javier Saurina, and Oscar Núñez. 2021. "Tea and Chicory Extract Characterization, Classification and Authentication by Non-Targeted HPLC-UV-FLD Fingerprinting and Chemometrics" Foods 10, no. 12: 2935. https://doi.org/10.3390/foods10122935
APA StylePons, J., Bedmar, À., Núñez, N., Saurina, J., & Núñez, O. (2021). Tea and Chicory Extract Characterization, Classification and Authentication by Non-Targeted HPLC-UV-FLD Fingerprinting and Chemometrics. Foods, 10(12), 2935. https://doi.org/10.3390/foods10122935








