A Novel Photoelectrochemical Aptamer Sensor Based on CdTe Quantum Dots Enhancement and Exonuclease I-Assisted Signal Amplification for Listeria monocytogenes Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Apparatus
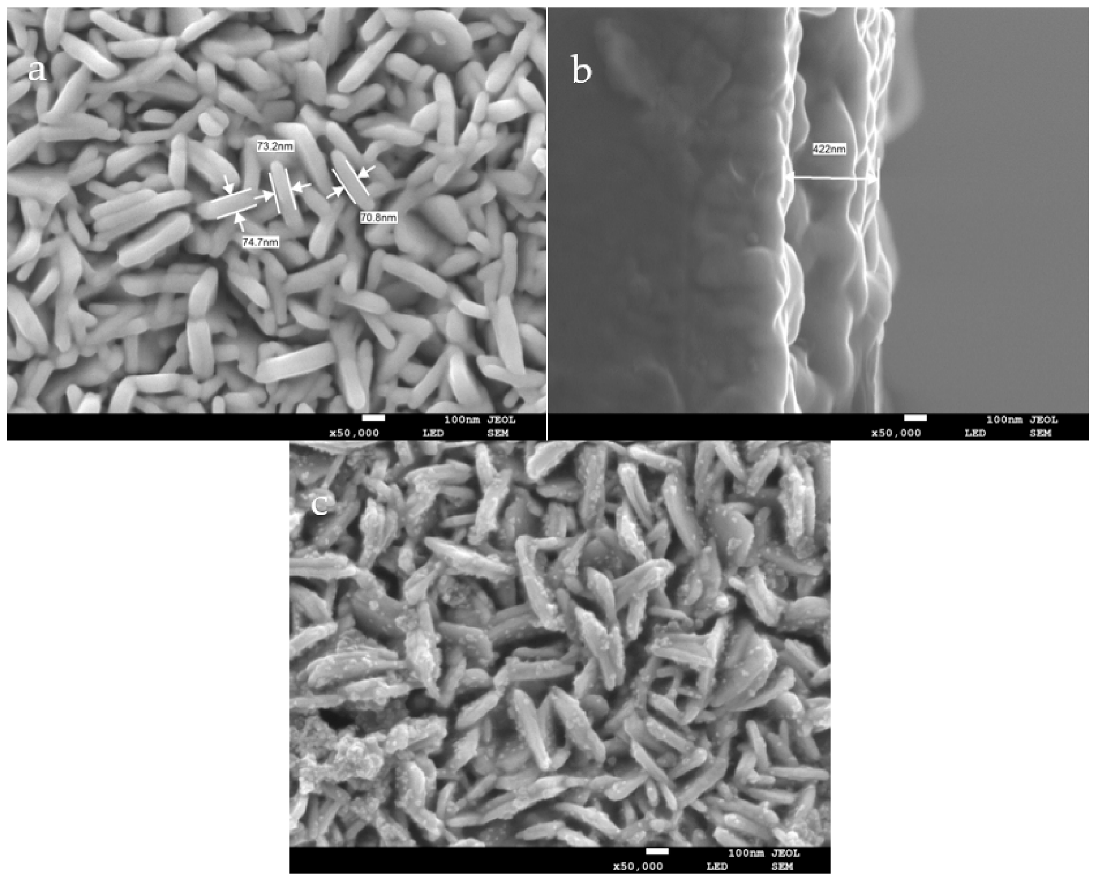
2.3. Preparation of WO3 Nanoplate
2.4. Preparation of Au NPs
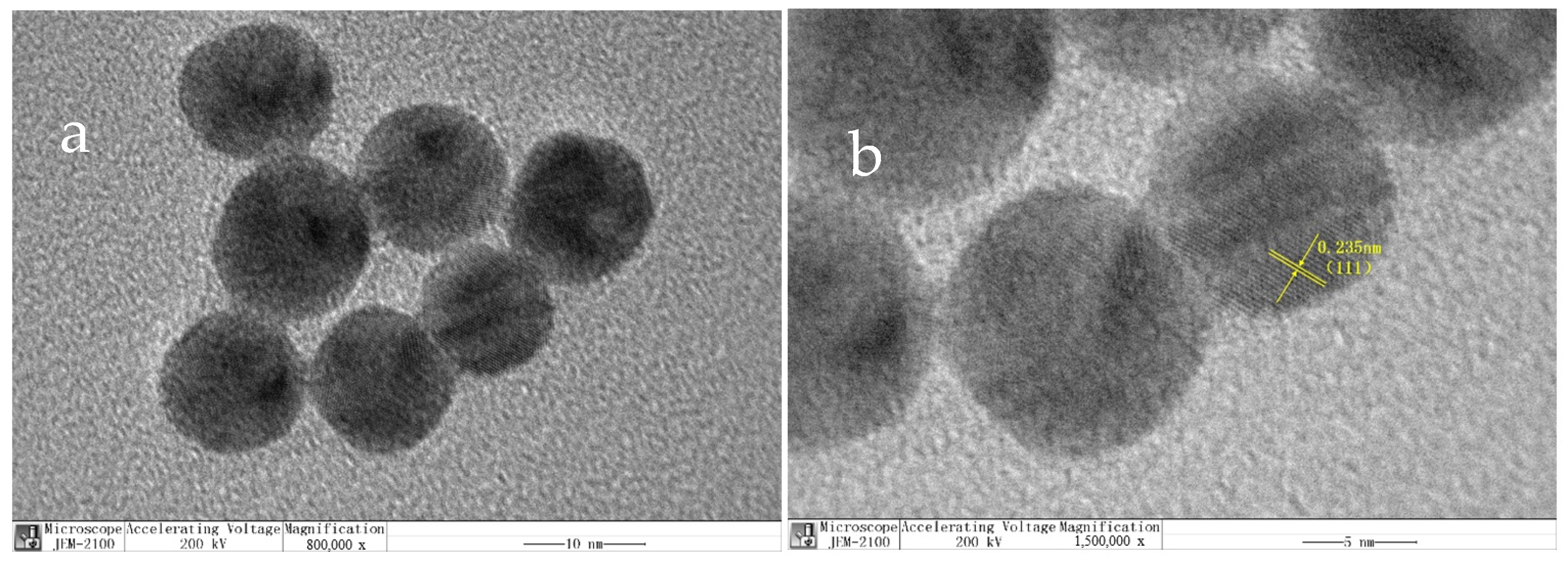
2.5. Preparation of CdTe QDs
2.6. Preparation of the CdTe QD–Ap Conjugates
2.7. The Culture Process of Listeria Monocytogenes
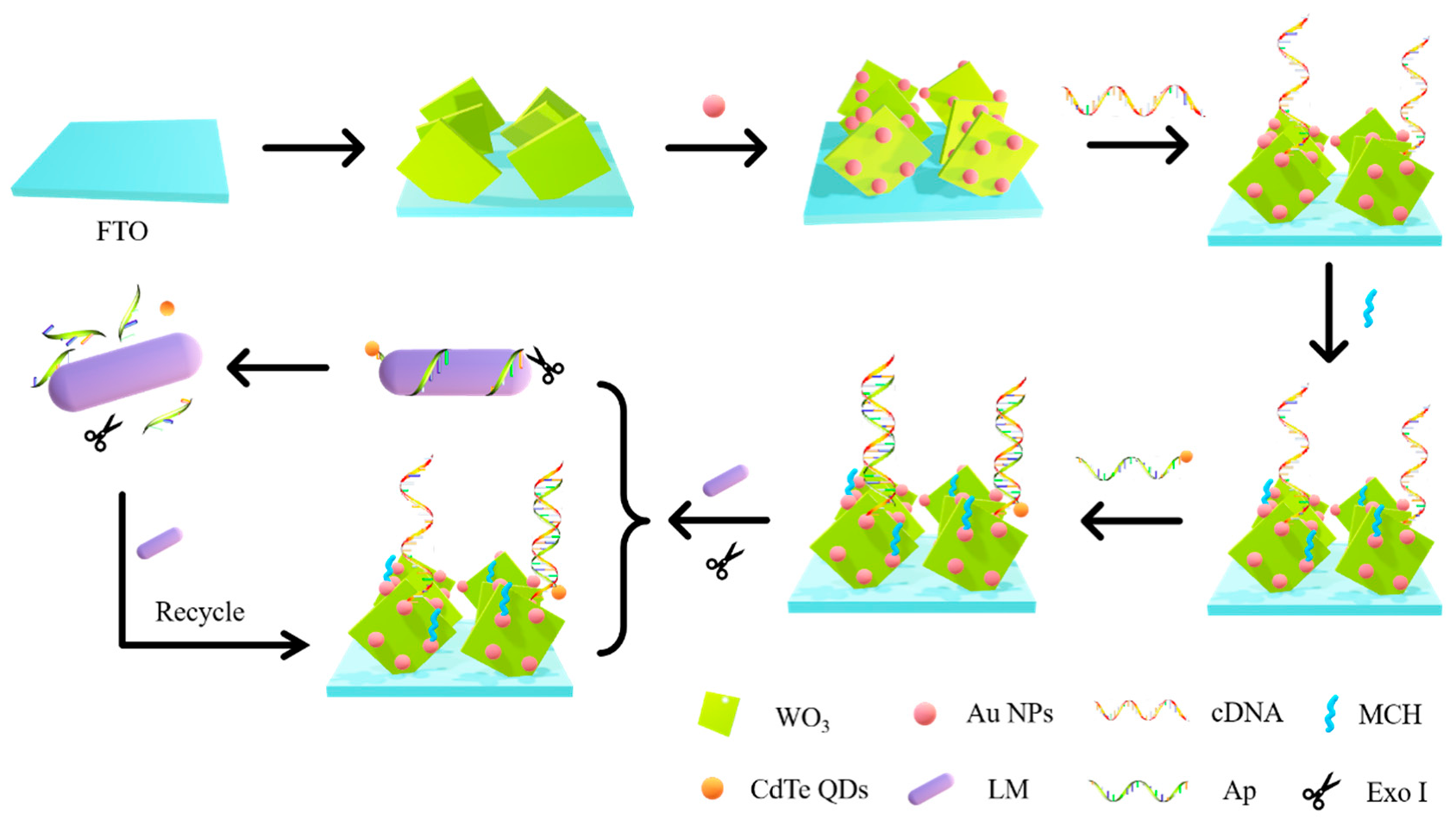
2.8. Fabrication of the Aptamer Sensor
2.9. Recovery Experiments
3. Results and Discussion
3.1. Photoelectrochemical Reaction Mechanism of the Sensor
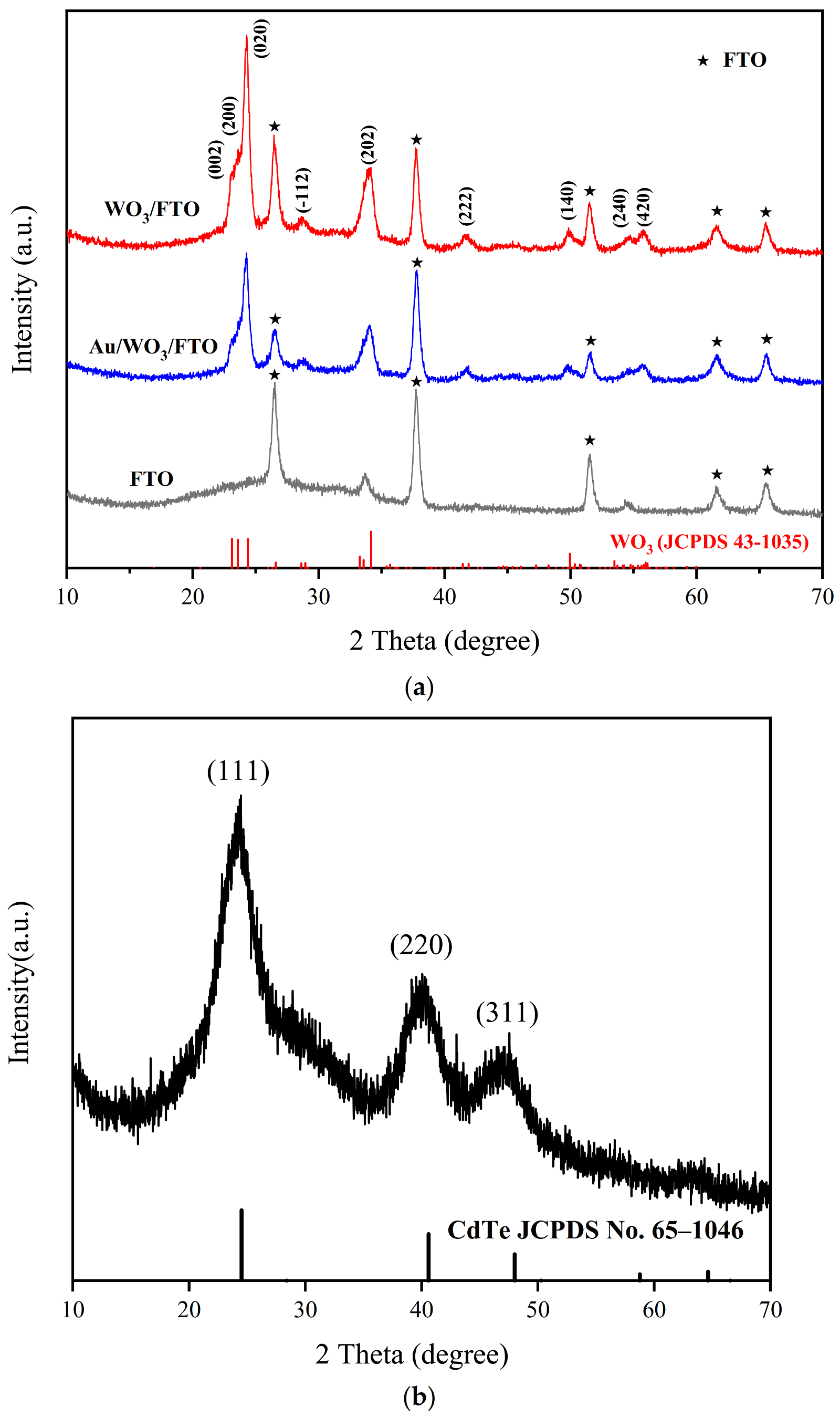
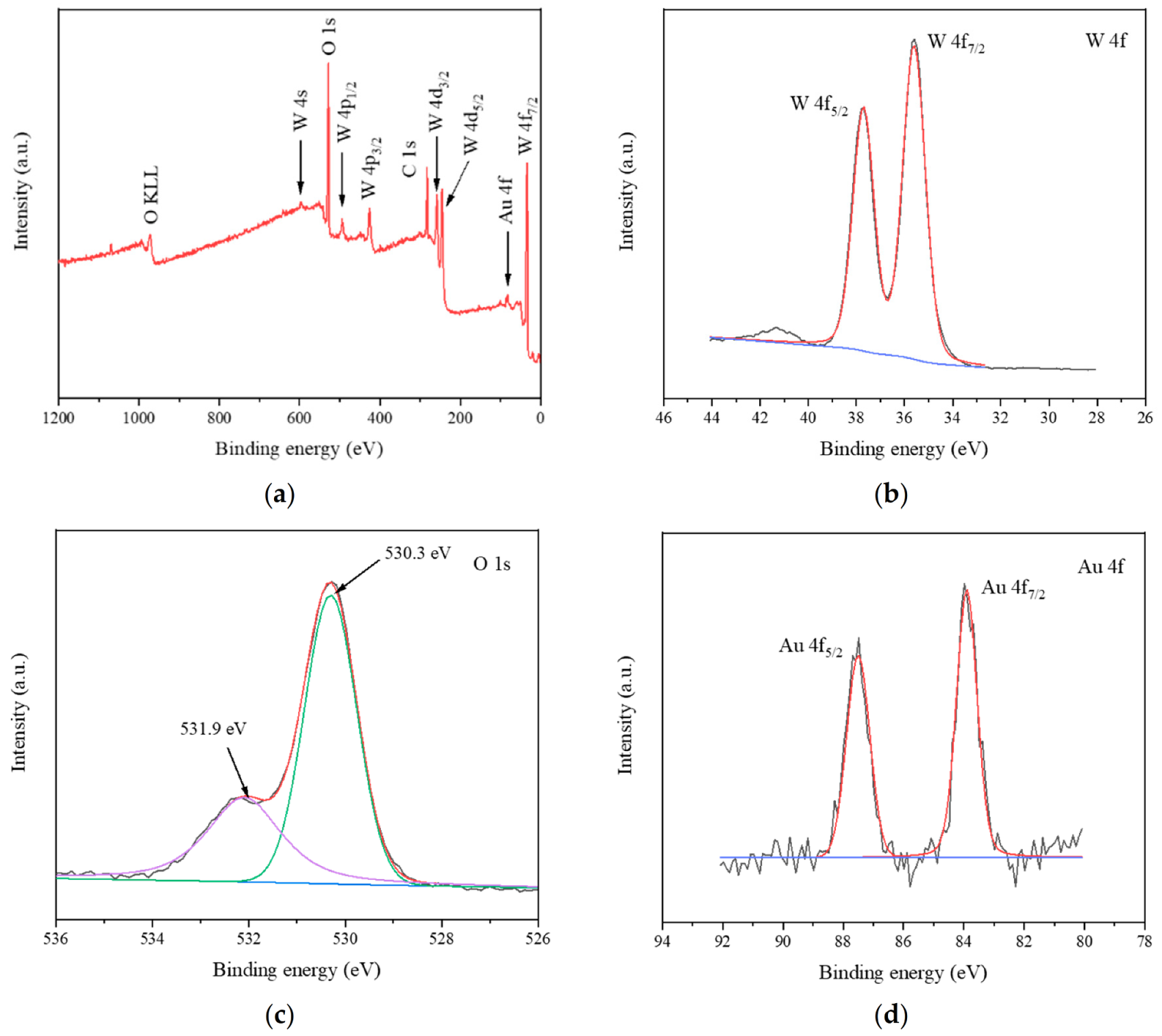
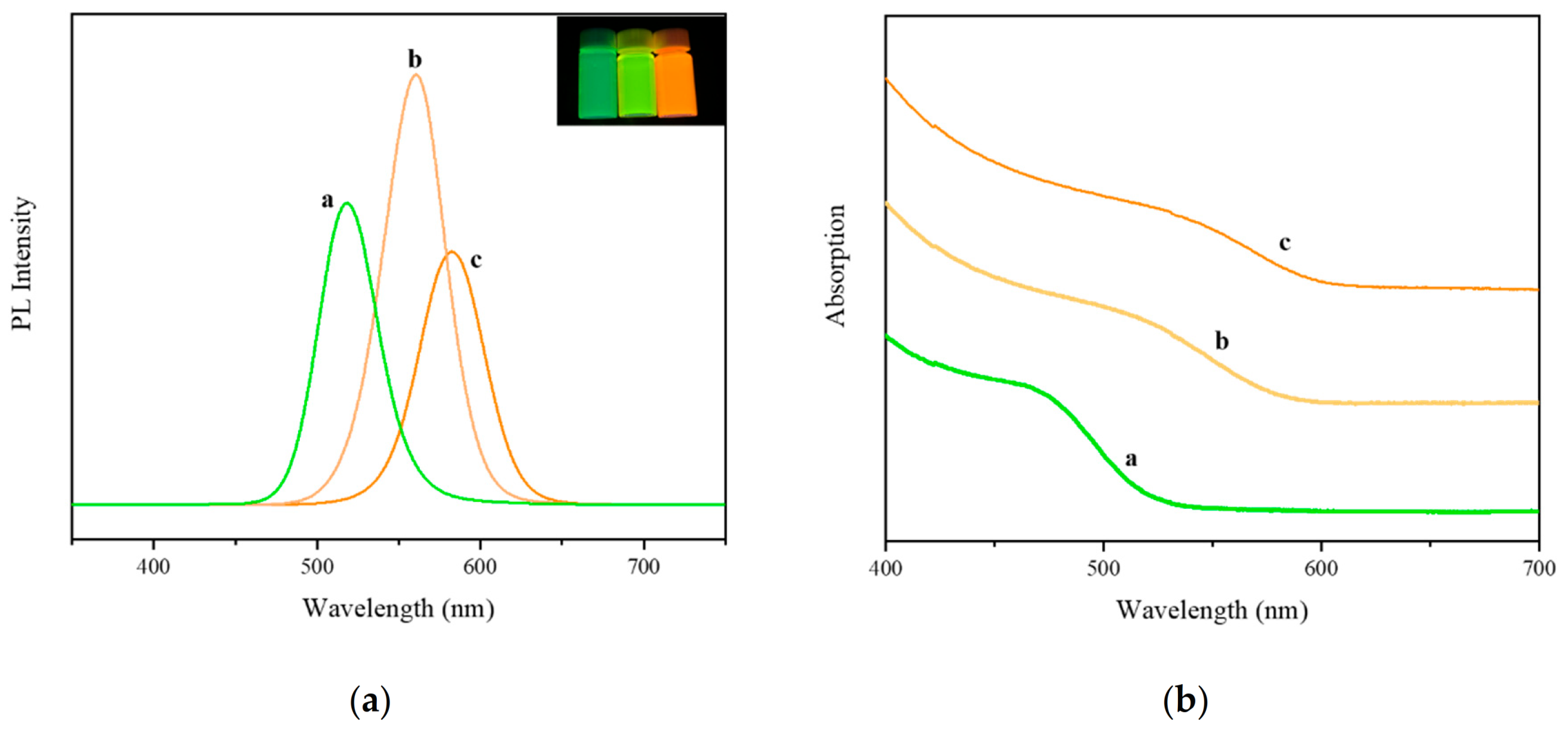
3.2. Characterization of Synthesized Materials
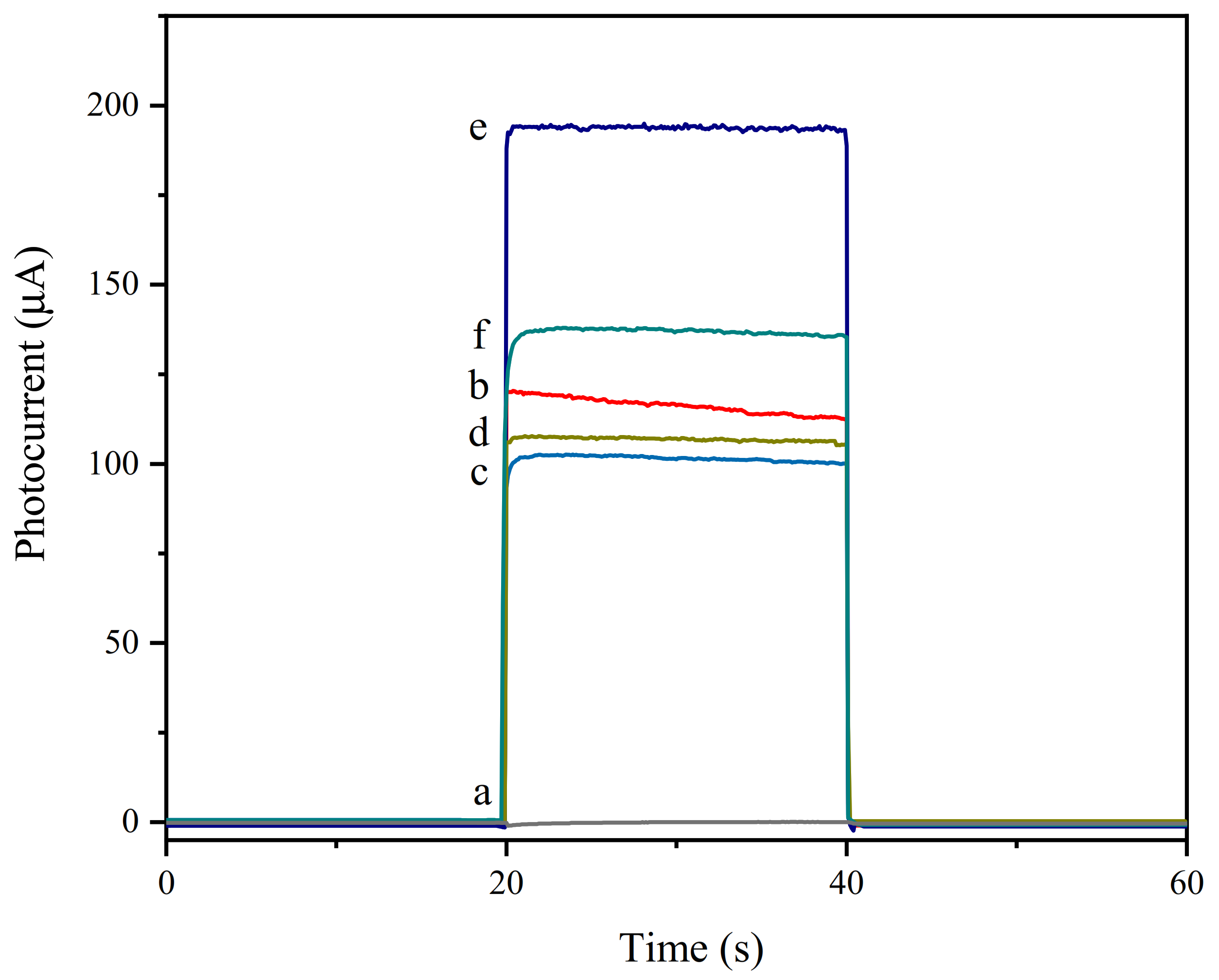
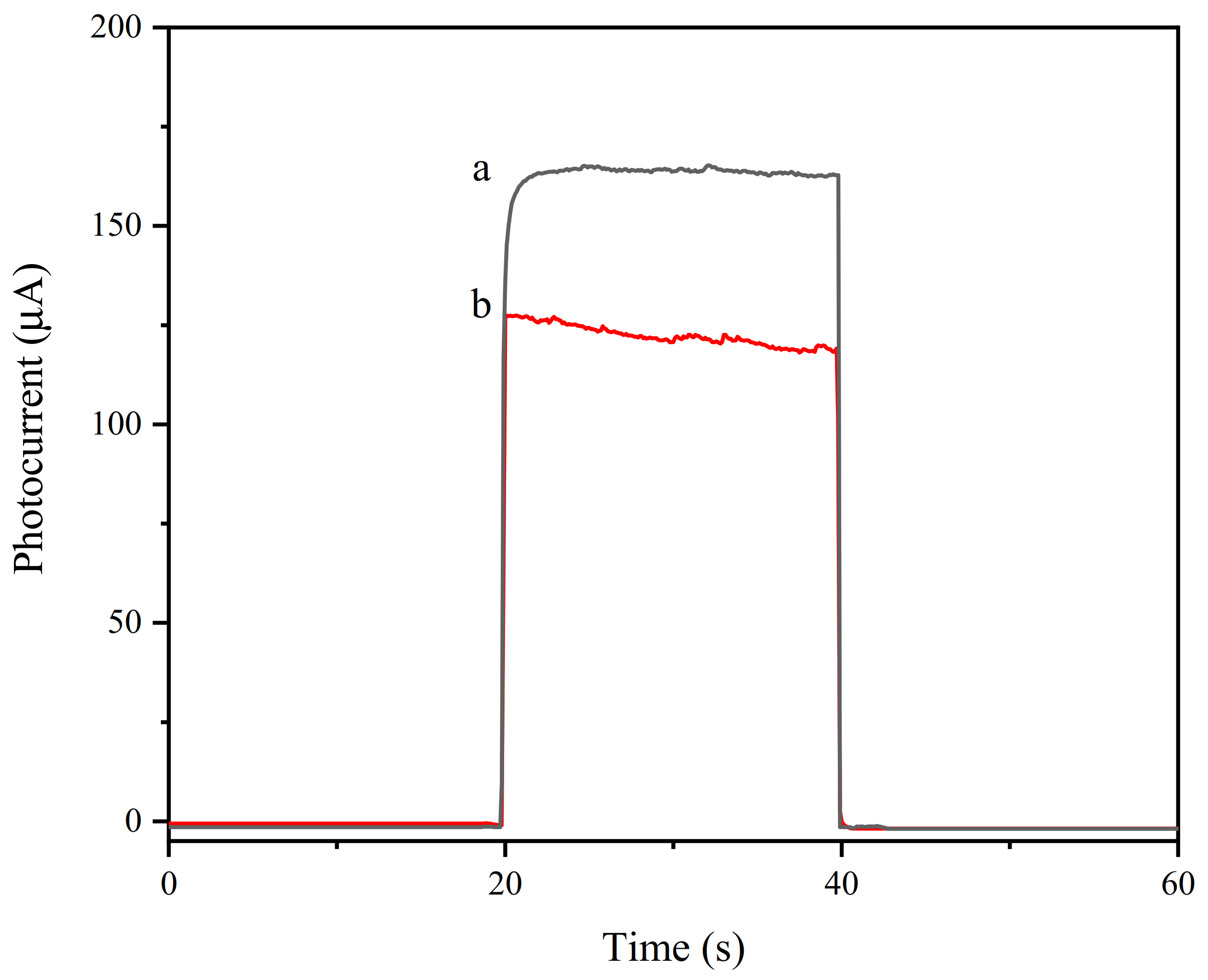
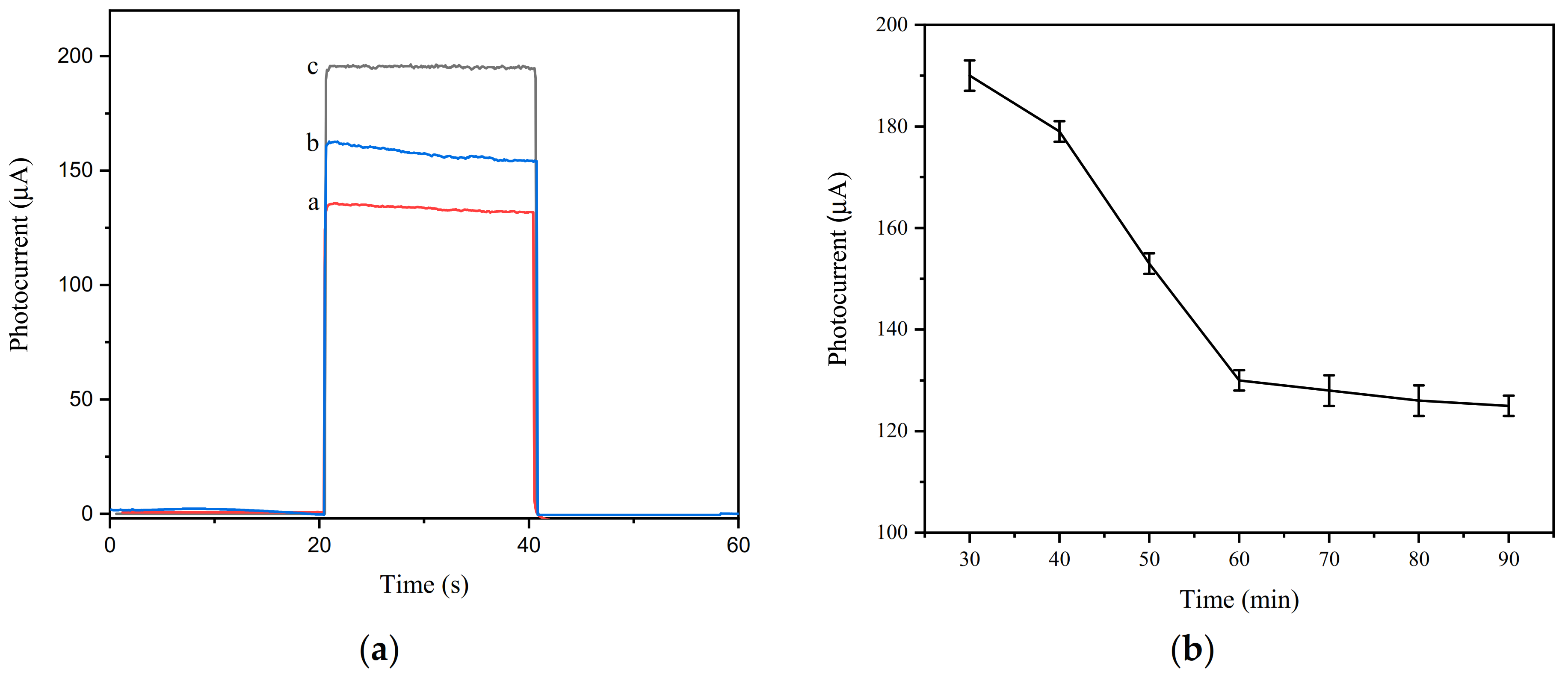
3.3. Photocurrent Characterization of the Aptamer Sensor
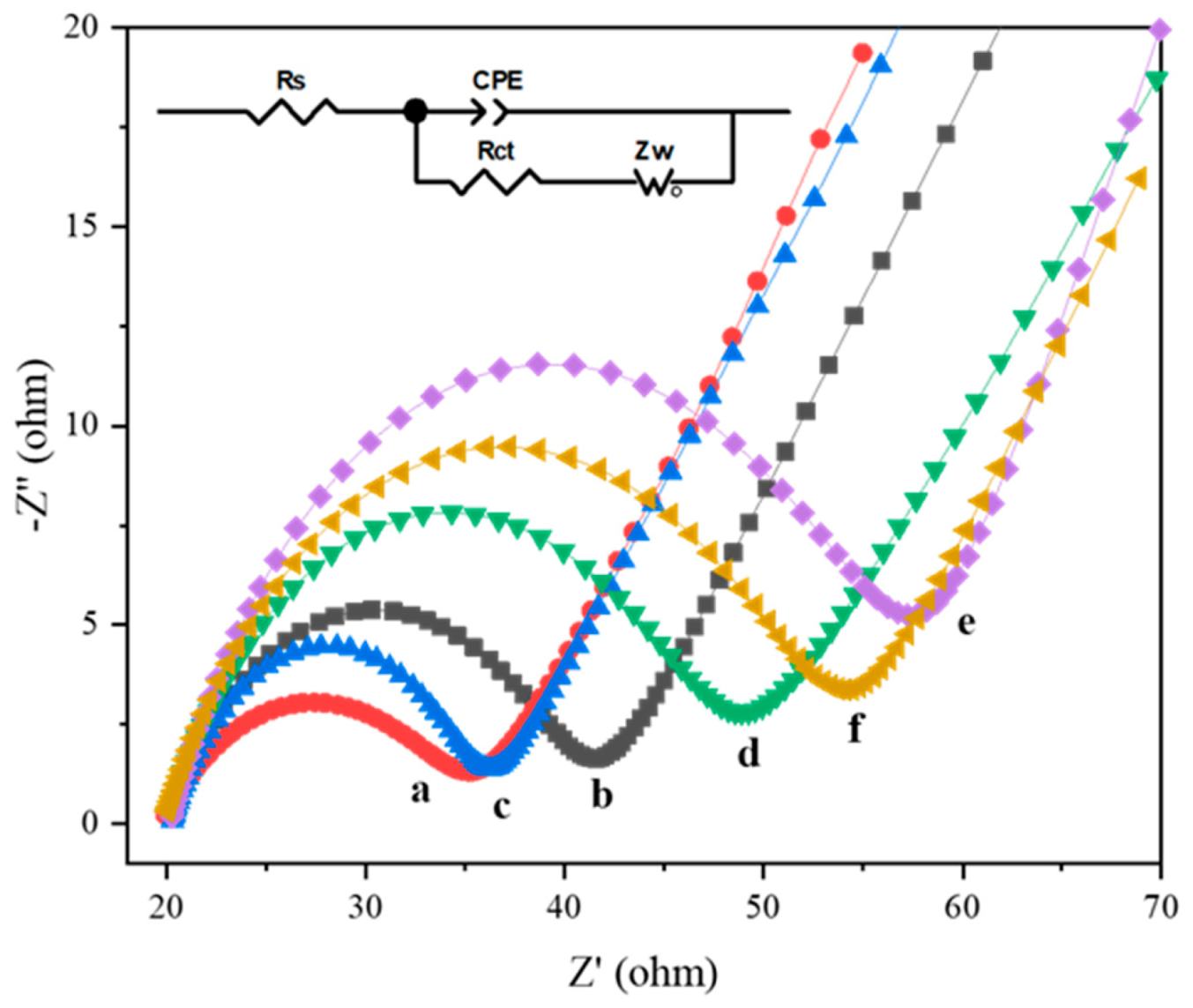
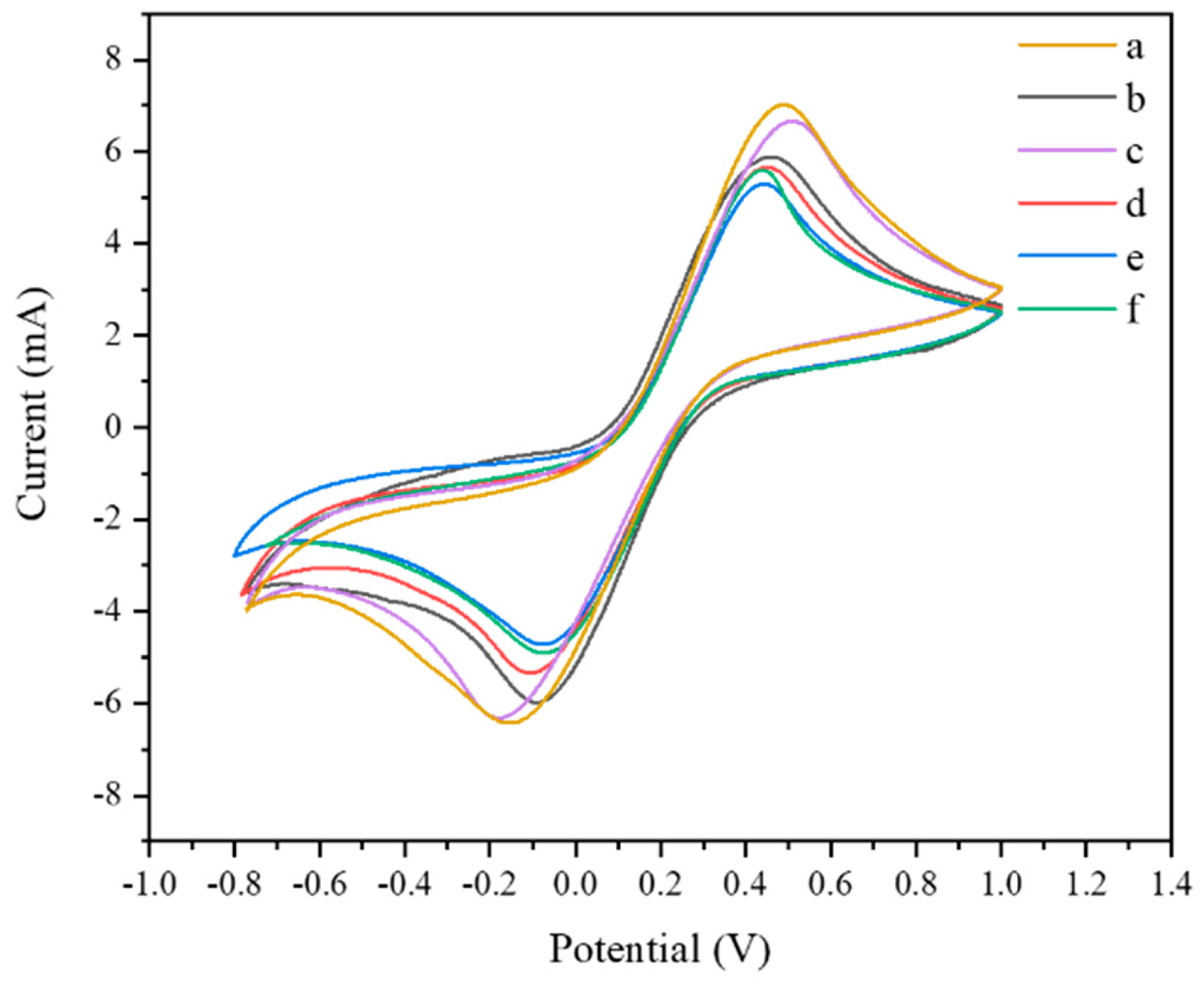
3.4. EIS and CV Characterization of Aptamer Sensors
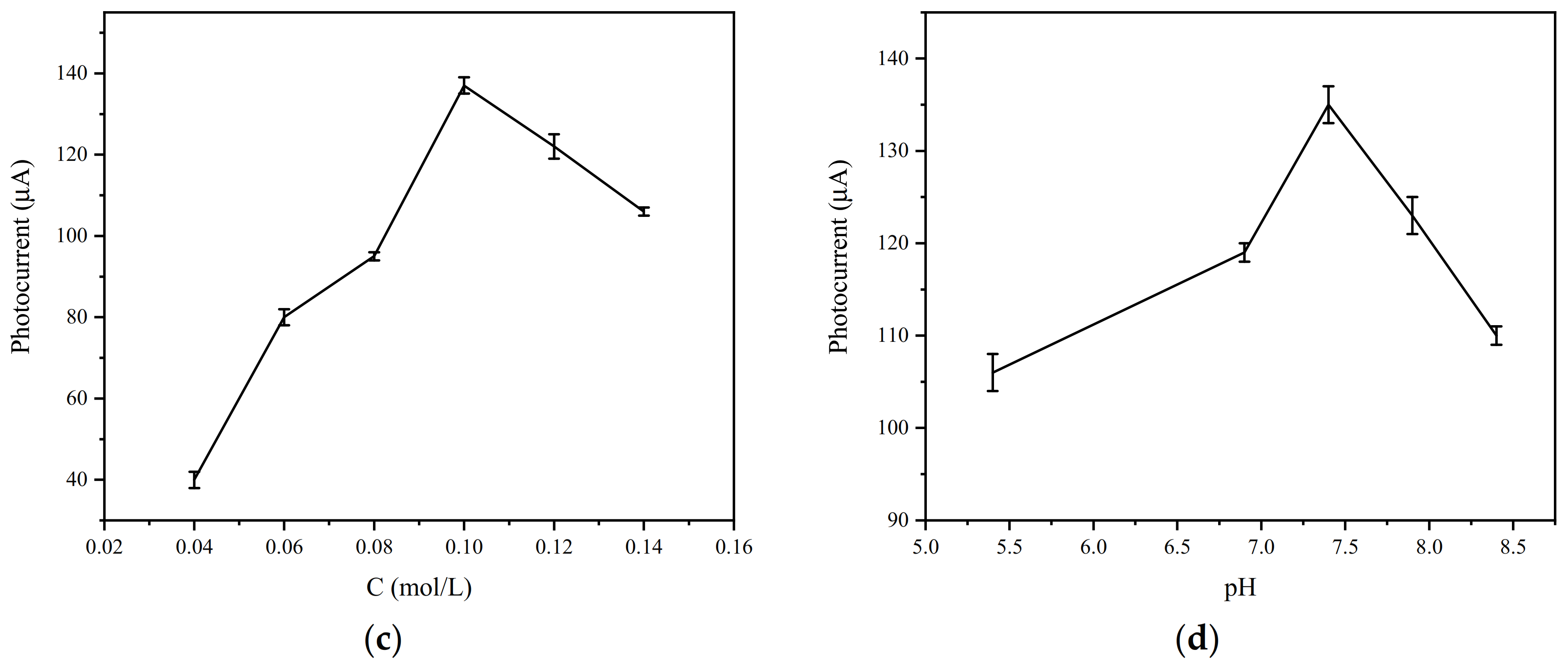
3.5. Optimization of Experimental Parameters
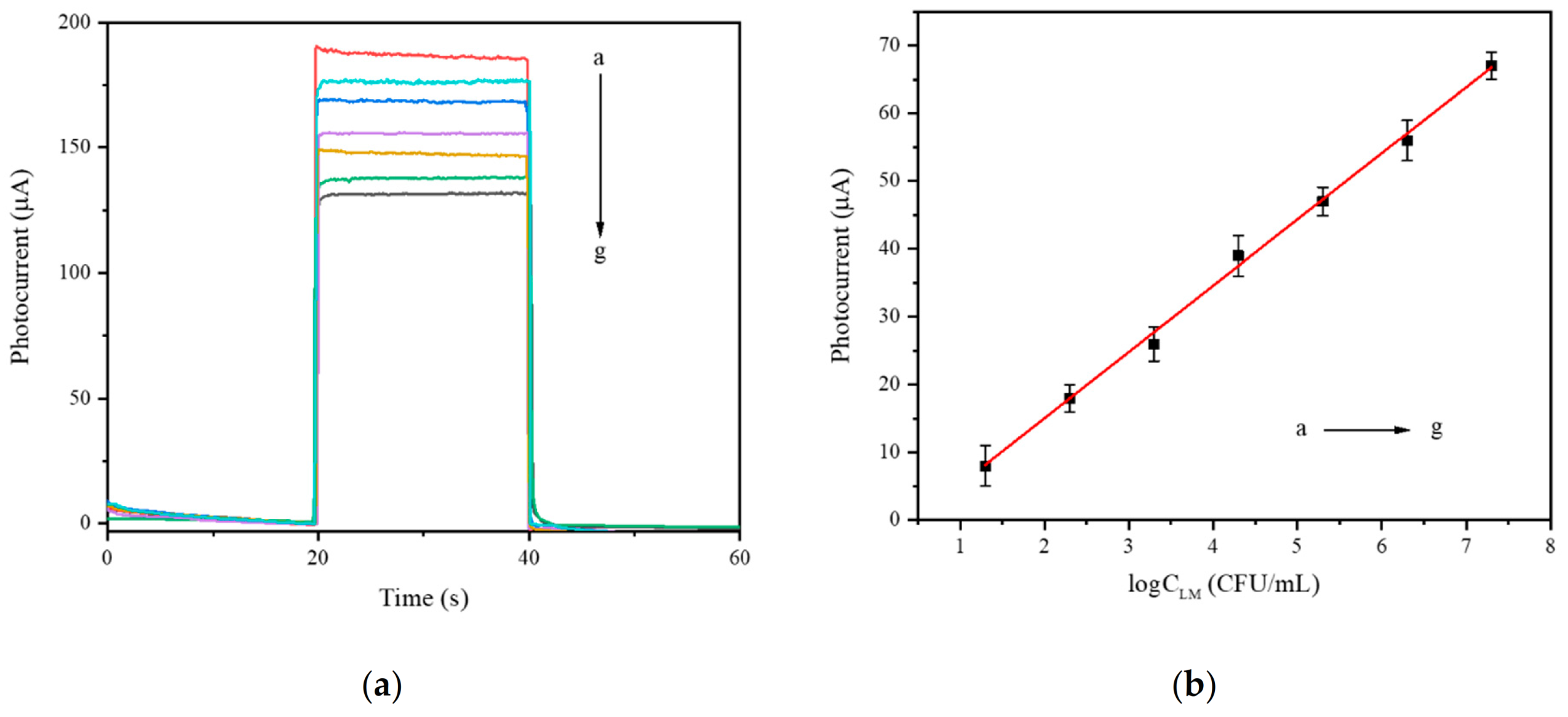
3.6. Analytical Performance of Aptamer Sensor
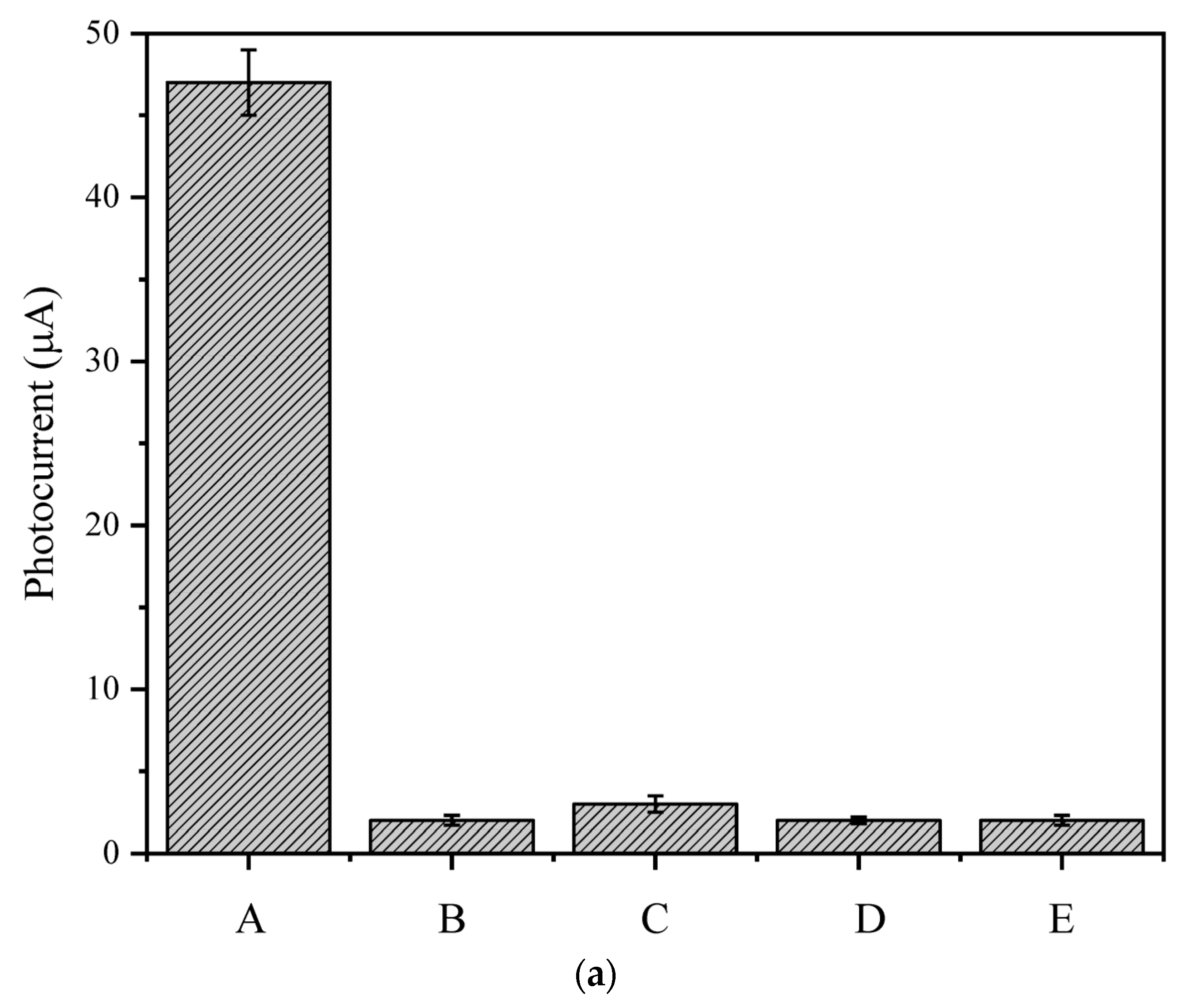
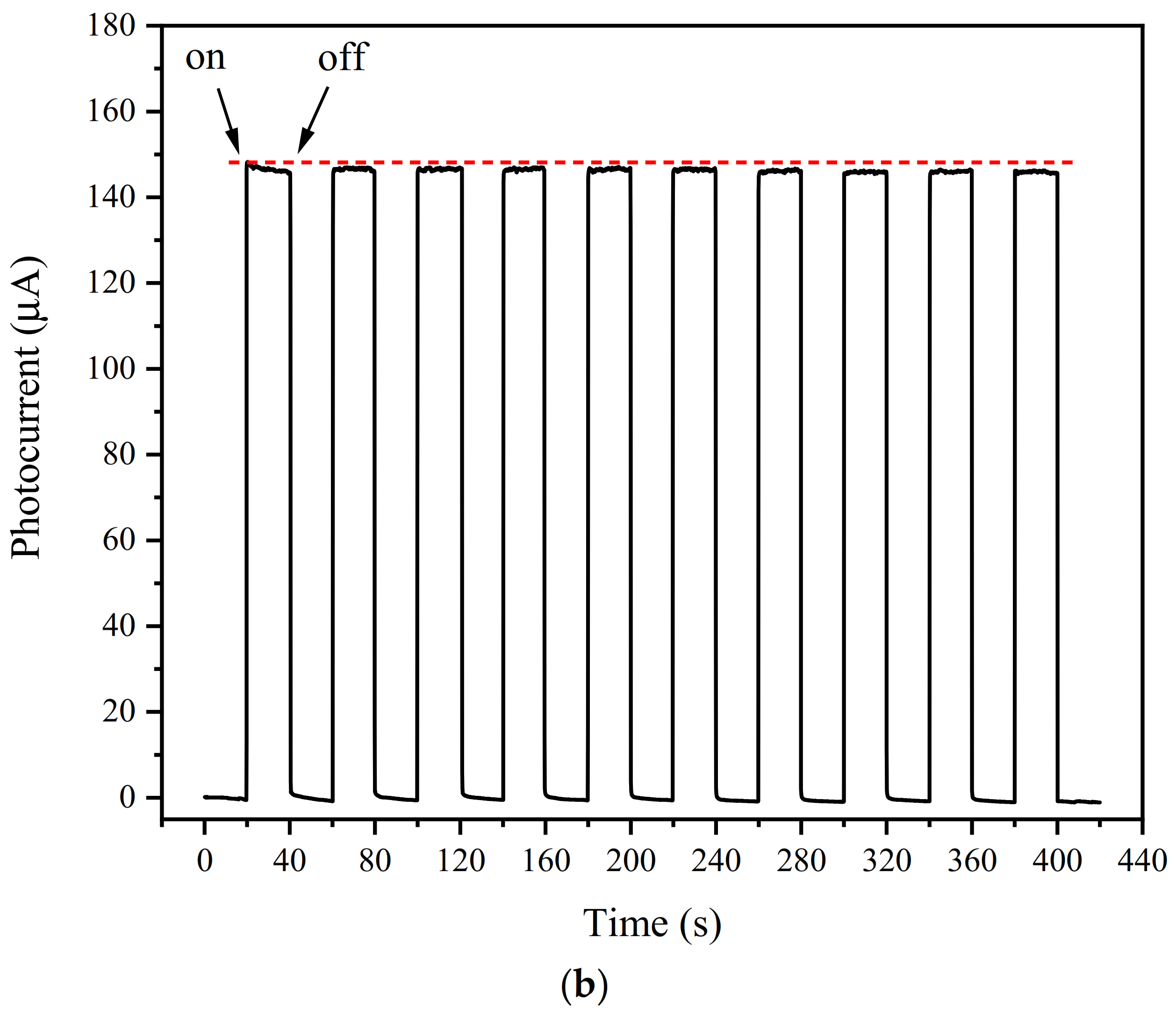
3.7. Selectivity, Stability, and Reproducibility of the PEC Sensing Platform
3.8. Analysis of Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, R.; Zhang, Y.; Ali, S.; Haruna, S.A.; He, P.; Li, H.; Ouyang, Q.; Chen, Q. Development of a fluorescence aptasensor for rapid and sensitive detection of Listeria monocytogenes in food. Food Control 2021, 122, 107808. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Chen, X.; Qu, C. Review controlling Listeria monocytogenes in ready-to-eat meat and poultry products: An overview of outbreaks, current legislations, challenges, and future prospects. Trends Food Sci. Technol. 2021, 116, 24–35. [Google Scholar] [CrossRef]
- Forauer, E.; Wu, S.T.; Etter, A.J. Listeria monocytogenes in the retail deli environment: A review. Food Control 2021, 119, 107443. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.G.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, T.; Liu, C.; Xu, Q.; Fang, S.; Wu, Y.; Wu, M.; Liu, Q. An ultrasensitive and contamination-free on-site nucleic acid detection platform for Listeria monocytogenes based on the CRISPR-Cas12a system combined with recombinase polymerase amplification. LWT 2021, 152, 112166. [Google Scholar] [CrossRef]
- Huang, Y.M.; Hsu, H.Y.; Hsu, C.L. Development of electrochemical method to detect bacterial count, Listeria monocytogenes, and somatic cell count in raw milk. J. Taiwan Inst. Chem. Eng. 2016, 62, 39–44. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Wang, Z.; Tu, K.; Pan, L.; Chen, Y. A magnetic relaxation DNA biosensor for rapid detection of Listeria monocytogenes using phosphatase-mediated Mn (VII)/Mn (II) conversion. Food Control 2021, 125, 107959. [Google Scholar] [CrossRef]
- Bundidamorn, D.; Supawasit, W.; Trevanich, S. Taqman® probe based multiplex RT-PCR for simultaneous detection of Listeria monocytogenes, Salmonella spp. and Shiga toxin-producing Escherichia coli in foods. LWT 2021, 147, 111696. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, C.; Yang, C.; Liu, Z.; Wan, S. Development of an in-situ signal amplified electrochemical assay for detection of Listeria monocytogenes with label-free strategy. Food Chem. 2021, 358, 129894. [Google Scholar] [CrossRef]
- Etty, M.C.; d’Auria, S.; Fraschini, C.; Salmieri, S.; Lacroix, M. Effect of the optimized selective enrichment medium on the expression of the p60 protein used as Listeria monocytogenes antigen in specific sandwich ELISA. Res. Microbiol. 2019, 17, 182–191. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Van Giau, V.; Vo, T.K. Multiplex PCR for simultaneous identification of E. coli O157: H7, Salmonella spp. and L. monocytogenes in food. 3 Biotech 2016, 6, 205. [Google Scholar] [PubMed] [Green Version]
- Qin, H.; Shi, X.; Yu, L.; Li, K.; Wang, J.; Chen, J.; Yang, F.; Xu, H.; Xu, H. Multiplex real-time PCR coupled with sodium dodecyl sulphate and propidium monoazide for the simultaneous detection of viable Listeria monocytogenes, Cronobacter sakazakii, Staphylococcus aureus and Salmonella spp. in milk. Int. Dairy J. 2020, 108, 104739. [Google Scholar] [CrossRef]
- Roumani, F.; Azinheiro, S.; Carvalho, J.; Prado, M.; Garrido-Maestu, A. Loop-mediated isothermal amplification combined with immunomagnetic separation and propidium monoazide for the specific detection of viable Listeria monocytogenes in milk products, with an internal amplification control. Food Control 2021, 125, 107975. [Google Scholar] [CrossRef]
- Sadighbayan, D.; Sadighbayan, K.; Tohid-Kia, M.R.; Khosroushahi, A.Y.; Hasanzadeh, M. Development of electrochemical biosensors for tumor marker determination towards cancer diagnosis: Recent progress. TrAC Trends Anal. Chem. 2019, 118, 73–88. [Google Scholar] [CrossRef]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent developments in biosensors for healthcare and biomedical applications: A review. Measurement 2021, 167, 108293. [Google Scholar] [CrossRef]
- Ren, R.; Cai, G.; Yu, Z.; Tang, D. Glucose-loaded liposomes for amplified colorimetric immunoassay of streptomycin based on enzyme-induced iron (II) chelation reaction with phenanthroline. Sens. Actuators B Chem. 2018, 265, 174–181. [Google Scholar] [CrossRef]
- Zeng, R.; Su, L.; Luo, Z.; Zhang, L.; Lu, M.; Tang, D. Ultrasensitive and label-free electrochemical aptasensor of kanamycin coupling with hybridization chain reaction and strand-displacement amplification. Anal. Chim. Acta 2018, 1038, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Niu, S.; Ge, J.; Luan, Q.; Jie, G. 3D DNA nanosphere-based photoelectrochemical biosensor combined with multiple enzyme-free amplification for ultrasensitive detection of cancer biomarkers. Biosens. Bioelectron. 2020, 147, 111778. [Google Scholar] [CrossRef]
- Shin, J.; Yan, Y.; Bai, W.; Xue, Y.; Gamble, P.; Tian, L.; Kandela, I.; Haney, C.R.; Spees, W.; Lee, Y.; et al. Bioresorbable pressure sensors protected with thermally grown silicon dioxide for the monitoring of chronic diseases and healing processes. Nat. Biomed. Eng. 2019, 3, 37–46. [Google Scholar] [CrossRef]
- Zhao, C.Q.; Ding, S.N. Perspective on signal amplification strategies and sensing protocols in photoelectrochemical immunoassay. Coord. Chem. Rev. 2019, 391, 1–14. [Google Scholar] [CrossRef]
- Li, N.; Sun, Y.; Wang, F.; Huang, C.; Fu, C.; Zhang, L.; Liu, Y.; Ge, S.; Yu, J. Target dual-recycling-induced bipedal DNA walker and Bi2WO6/Bi2S3 cascade amplification strategy in photoelectrochemical biosensor for TP53 detection. Sens. Actuators B Chem. 2021, 345, 130386. [Google Scholar] [CrossRef]
- Wang, H.H.; Li, M.J.; Tu, Y.P.; Wang, H.J.; Chai, Y.Q.; Li, Z.H.; Yuan, R. Fullerenol as a photoelectrochemical nanoprobe for discrimination and ultrasensitive detection of amplification-free single-stranded DNA. Biosens. Bioelectron. 2021, 173, 112802. [Google Scholar] [CrossRef]
- Wen, Y.X.; Qing, M.; Chen, S.L.; Luo, H.Q.; Li, N.B. Y-type DNA structure stabilized p-type CuS quantum dots to quench photocurrent of ternary heterostructure for sensitive photoelectrochemical detection of miRNA. Sens. Actuators B Chem. 2021, 329, 129257. [Google Scholar] [CrossRef]
- Song, W.; Zhang, F.; Song, P.; Zhang, Z.; He, P.; Li, Y.; Zhang, X. Untrasensitive photoelectrochemical sensor for microRNA detection with DNA walker amplification and cation exchange reaction. Sens. Actuators B Chem. 2021, 327, 128900. [Google Scholar] [CrossRef]
- Kang, Q.; Zhang, Q.; Zang, L.; Zhao, M.; Chen, X.; Shen, D. Enhancement anti-interference ability of photoelectrochemical sensor via differential molecularly imprinting technique demonstrated by dopamine determination. Anal. Chim. Acta 2020, 1125, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Dashtian, K.; Hajati, S.; Ghaedi, M. Ti-based solid-state imprinted-Cu2O/CuInSe2 heterojunction photoelectrochemical platform for highly selective dopamine monitoring. Sens. Actuators B Chem. 2021, 326, 128824. [Google Scholar] [CrossRef]
- Liu, Q.; Kim, J.; Cui, T. A highly sensitive photoelectrochemical sensor with polarity-switchable photocurrent for detection of trace hexavalent chromium. Sens. Actuators B Chem. 2020, 317, 128181. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, L.; Li, P.; Chen, X.; Jiang, J.; Zhang, S.; Zhang, C.; Zhang, A.; Chen, G.; Wang, H. Direct Z-scheme photocatalyst of hollow CoSx@ CdS polyhedron constructed by ZIF-67-templated one-pot solvothermal route: A signal-on photoelectrochemical sensor for mercury (II). Chem. Eng. J. 2020, 395, 125072. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, H.; Wei, X.; Wu, Y.; Gu, W.; Hu, L.; Zhu, C. Efficient BiVO4 photoanode decorated with Ti3C2TX MXene for enhanced photoelectrochemical sensing of Hg (II) ion. Anal. Chim. Acta 2020, 1119, 11–17. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, Y.X.; Wang, B.; Ren, S.W.; Cao, J.T.; Liu, Y.M. CdS: Mn-sensitized 2D/2D heterostructured g-C3N4-MoS2 with excellent photoelectrochemical performance for ultrasensitive immunosensing platform. Talanta 2020, 207, 120288. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Gao, Y.; Zhang, J.; Yan, J.; Song, W. Signal-on cathodic photoelectrochemical aptasensing of insulin: Plasmonic Au activated amorphous MoSx photocathode coupled with target-induced sensitization effect. Biosens. Bioelectron. 2020, 165, 112359. [Google Scholar] [CrossRef]
- Yan, P.; Jiang, D.; Tian, Y.; Xu, L.; Qian, J.; Li, H.; Xia, J.; Li, H. A sensitive signal-on photoelectrochemical sensor for tetracycline determination using visible-light-driven flower-like CN/BiOBr composites. Biosens. Bioelectron. 2018, 111, 74–81. [Google Scholar] [CrossRef]
- Zhang, K.; Lv, S.; Zhou, Q.; Tang, D. CoOOH nanosheets-coated g-C3N4/CuInS2 nanohybrids for photoelectrochemical biosensor of carcinoembryonic antigen coupling hybridization chain reaction with etching reaction. Sens. Actuators B Chem. 2020, 307, 127631. [Google Scholar] [CrossRef]
- Li, Y.; Bu, Y.; Jiang, F.; Dai, X.; Ao, J.P. Fabrication of ultra-sensitive photoelectrochemical aptamer biosensor: Based on semiconductor/DNA interfacial multifunctional reconciliation via 2D-C3N4. Biosens. Bioelectron. 2020, 150, 111903. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Xu, L.; Han, Z.; Yin, H.; Ai, S. Photoelectrochemical apta-biosensor for zeatin detection based on graphene quantum dots improved photoactivity of graphite-like carbon nitride and streptavidin induced signal inhibition. Sens. Actuators B Chem. 2018, 257, 237–244. [Google Scholar] [CrossRef]
- Zargazi, M.; Entezari, M.H. Ultrasound assisted deposition of highly stable self-assembled Bi2MoO6 nanoplates with selective crystal facet engineering as photoanode. Ultrason. Sonochem. 2020, 67, 105145. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, H.; Ma, X.; Zhang, X.; Zhu, Y.; Zhang, A.; Cao, Y.; Yang, P. Construction of AgI/Bi2MoO6/AgBi (MoO4)2 multi-heterostructure composite nanosheets for visible-light photocatalysis. Mater. Today Commun. 2020, 23, 100903. [Google Scholar] [CrossRef]
- Miodyńska, M.; Mikolajczyk, A.; Bajorowicz, B.; Zwara, J.; Klimczuk, T.; Lisowski, W.; Trykowski, G.; Pinto, H.P.; Zaleska-Medynska, A. Urchin-like TiO2 structures decorated with lanthanide-doped Bi2S3 quantum dots to boost hydrogen photogeneration performance. Appl. Catal. B Environ. 2020, 272, 118962. [Google Scholar] [CrossRef]
- Shen, Y.; Li, T.; Zhong, X.; Li, G.; Li, A.; Wei, D.; Zhang, Y.; Wei, K. Ppb-level NO2 sensing properties of Au-doped WO3 nanosheets synthesized from a low-grade scheelite concentrate. Vacuum 2020, 172, 109036. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Wang, Y.; Zhang, J. Two-photon induced NIR active core-shell structured WO3/CdS for enhanced solar light photocatalytic performance. Appl. Catal. B Environ. 2020, 272, 118979. [Google Scholar] [CrossRef]
- Pei, F.; Feng, S.; Wu, Y.; Lv, X.; Wang, H.; Chen, S.-M.; Hao, Q.; Cao, Y.; Lei, W.; Tong, Z. Label-free photoelectrochemical immunosensor for aflatoxin B1 detection based on the Z-scheme heterojunction of g-C3N4/Au/WO3. Biosens. Bioelectron. 2021, 189, 113373. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, Z.; Liu, J.; Liu, H.; Bian, W.; Tian, D.; Xia, F.; Zhou, C. A novel electrochemiluminescence aptasensor based CdTe QDs@ NH2-MIL-88 (Fe) for signal amplification. Electrochim. Acta 2020, 354, 136644. [Google Scholar] [CrossRef]
- Shahdost-Fard, F.; Fahimi-Kashani, N.; Hormozi-Nezhad, M.R. A ratiometric fluorescence nanoprobe using CdTe QDs for fast detection of carbaryl insecticide in apple. Talanta 2021, 221, 121467. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Wu, S.; Dai, S.; Gu, H.; Hao, L.; Ye, H.; Wang, Z. Advances in aptasensors for the detection of food contaminants. Analyst 2016, 141, 3942–3961. [Google Scholar] [CrossRef]
- Cong, X.; Fan, G.C.; Wang, X.; Abdel-Halim, E.S.; Zhu, J.J. Enhanced photoelectrochemical aptasensing platform amplified through the sensitization effect of CdTe@ CdS core–shell quantum dots coupled with exonuclease-I assisted target recycling. J. Mater. Chem. B 2016, 4, 6117–6124. [Google Scholar] [CrossRef]
- Lee, S.H.; Ahn, J.Y.; Lee, K.A.; Um, H.J.; Sekhon, S.S.; Park, T.S.; Kim, Y.H. Analytical bioconjugates, aptamers, enable specific quantitative detection of Listeria monocytogenes. Biosens. Bioelectron. 2015, 68, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Fu, H.; Wang, W.; Han, D.; An, X. Experimental and theoretical study on the excellent amine-sensing performance of Au decorated WO3 needle-like nanocomposites. Mater. Chem. Phys. 2019, 234, 122–132. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Y.; Kong, F.; Wang, S.; Zhu, B.; Guo, X.; Zhang, J.; Wu, S. Au-doped WO3-based sensor for NO2 detection at low operating temperature. Sens. Actuators B Chem. 2008, 134, 133–139. [Google Scholar] [CrossRef]
- Fan, G.C.; Zhao, M.; Zhu, H.; Shi, J.J.; Zhang, J.R.; Zhu, J.J. Signal-on photoelectrochemical aptasensor for adenosine triphosphate detection based on sensitization effect of CdS: Mn@ Ru(bpy)2(dcbpy) nanocomposites. J. Phys. Chem. C 2016, 120, 15657–15665. [Google Scholar] [CrossRef]

| Samples | Added (CFU/mL) | Found (CFU/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 1 × 102 | 0.91 × 102 | 91 | 2.2 |
| 2 | 1 × 105 | 1.12 × 105 | 112 | 3.4 |
| 3 | 1 × 107 | 0.89 × 107 | 89 | 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Hao, H.; Ding, C.; Gan, H.; Jiang, S.; Zhang, G.; Bi, J.; Yan, S.; Hou, H. A Novel Photoelectrochemical Aptamer Sensor Based on CdTe Quantum Dots Enhancement and Exonuclease I-Assisted Signal Amplification for Listeria monocytogenes Detection. Foods 2021, 10, 2896. https://doi.org/10.3390/foods10122896
Zhu L, Hao H, Ding C, Gan H, Jiang S, Zhang G, Bi J, Yan S, Hou H. A Novel Photoelectrochemical Aptamer Sensor Based on CdTe Quantum Dots Enhancement and Exonuclease I-Assisted Signal Amplification for Listeria monocytogenes Detection. Foods. 2021; 10(12):2896. https://doi.org/10.3390/foods10122896
Chicago/Turabian StyleZhu, Liangliang, Hongshun Hao, Chao Ding, Hanwei Gan, Shuting Jiang, Gongliang Zhang, Jingran Bi, Shuang Yan, and Hongman Hou. 2021. "A Novel Photoelectrochemical Aptamer Sensor Based on CdTe Quantum Dots Enhancement and Exonuclease I-Assisted Signal Amplification for Listeria monocytogenes Detection" Foods 10, no. 12: 2896. https://doi.org/10.3390/foods10122896
APA StyleZhu, L., Hao, H., Ding, C., Gan, H., Jiang, S., Zhang, G., Bi, J., Yan, S., & Hou, H. (2021). A Novel Photoelectrochemical Aptamer Sensor Based on CdTe Quantum Dots Enhancement and Exonuclease I-Assisted Signal Amplification for Listeria monocytogenes Detection. Foods, 10(12), 2896. https://doi.org/10.3390/foods10122896







