Acrylamide in Baby Foods: A Probabilistic Exposure Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Food Sampling
2.2. Reagents and Equipment
2.3. Analytical Standards
2.4. Analytical Method
2.5. Bromination Procedure
2.6. GC/MS Detection
2.7. Analytical Quality Assurance and Method Performance
2.8. Monte Carlo Simulations
2.9. Dietary Exposure to Acrylamide
3. Results and Discussion
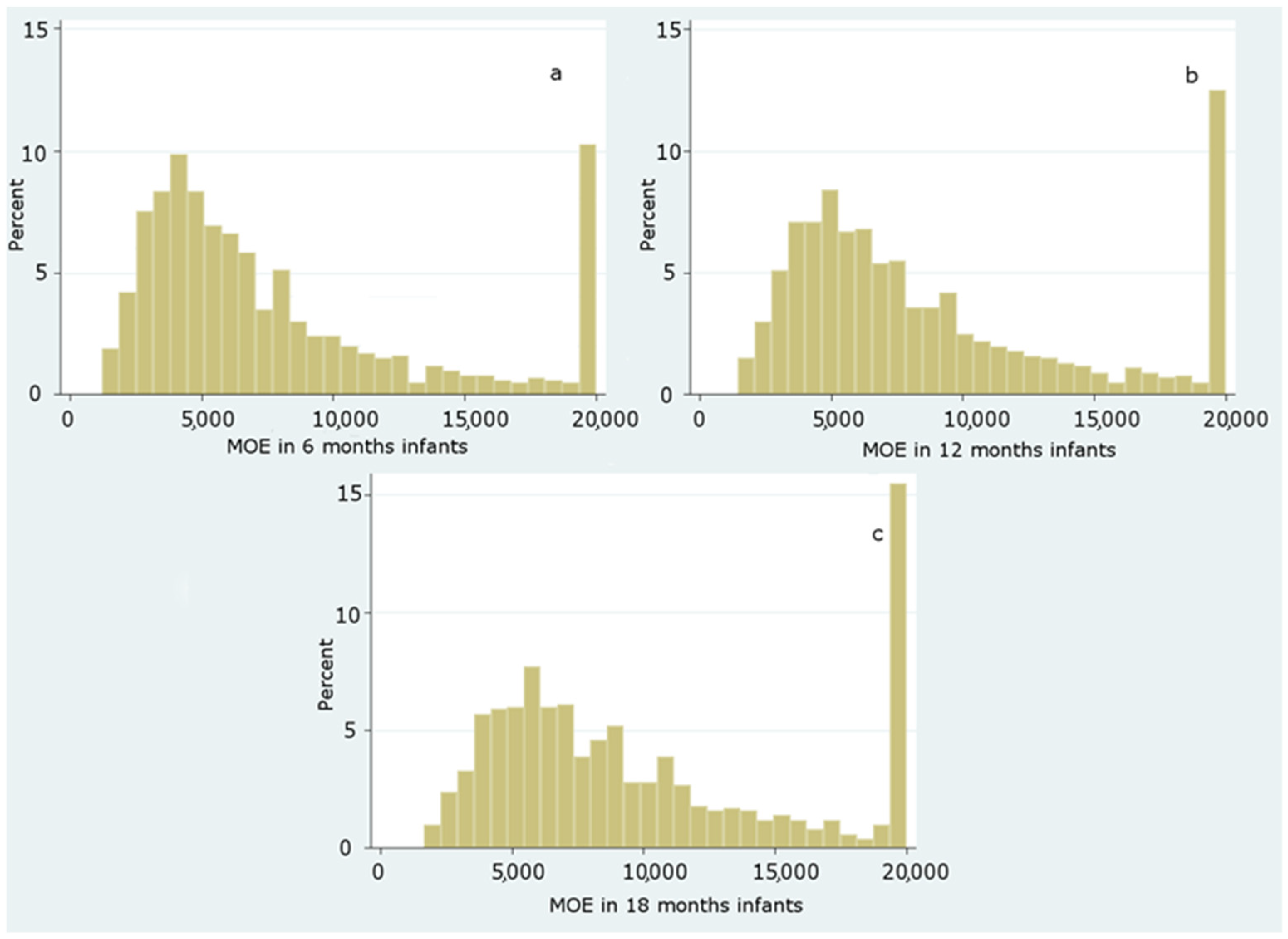
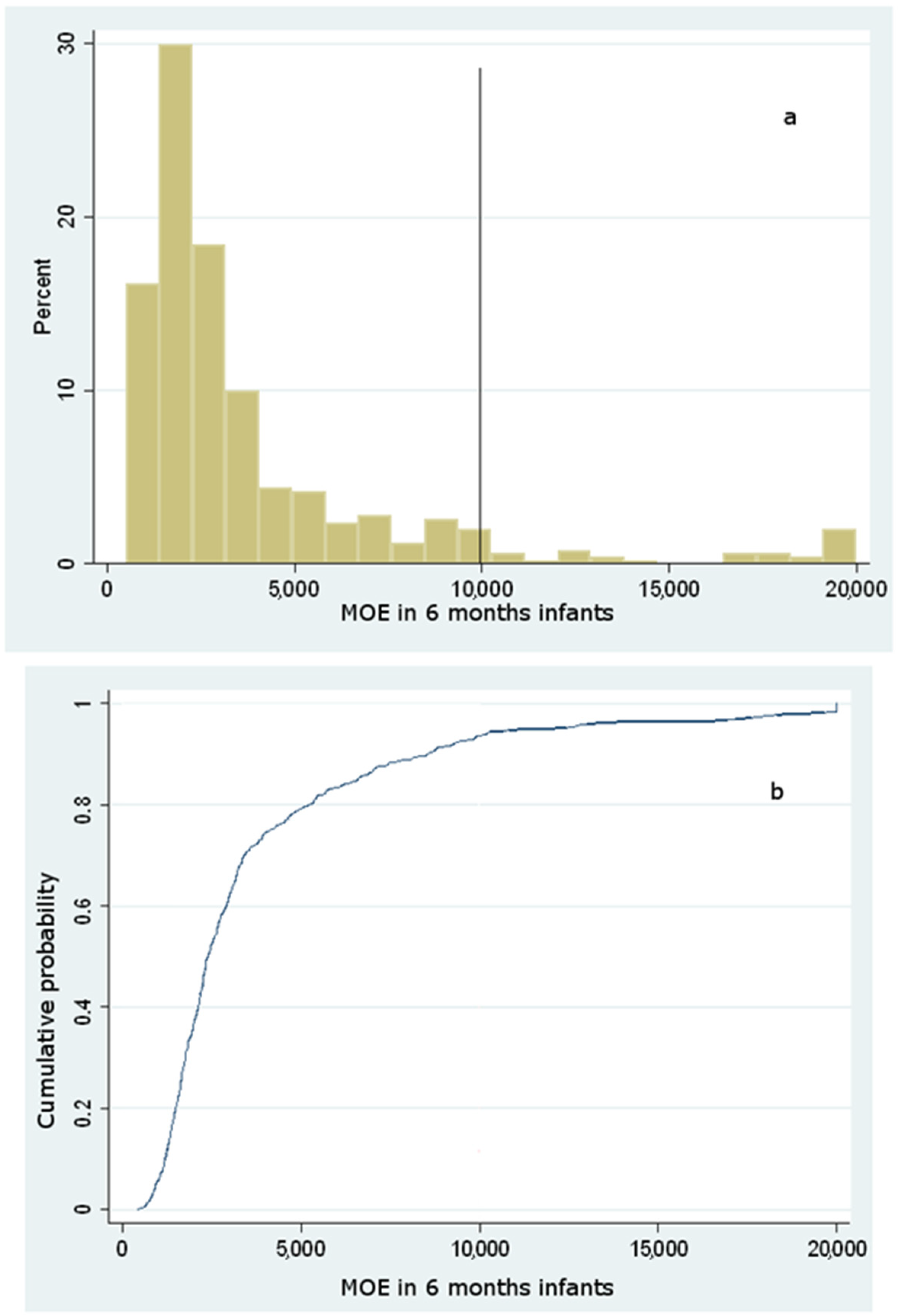
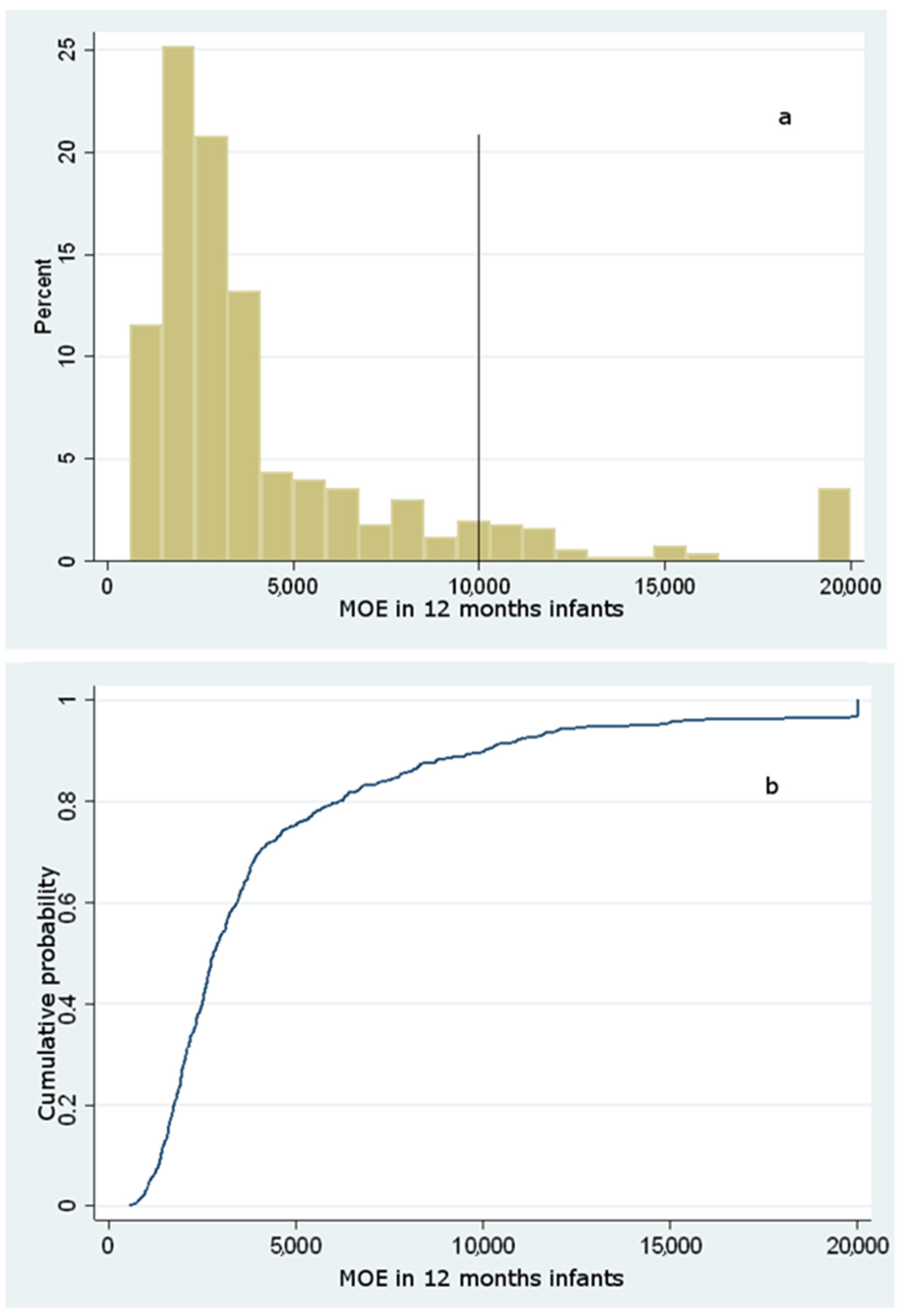
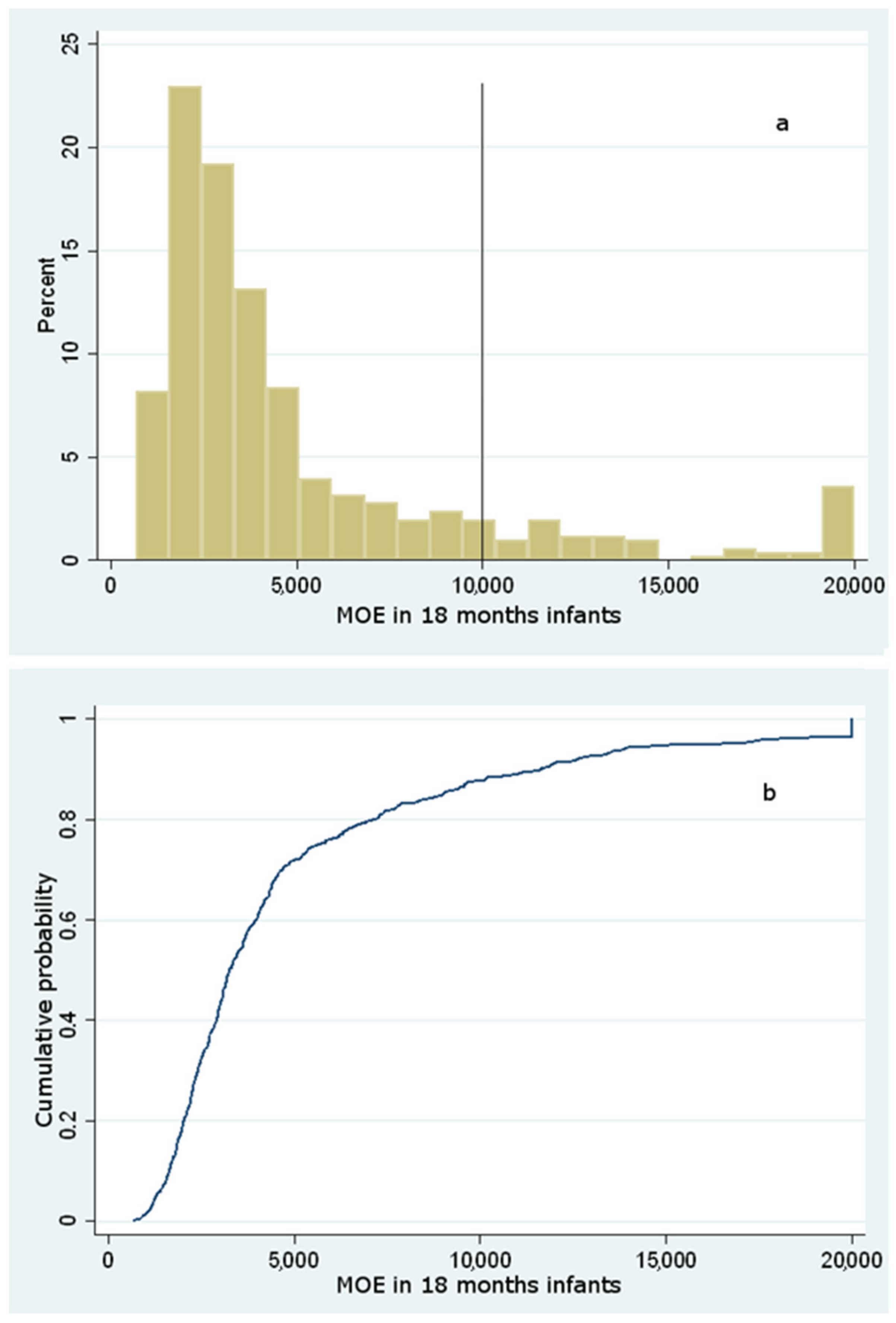
Dietary Exposure to Acrylamide of Infants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Esposito, F.; Nardone, A.; Fasano, E.; Triassi, M.; Cirillo, T. Determination of Acrylamide Levels in Potato Crisps and Other Snacks and Exposure Risk Assessment through a Margin of Exposure Approach. Food Chem. Toxicol. 2017, 108, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rifai, L.; Saleh, F.A. A Review on Acrylamide in Food: Occurrence, Toxicity, and Mitigation Strategies. Int. J. Toxicol. 2020, 39, 93–102. [Google Scholar] [CrossRef]
- Branciari, R.; Roila, R.; Ranucci, D.; Altissimi, M.S.; Mercuri, M.L.; Haouet, N.M. Estimation of Acrylamide Exposure in Italian Schoolchildren Consuming a Canteen Menu: Health Concern in Three Age Groups. Int. J. Food Sci. Nutr. 2020, 71, 122–131. [Google Scholar] [CrossRef]
- Surma, M.; Sadowska-Rociek, A.; Cieślik, E.; Sznajder-Katarzyńska, K. Optimization of QuEChERS Sample Preparation Method for Acrylamide Level Determination in Coffee and Coffee Substitutes. Microchem. J. 2017, 131, 98–102. [Google Scholar] [CrossRef]
- Swedish National Food Administration (SNFA). Analytical Methodology and Survey Results for Acrylamide in Foods. 2002. Press release, 24 April 2002. Available online: http://www.slv.se/engdefault.asp (accessed on 25 June 2021).
- Shahrbabki, P.E.; Hajimohammadi, B.; Shoeibi, S.; Elmi, M.; Yousefzadeh, A.; Conti, G.O.; Ferrante, M.; Amirahmad, M.; Fakhri, Y.; Khaneghah, A.M. Probabilistic Non-Carcinogenic and Carcinogenic Risk Assessments (Monte Carlo Simulation Method) of the Measured Acrylamide Content in Tah-dig Using QuEChERS Extraction and UHPLC-MS/MS. Food Chem. Toxicol. 2018, 118, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Mojska, H.; Gielecińska, I.; Stoś, K. Determination of Acrylamide Level in Commercial Baby Foods and an Assessment of Infant Dietary Exposure. Food Chem. Toxicol. 2012, 50, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Acrylamide: A Cooking Carcinogen? Chem. Res. Toxicol. 2000, 13, 517–522. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of Acrylamide, a Carcinogen Formed in Heated Foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Friedman, M.A.; Dulak, L.H.; Stedham, M.A. A Lifetime Oncogenicity Study in Rats with Acrylamide. Toxicol. Sci. 1995, 27, 95–105. [Google Scholar] [CrossRef]
- Johnson, K.A.; Gorzinski, S.J.; Bodner, K.M.; Campbell, R.A.; Wolf, C.H.; Friedman, M.A.; Mast, R.W. Chronic Toxicity and Oncogenicity Study on Acrylamide Incorporated in the Drinking Water of Fischer 344 Rats. Toxicol. Appl. Pharmacol. 1986, 85, 154–168. [Google Scholar] [CrossRef]
- IARC. IARC monographs on the evaluation of carcinogenic risks to humans. In Textbook of Some Industrial Chemicals; IARC: Lyon, France, 1994; Volume 60, pp. 389–433. [Google Scholar]
- Raju, J.; Roberts, J.; Taylor, M.; Patry, D.; Chomyshyn, E.; Caldwell, D.; Cooke, G.; Mehta, R. Toxicological Effects of Short-Term Dietary Acrylamide Exposure in Male F344 Rats. Environ. Toxicol. Pharmacol. 2015, 39, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.; Latendresse, J.R.; Muskhelishvili, L.; Patton, R.; Bowyer, J.F.; Thomas, M.; Doerge, D.R. Effects of Acrylamide Exposure on Serum Hormones, Gene Expression, Cell Proliferation, and Histopathology in Male Reproductive Tissues of Fischer 344 Rats. Toxicol. Lett. 2012, 211, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; El-Demerdash, F.M. Acrylamide-Induced Oxidative Stress and Biochemical Perturbations in Rats. Toxicology 2006, 219, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Zakłos-Szyda, M.; Żyżelewicz, D.; Koszucka, A.; Motyl, I. Acrylamide Decreases Cell Viability, and Provides Oxidative Stress, DNA Damage, and Apoptosis in Human Colon Adenocarcinoma Cell Line Caco-2. Molecules 2020, 25, 368. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Das, S.; Teoh, S.L. Dietary Acrylamide and the Risks of Developing Cancer: Facts to Ponder. Front. Nutr. 2018, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Kadawathagedara, M.; Tong, A.C.H.; Heude, B.; Forhan, A.; Charles, M.A.; Sirot, V.; Botton, J.; Eden Mother-Child Cohort Study Group. Dietary Acrylamide Intake during Pregnancy and Anthropometry at Birth in the French EDEN Mother-Child Cohort Study. Environ. Res. 2016, 149, 189–196. [Google Scholar] [CrossRef]
- Pelucchi, C.; Bosetti, C.; Galeone, C.; La Vecchia, C. Dietary Acrylamide and Cancer Risk: An Updated Meta-Analysis. Int. J. Cancer 2015, 136, 2912–2922. [Google Scholar] [CrossRef] [PubMed]
- Belhadj Benziane, A.; Bouras, A.D.; Mezaini, A.; Belhadri, A.; Benali, M. Effect of Oral Exposure to Acrylamide on Biochemical and Hematologic Parameters in Wistar Rats. Drug Chem. Toxicol. 2019, 42, 157–166. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Acrylamide in Food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef] [Green Version]
- Esposito, F.; Fasano, E.; De Vivo, A.; Velotto, S.; Sarghini, F.; Cirillo, T. Processing Effects on Acrylamide Content in Roasted Coffee Production. Food Chem. 2020, 319, 126550. [Google Scholar] [CrossRef]
- Esposito, F.; Velotto, S.; Rea, T.; Stasi, T.; Cirillo, T. Occurrence of Acrylamide in Italian Baked Products and Dietary Exposure Assessment. Molecules 2020, 25, 4156. [Google Scholar] [CrossRef]
- Eslamizad, S.; Kobarfard, F.; Tsitsimpikou, C.; Tsatsakis, A.; Tabib, K.; Yazdanpanah, H. Health Risk Assessment of Acrylamide in Bread in Iran Using LC-MS/MS. Food Chem. Toxicol. 2019, 126, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Galani, J.H.; Patel, N.J.; Talati, J.G. Acrylamide-Forming Potential of Cereals, Legumes and Roots and Tubers Analyzed by UPLC-UV. Food Chem. Toxicol. 2017, 108, 244–248. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Y.; Zhu, F.; Ma, Y.; Li, X.; Miao, H.; Wu, Y. Dietary Exposure of Acrylamide from the Fifth Chinese Total Diet Study. Food Chem. Toxicol. 2016, 87, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.S.; Briddon, A.; Dodson, A.T.; Muttucumaru, N.; Halford, N.G.; Mottram, D.S. Acrylamide in Potato Crisps Prepared from 20 UK-Grown Varieties: Effects of Variety and Tuber Storage Time. Food Chem. 2015, 182, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mesías, M.; Morales, F.J. Acrylamide in Commercial Potato Crisps from Spanish Market: Trends from 2004 to 2014 and Assessment of the Dietary Exposure. Food Chem. Toxicol. 2015, 81, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnakumar, T.; Visvanathan, R. Acrylamide in Food Products: A Review. Int. J. Food Process. Technol. 2014, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Başaran, B.; Aydın, F.; Kaban, G. The Determination of Acrylamide Content in Brewed Coffee Samples Marketed in Turkey. Food Addit. Contam. Part A 2020, 37, 280–287. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Fakhri, Y.; Nematollahi, A.; Seilani, F.; Vasseghian, Y. The Concentration of Acrylamide in Different Food Products: A Global Systematic Review, Meta-Analysis, and Meta-Regression. Food Rev. Int. 2020, 1–19. [Google Scholar] [CrossRef]
- EC Commission Regulation (EU) 2017/2158: Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food. Off. J. Eur. Union 2017, 2017, 24–44.
- Tateo, F.; Bononi, M. Preliminary Study on Acrylamide in Baby Foods on the Italian Market. Ital. J. Food Sci. 2003, 15, 593–599. [Google Scholar]
- Capei, R.; Pettini, L.; Nostro, A.L.; Pesavento, G. Occurrence of Acrylamide in Breakfast Cereals and Biscuits Available in Italy. J. Prev. Med. Public Health 2015, 56, E190. [Google Scholar] [CrossRef]
- Fernandes, J.O.; Soares, C. Application of Matrix Solid-Phase Dispersion in the Determination of Acrylamide in Potato Chips. J. Chromatogr. A 2007, 1175, 1–6. [Google Scholar] [CrossRef]
- Hensawang, S.; Chanpiwat, P. Analysis and Probabilistic Risk Assessment of Bioaccessible Arsenic in Polished and Husked Jasmine Rice Sold in Bangkok. Chemosphere 2018, 207, 637–648. [Google Scholar] [CrossRef] [PubMed]
- WHO. Child Growth Standards. 2006. Available online: http://www.who.int/childgrowth/standards/weight_for_age/en/ (accessed on 25 June 2021).
- Mojska, H.; Gielecińska, I.; Szponar, L.; Ołtarzewski, M. Estimation of the Dietary Acrylamide Exposure of the Polish Population. Food Chem. Toxicol. 2010, 48, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Michalak, J.; Gujska, E.; Kuncewicz, A. RP-HPLC-DAD Studies on Acrylamide in Cereal-Based Baby Foods. J. Food Compos. Anal. 2013, 32, 68–73. [Google Scholar] [CrossRef]
- Cengiz, M.F.; Gündüz, C.P.B. An Eco-Friendly, Quick and Cost-Effective Method for the Quantification of Acrylamide in Cereal-Based Baby Foods. J. Sci. Food Agric. 2014, 94, 2534–2540. [Google Scholar] [CrossRef]
- Başaran, B.; Aydın, F. Determination of Acrylamide Levels in Infant Formulas and Baby Biscuits Sold in Turkey. Lett. Appl. NanoBioScience 2022, 11, 3155–3165. [Google Scholar] [CrossRef]
- Lambert, M.; Inthavong, C.; Hommet, F.; Leblanc, J.C.; Hulin, M.; Guérin, T. Levels of Acrylamide in Foods Included in “The First French Total Diet Study on Infants and Toddlers”. Food Chem. 2018, 240, 997–1004. [Google Scholar] [CrossRef]
- Constantin, O.E.; Kukurova, K.; Dasko, L.; Stanciuc, N.; Ciesarova, Z.; Croitoru, C.; Rapeanu, G. Effect of Thermal Processing on Simultaneous Formation of Acrylamide and Hydroxymethylfurfural in Plum Puree. Polish J. Food Nutr. Sci. 2019, 69, 179–189. [Google Scholar] [CrossRef]
- Becalski, A.; Brady, B.; Feng, S.; Gauthier, B.R.; Zhao, T. Formation of Acrylamide at Temperatures Lower Than 100 °C: The Case of Prunes and a Model Study. Food Addit. Contam. 2011, 28, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.; Roasto, M.; Reinik, M.; Nelis, K.; Nurk, E.; Elias, T. Acrylamide in Commercial Foods and Intake by Infants in Estonia. Food Addit. Contam. Part A 2017, 34, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
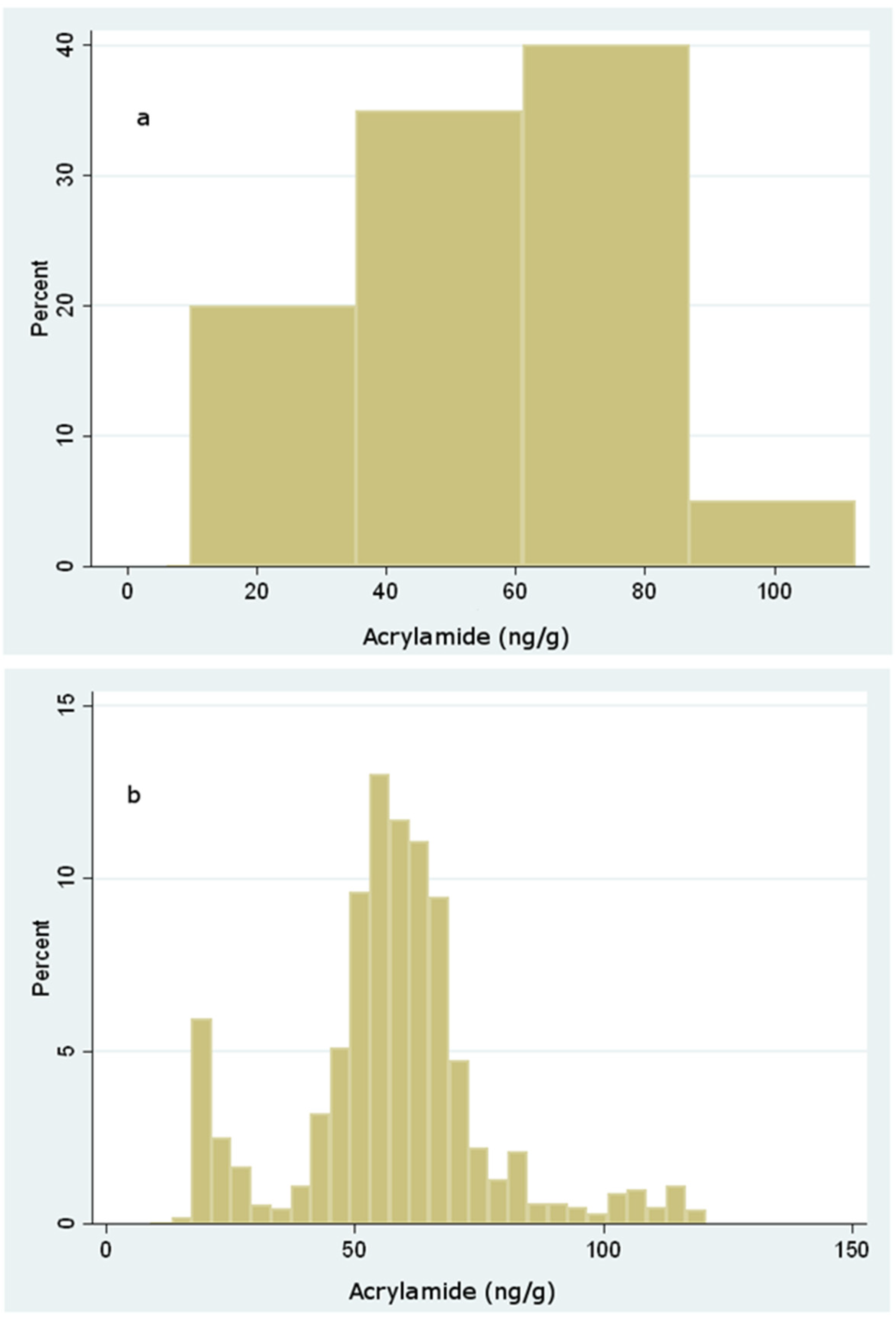
| Sample | Acrylamide (ng/g) | ||||
|---|---|---|---|---|---|
| Mean ± SD | Median | 95th Percentile | Min | Max | |
| Biscuits (n = 20) | 61 ± 20 | 61 | 79 | <LOQ | 109 |
| Ground biscuits (n = 20) | 39 ± 11 | 36 | 63 | <LOQ | 55 |
| Multigrain meal (n = 14) | <LOD | <LOD | <LOD | <LOD | <LOD |
| Sweet snacks (n = 12) | <LOQ | <LOQ | <LOQ | <LOD | <LOQ |
| Savory snacks (n = 12) | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Plum puree (n = 12) | <LOQ | <LOQ | 32 | <LOD | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, F.; Nolasco, A.; Caracciolo, F.; Velotto, S.; Montuori, P.; Romano, R.; Stasi, T.; Cirillo, T. Acrylamide in Baby Foods: A Probabilistic Exposure Assessment. Foods 2021, 10, 2900. https://doi.org/10.3390/foods10122900
Esposito F, Nolasco A, Caracciolo F, Velotto S, Montuori P, Romano R, Stasi T, Cirillo T. Acrylamide in Baby Foods: A Probabilistic Exposure Assessment. Foods. 2021; 10(12):2900. https://doi.org/10.3390/foods10122900
Chicago/Turabian StyleEsposito, Francesco, Agata Nolasco, Francesco Caracciolo, Salvatore Velotto, Paolo Montuori, Raffaele Romano, Tommaso Stasi, and Teresa Cirillo. 2021. "Acrylamide in Baby Foods: A Probabilistic Exposure Assessment" Foods 10, no. 12: 2900. https://doi.org/10.3390/foods10122900






