Toxic Metals in Wild Ungulates and Domestic Meat Animals Slaughtered for Food Purposes: A Systemic Review
Abstract
1. Introduction
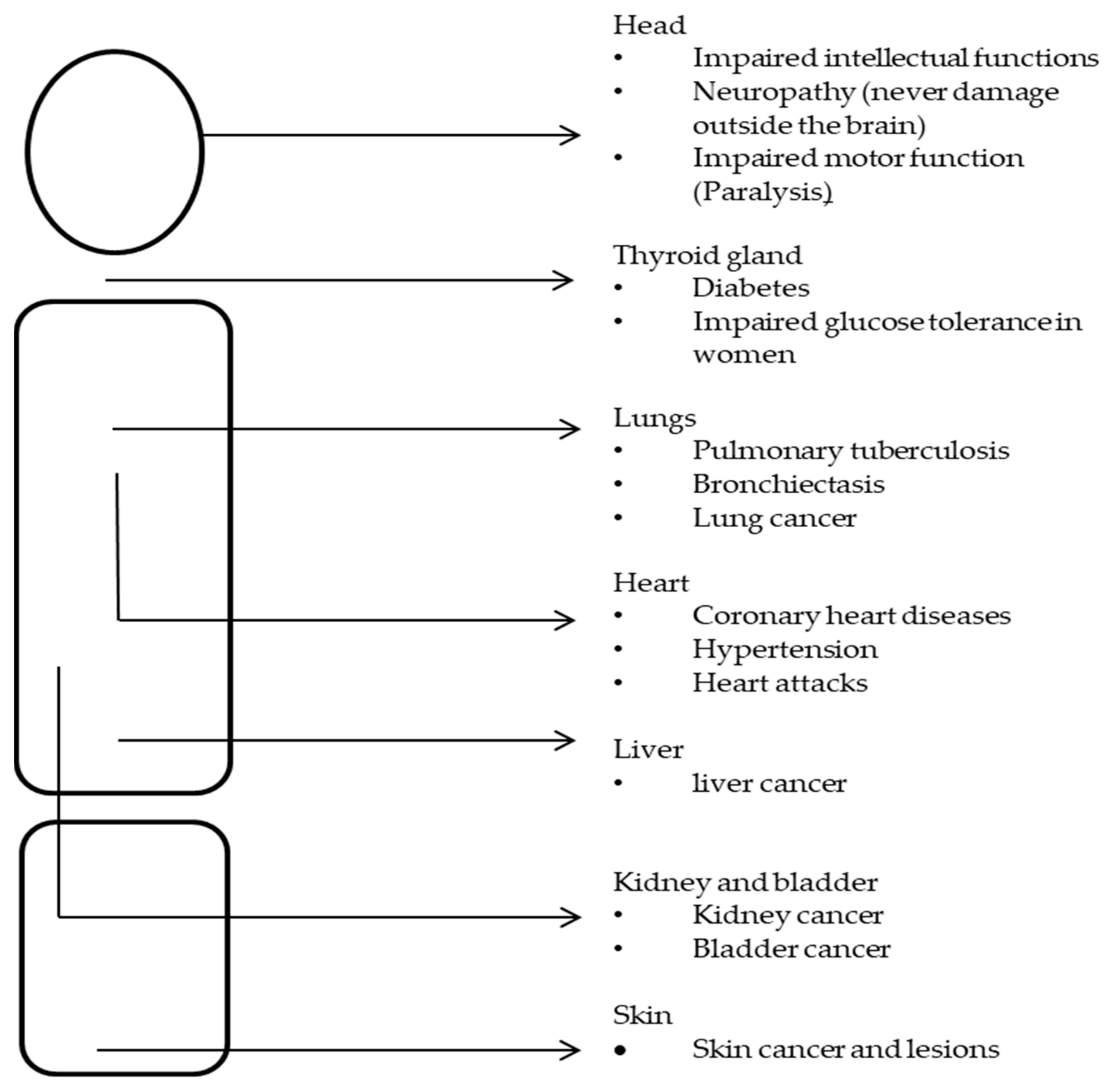
General Health Effects of Toxic Metals
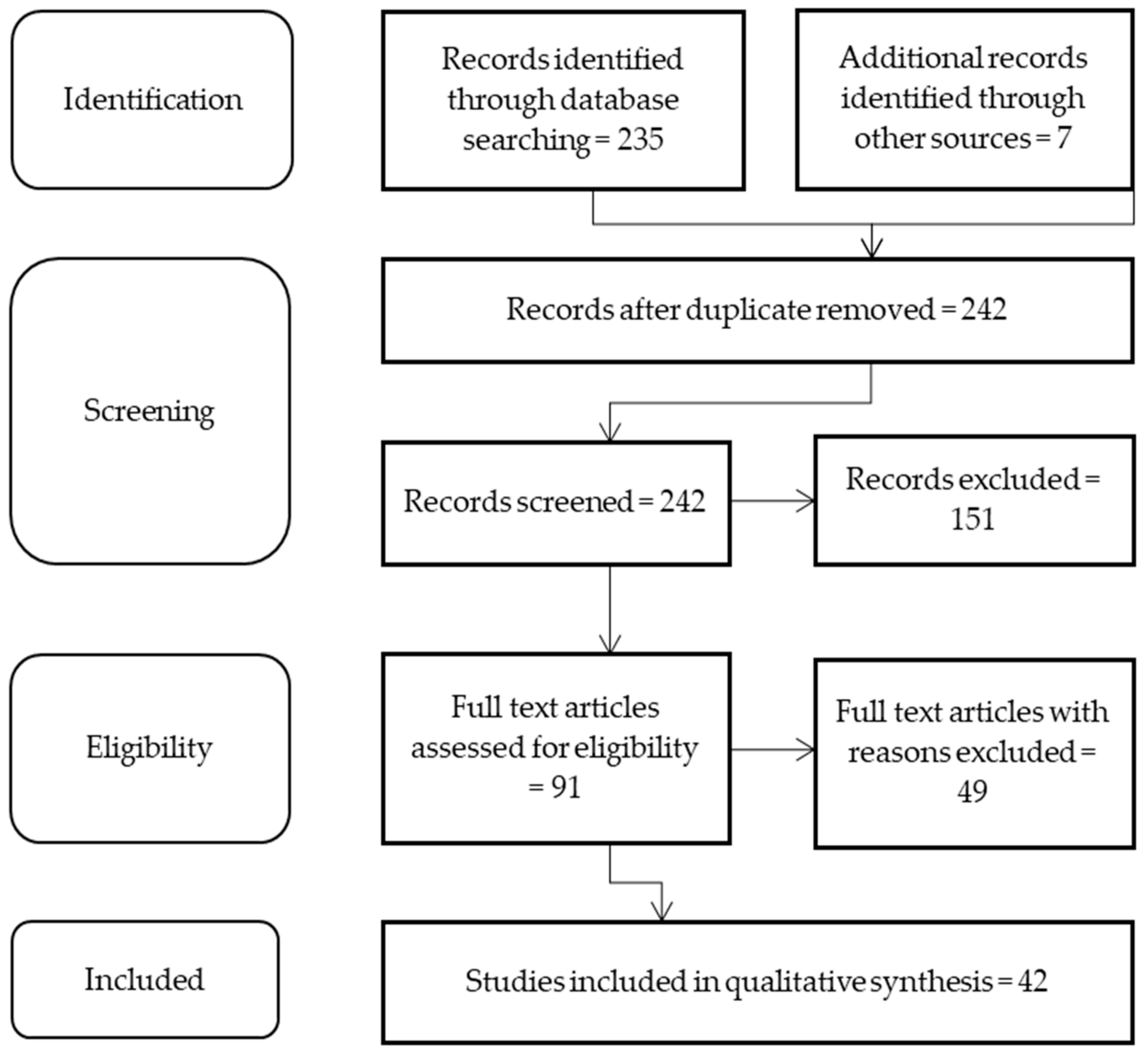
2. Materials and Methods
3. Results
4. Discussion
- “One Health” concepts: According to the World Health Organisation (WHO) (www.who.org, accessed on 10 June 2021), “One Health is an approach to designing and implementing programs, policies, legislation and research in which multiple sectors communicate and work together to achieve better public health outcomes” [78]. The adoption of these concepts in the production of meat provides a structured way of investigating meat safety risks [92,93].
- Stakeholder’s involvement: Studies by Shrivastava and Shrivastava [94] and Duc and Toribio [95] concluded that when different stakeholders are identified or identifiable, information generally flows efficiently within different categories. This information could be for training purposes, ideas sharing and early warning systems to identify changes in contamination levels in the environment [96].
- Good Agricultural Practices (GAP): With the adoption of meat safety strategies during meat animals farming, a better foundation of toxic identification exists [97]. The same is applicable to game farming and the production of meat animals ready for slaughter. Measures such as compliance of feed, water and medication used; detoxification of industrial effluents before being released to the environments and monitoring of toxic metals in meat animals must be put in place to facilitate the process of meat animals production [98].
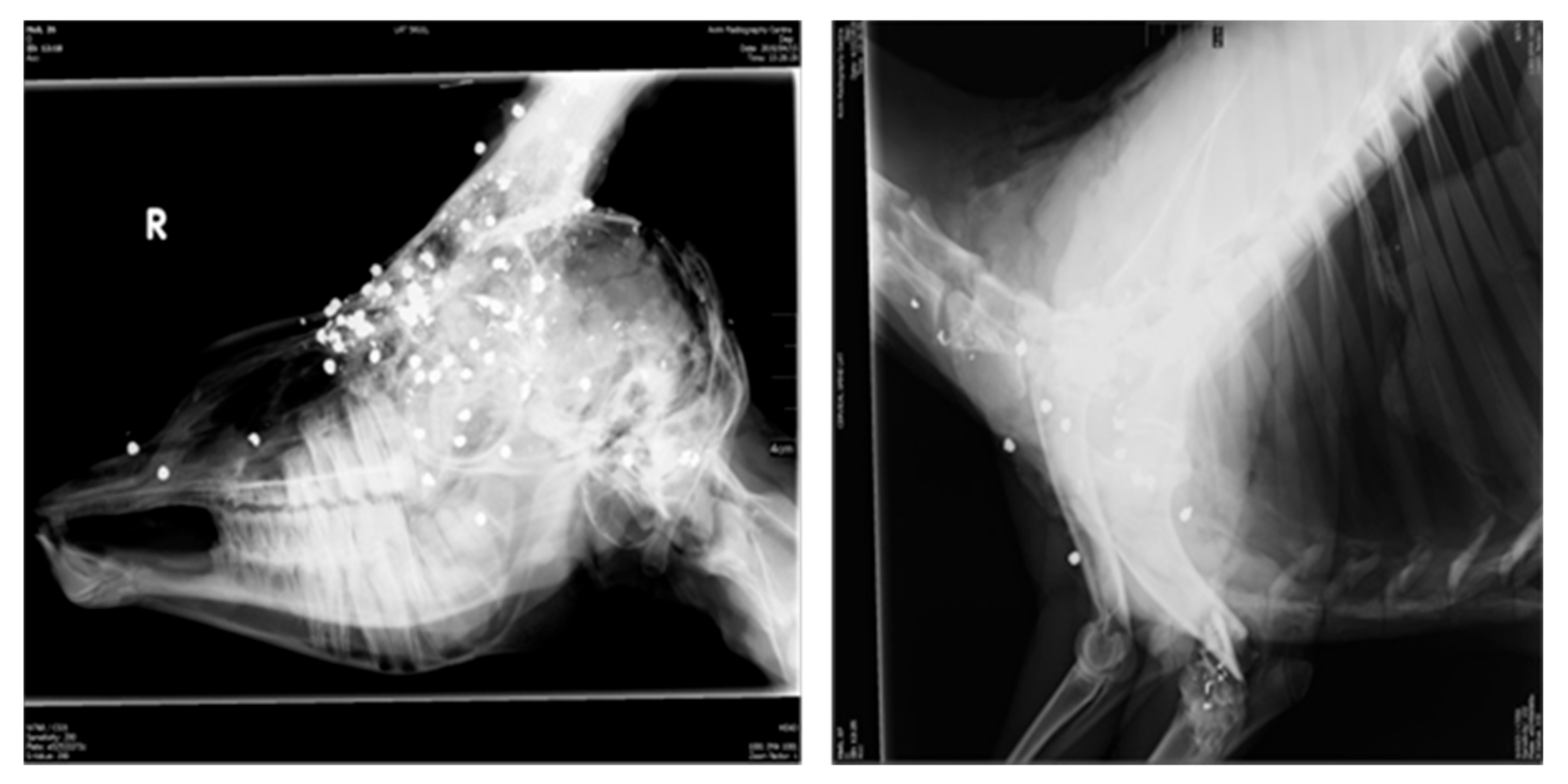
- Enforcement: Law enforcement would entail environmental pollution control, hunting and slaughter/processing control and the banning of generally used products with high concentration of toxic metals (e.g., fuel, paint) but, specifically, lead bullets and pellets used for hunting. This will ensure that secondary contamination of meat animals by these products is minimised [99,100].
- Bio-indicators: Environmental monitoring of toxic metals by using animals and plants as bio-indicators is an effective method of environmental pollution monitoring [101]. The concentration of toxic metals of interest could be found on leaves, plants, feathers, fur and skin of other animals [102,103]. The presence of specific plants in an ecosystem could be used to indicate toxic metals contamination in the environment [104].
- Risk assessment: Risk assessment from farm to fork could be the best tool to be used for the identification, evaluation and control of food hazards in a food supply chain [99,105]. The situation is no different for the game meat production systems and food safety hazards such as toxic metals must be controlled at farms, killing, slaughter and dressing processes and preparation by consumers [106]. For example, Food Safety Management Systems, such as ISO 22000 [9], require that suppliers of raw materials (including live animals sent for slaughter) must provide information such as risks to an extent that it will allow the next entity in the supply chain to conduct a hazard analysis.
- Monitoring: Similar to enforcement, monitoring of the presence and concentration of these toxic metals must be done to facilitate the detection levels of toxic metals in the environment (soil, vegetation, water and meat products) [107,108]. It is also a requirement of ISO 22000, the Codex Alimentarius standards, European Food Safety Authority and the United States Food and Drug Administration [36,38,39] that toxic metals hazards must be monitored at all times during food production. Known levels of toxic metals will assist with controls and decisions that need to be made to ensure safe meat [53,109]. While these could be done by different governments, industries must also play a significant role in developing monitoring systems.
- Further research: Wild angulate meat animals must be further researched as a means of ensuring that meat from these animals meet the requirements to be certified safe for human consumption [104]. Strategies employable to reduce meat contamination from toxic metals must be developed; these may include the promotion of the “One Health” approach in meat production from farm to fork [110].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hoffman, L.C.; Wiklund, E. Game and venison–meat for the modern consumer. Meat Sci. 2006, 74, 197–208. [Google Scholar] [CrossRef] [PubMed]
- White, P.A.; Belant, J.L. Provisioning of Game Meat to Rural Communities as a Benefit of Sport Hunting in Zambia. PLoS ONE 2015, 10, e0117237. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, M.M.; Munteanu, M.; Postolache, A.N.; Boișteanu, P.C. Toxic heavy metals content in wild boar and venison meat: A brief review. Sci. Pap. Ser. D Anim. Sci. Int. Session Sci. Commun. Fac. Anim. Sci. 2020, 63, 1. [Google Scholar]
- Hoffman, L.C. Game as an alternative protein source, feed science. AFMA Matrix 2016, 25, 54–55. [Google Scholar]
- Taylor, A.; Lindsey, P.; Davies-Mostert, H.; Goodman, P. An assessment of the economic, social and conservation value of the wildlife ranching industry and its potential to support the green economy in South Africa. Endanger. Wildl. Trust Johannesbg. 2016. Available online: https://www.sagreenfund.org.za/wordpress/wp-content/uploads/2016/04/EWT-Research-report.pdf (accessed on 12 November 2020).
- Niewiadomska, K.; Kosicka-Gębska, M.; Gębski, J.; Gutkowska, K.; Jeżewska-Zychowicz, M.; Sułek, M. Game Meat Consumption—Conscious Choice or Just a Game? Foods 2020, 9, 1357. [Google Scholar] [CrossRef]
- Bekker, J.L.; Hoffman, L.C.; Jooste, P.J. Essential food safety management points in the supply chain of game meat in South Africa. In Game Meat Hygiene in Focus; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011; pp. 39–65. [Google Scholar] [CrossRef]
- Nkosi, D.V.; Bekker, J.L.; Hoffman, L.C. The Use of Organic Acids (Lactic and Acetic) as a Microbial Decontaminant during the Slaughter of Meat Animal Species: A Review. Foods 2021, 10, 2293. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Chen, Y.; Chen, C.; Yang, H.; Chen, Y. Food safety management systems based on ISO 22000:2018 methodology of hazard analysis compared to ISO 22000:2005. Accredit. Qual. Assur. 2019, 25, 23–37. [Google Scholar] [CrossRef]
- Falandysz, J.; Szymczyk–Kobrzyńska, K.; Brzostowski, A.; Zalewski, K.; Zasadowski, A. Concentrations of heavy metals in the tissues of red deer (Cervus elaphus) from the region of Warmia and Mazury, Poland. Food Addit. Contam. 2005, 22, 141–149. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand (FSANZ). Contaminants and Natural Toxicants Federal Register of Legislative Instruments; Food Standards Australia New Zealand (FSANZ): Kingston, Australia; Wellington, New Zealand, 2015.
- Irschik, I.; Wanek, C.; Bauer, F.; Sager, M.; Paulsen, P. 31. Composition of bullets used for hunting and food safety considerations. In Trends in Game Meat Hygiene; Wageningen Academic Publishers: Wageningen, The Netherlands, 2014; pp. 363–370. [Google Scholar] [CrossRef]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Pinedo-Hernández, J.; Díez, S. Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environ. Res. 2017, 154, 380–388. [Google Scholar] [CrossRef]
- Lü, J.; Jiao, W.-B.; Qiu, H.-Y.; Chen, B.; Huang, X.-X.; Kang, B. Origin and spatial distribution of heavy metals and carcinogenic risk assessment in mining areas at You’xi County southeast China. Geoderma 2018, 310, 99–106. [Google Scholar] [CrossRef]
- Levin, R.; Vieira, C.L.Z.; Mordarski, D.C.; Rosenbaum, M.H. Lead seasonality in humans, animals, and the natural environment. Environ. Res. 2020, 180, 108797. [Google Scholar] [CrossRef] [PubMed]
- Altarez, R.D.D.; Sedigo, N.A. Existing land use and extent of lead (Pb) contamination in the grazing food chain of the closed Carmona sanitary landfill in the Philippines. Heliyon 2019, 5, e01680. [Google Scholar] [CrossRef]
- Cooper, S.M.; Van der Merwe, M. Game ranching for meat production in marginal African agricultural lands. J. Arid Land Stud. 2014, 24, 249–252. [Google Scholar]
- Doabi, S.A.; Karami, M.; Afyuni, M.; Yeganeh, M. Pollution and health risk assessment of heavy metals in agricultural soil, atmospheric dust and major food crops in Kermanshah province, Iran. Ecotoxicol. Environ. Saf. 2018, 163, 153–164. [Google Scholar] [CrossRef]
- Kamunda, C.; Mathuthu, M.; Madhuku, M. Health Risk Assessment of Heavy Metals in Soils from Witwatersrand Gold Mining Basin, South Africa. Int. J. Environ. Res. Public Health 2016, 13, 663. [Google Scholar] [CrossRef]
- Fowler, B.A.; Alexander, J.; Oskarsson, A. Toxic metals in food. In Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2015; pp. 123–140. [Google Scholar]
- Prashanth, L.; Kattapagari, K.K.; Chitturi, R.; Baddam, V.R.; Prasad, L. A review on role of essential trace elements in health and disease. J. Dr. NTR Univ. Health Sci. 2015, 4, 75. [Google Scholar] [CrossRef]
- Mudgal, V.; Madaan, N.; Mudgal, A.; Singh, R.; Mishra, S. Effect of toxic metals on human health. Open Nutraceuticals J. 2010, 3, 1. [Google Scholar] [CrossRef]
- Durkalec, M.; Szkoda, J.; Kolacz, R.; Opalinski, S.; Nawrocka, A.; Zmudzki, J. Bioaccumulation of Lead, Cadmium and Mercury in Roe Deer and Wild Boars from Areas with Different Levels of Toxic Metal Pollution. Int. J. Environ. Res. 2015, 9, 205–212. [Google Scholar] [CrossRef]
- Adejumo, O.E.; Fasinu, P.S.; Odion, J.E.; Silva, B.O.; Fajemirokun, T.O. High Cadmium Levels in Cured Meat Products Marketed in Nigeria—Implications for Public Health. Asian Pac. J. Cancer Prev. 2016, 17, 1933–1936. [Google Scholar] [CrossRef]
- Gerofke, A.; Martin, A.; Schlichting, D.; Gremse, C.; Müller-Graf, C. Heavy metals in game meat. In Chemical Hazards in Foods of Animal Origin; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; pp. 435–439. [Google Scholar]
- Taggart, M.A.; Reglero, M.M.; Camarero, P.R.; Mateo, R. Should legislation regarding maximum Pb and Cd levels in human food also cover large game meat? Environ. Int. 2011, 37, 18–25. [Google Scholar] [CrossRef]
- Phillips, L. Bringing more South African game meat to dinner tables. Farmer’s Wkly. 2019, 19031, 30–32. [Google Scholar]
- Fan, Y.; Zhu, T.; Li, M.; He, J.; Huang, R. Heavy Metal Contamination in Soil and Brown Rice and Human Health Risk Assessment near Three Mining Areas in Central China. J. Health Eng. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Szkoda, J.; Durkalec, M.; Kołacz, R.; Opaliński, S.; Żmudzki, J. Content of cadmium, lead and mercury in the tissues of game animals. Med. Weter. 2012, 68, 689–692. [Google Scholar]
- Mateo, R.; Kanstrup, N. Regulations on lead ammunition adopted in Europe and evidence of compliance. Ambio 2019, 48, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolska, A.; Melosik, M. Bullet-derived lead in tissues of the wild boar (Sus scrofa) and red deer (Cervus elaphus). Eur. J. Wildl. Res. 2007, 54, 231–235. [Google Scholar] [CrossRef]
- Kanstrup, N.; Thomas, V.G. Transitioning to lead-free ammunition use in hunting: Socio-economic and regulatory considerations for the European Union and other jurisdictions. Environ. Sci. Eur. 2020, 32, 1–11. [Google Scholar] [CrossRef]
- Juric, A.K.; Batal, M.; David, W.; Sharp, D.; Schwartz, H.; Ing, A.; Fediuk, K.; Black, A.; Tikhonov, C.; Chan, H.M. A total diet study and probabilistic assessment risk assessment of dietary mercury exposure among First Nations living on-reserve in Ontario, Canada. Environ. Res. 2017, 158, 409–420. [Google Scholar] [CrossRef]
- Department of Health, Minister of Health, Government of South Africa. Department of Health. Food Stuff, Cosmetics and Disinfactant Act. (Act. 54 of 1972): Regulations Relating to Maximum Levels of Metals in Food; Department of Health, Minister of Health, Government of South Africa: Pretoria, South Africa, 2018.
- European Food Safety Authority. Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety; European Food Safety Authority: Parma, Italy, 2002. [Google Scholar]
- Ministry of Health, People’s Republic of China. National Food Safety Standard: Maximum Levels of Contaminants in Food, GB2762-2012; Ministry of Health, People’s Republic of China: Beijing, China, 2013.
- United States Food and Drugs Administration, FDA. Potential Hazards Associated with the Manufacturing, Processing, Packing, and Holding of Human Food. In Food Safety in the Center for Food Safety and Applied Nutrition at the U.S. Food and Drug Administration; United States Food and Drugs Administration, FDA: Washington, DC, USA, 2018. [Google Scholar]
- Codex Alimentarius Commission. Codex General Standard for Contaminants and Toxins in Food and Feed; World Health Organisation: Geneva, Switzerland, 2010. [Google Scholar]
- Uhart, M.; del Valle Ferreyra, H.; Romano, M.; Muchiutti, A.; Alzuagaray, S.; Santiago, M.; Caselli, A. Lead pollution from hunting ammunition in Argentina and current state of lead shot replacement efforts. Ambio 2019, 48, 1015–1022. [Google Scholar] [CrossRef]
- MacLachlan, D.; Budd, K.; Connolly, J.; Derrick, J.; Penrose, L.; Tobin, T. Arsenic, cadmium, cobalt, copper, lead, mercury, molybdenum, selenium and zinc concentrations in liver, kidney and muscle in Australian sheep. J. Food Compos. Anal. 2016, 50, 97–107. [Google Scholar] [CrossRef]
- Hampton, J.O.; Laidlaw, M.; Buenz, E.; Arnemo, J.M. Heads in the sand: Public health and ecological risks of lead-based bullets for wildlife shooting in Australia. Wildl. Res. 2018, 45, 287. [Google Scholar] [CrossRef]
- Bond, A.L.; Robertson, G.; Lavers, J.L.; Hobson, K.A.; Ryan, P.C. Trace element concentrations in harvested auks from Newfoundland: Toxicological risk of a traditional hunt. Ecotoxicol. Environ. Saf. 2015, 115, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Laird, B.D.; Chan, H.M. Bioaccessibility of metals in fish, shellfish, wild game, and seaweed harvested in British Columbia, Canada. Food Chem. Toxicol. 2013, 58, 381–387. [Google Scholar] [CrossRef]
- Legagneux, P.; Suffice, P.; Messier, J.-S.; Lelievre, F.; Tremblay, J.A.; Maisonneuve, C.; Saint-Louis, R.; Bêty, J. High Risk of Lead Contamination for Scavengers in an Area with High Moose Hunting Success. PLoS ONE 2014, 9, e111546. [Google Scholar] [CrossRef]
- Ballová, Z.; Janiga, M.; Hančinský, R. Comparison of Element Concentrations (Ba, Mn, Pb, Sr, Zn) in the Bones and Teeth of Wild Ruminants from the West Carpathians and the Tian-Shan Mountains as Indicators of Air Pollution. Atmosphere 2019, 10, 64. [Google Scholar] [CrossRef]
- Thomas, V.G.; Pain, D.J.; Kanstrup, N.; Green, R.E. Setting maximum levels for lead in game meat in EC regulations: An adjunct to replacement of lead ammunition. Ambio 2020, 49, 2026–2037. [Google Scholar] [CrossRef] [PubMed]
- Trinogga, A.L.; Courtiol, A.; Krone, O. Fragmentation of lead-free and lead-based hunting rifle bullets under real life hunting conditions in Germany. Ambio 2019, 48, 1056–1064. [Google Scholar] [CrossRef]
- Grúz, A.; Mackle, O.; Bartha, A.; Szabó, R.; Déri, J.; Budai, P.; Lehel, J. Biomonitoring of toxic metals in feathers of predatory birds from eastern regions of Hungary. Environ. Sci. Pollut. Res. 2019, 26, 26324–26331. [Google Scholar] [CrossRef]
- Lehel, J.; Laczay, P.; Gyurcsó, A.; Jánoska, F.; Majoros, S.; Lányi, K.; Marosán, M. Toxic heavy metals in the muscle of roe deer (Capreolus capreolus)—food toxicological significance. Environ. Sci. Pollut. Res. 2016, 23, 4465–4472. [Google Scholar] [CrossRef]
- Sinkakarimi, M.H.; Pourkhabbaz, A.R.; Hassanpour, M.; Levengood, J.M.; Ghasempouri, S.M. Potential human health risk assessment of heavy metals in the flesh of mallard and pochard in the South Eastern Caspian Sea region of Iran. J. Adv. Environ. Health Res. 2015, 3, 139–145. [Google Scholar]
- Determination of chromium, lead and cadmium levels in edible organs of marketed chickens in Mashhad, Iran. J. Food Qual. Hazards Control. 2015, 2, 134–138.
- Hashemi, M.; Salehi, T.; Aminzare, M.; Raeisi, M.; Afshari, A. Contamination of toxic heavy metals in various foods in Iran: A review. J. Pharm. Sci. Res. 2017, 9, 1692–1697. [Google Scholar]
- Abd-ElGhany, S.M.; Mohammed, M.A.; Abdelkhalek, A.; Saad, F.S.S.; Sallam, K.I. Health Risk Assessment of Exposure to Heavy Metals from Sheep Meat and Offal in Kuwait. J. Food Prot. 2020, 83, 503–510. [Google Scholar] [CrossRef]
- Adán, E.-G.; Gerardo, G.-B. Lead Concentrations in Sediments and Blue-Winged Teals (Anas discors) From El Palmar State Reserve, Yucatan, Mexico. Arch. Environ. Contam. Toxicol. 2013, 65, 588–597. [Google Scholar] [CrossRef]
- Hiller, B.J.; Barclay, J.S. Concentrations of Heavy Metals in American Woodcock Harvested in Connecticut. Arch. Environ. Contam. Toxicol. 2011, 60, 156–164. [Google Scholar] [CrossRef]
- Magwedere, K.; Shimwino, J.; Hemberger, Y.; Hoffman, L.; Midzi, E.; Dziva, F. Lead and cadmium levels in liver, kidney and muscle of harvested wild springbok (Antidorcus marsupialis) under extensive management in southern and southeastern Namibia. Afr. J. Wildl. Res. 2013, 43, 52–60. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.; Al-Amer, S.; Gooneratne, R.; Mason, S.L.; Osman, K.A.; Clucas, L. Concentrations of trace elementals and organochlorines in Mutton bird (Puffinus griseus). Ecotoxicol. Environ. Saf. 2011, 74, 1742–1746. [Google Scholar] [CrossRef]
- Abel, E. Evaluation of Some Heavy Metals’ Concentration Levels in Fresh and Smoke-Dried Porcupine (Atherur africanus) Meat. EC Nutr. 2016, 4, 927–936. [Google Scholar]
- Lucky, L.N.; Temitayo, L.O.; Nwidu, L.L.; Ohemu, T.L. Hematotoxicity status of lead and three other heavy metals in cow slaughtered for human consumption in Jos, Nigeria. J. Toxicol. Environ. Health Sci. 2017, 9, 83–91. [Google Scholar] [CrossRef][Green Version]
- Oloruntoba, A.; Nathaniel, I.A. Assessment of Heavy Metal Levels in Offal Meats (Kidney and Liver) of Beef Sold at Gwagwalada Market, Abuja, Nigeria. Asian J. Phys. Chem. Sci. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Hassan, A.E.A. Nutrients and Toxic Elements in Semidomesticated Reindeer in Norway: Nutritional and Food Safety Aspects; UiT Munin: Tromsø, Norway, 2012. [Google Scholar]
- Khan, M.A.; Lalarukh, I.; Gaitee, J. Detection of Heavy Metal Contents in Meat and Milk of Buffaloes from area of Ring Road and Kasur Lahore. Appl. Zool. 2016, 96, 41851–41858. [Google Scholar]
- Lanocha-Arendarczyk, N.; Kalisinska, E.; Kosik-Bogacka, D.I.; Budis, H.; Podlasinska, J.; Jedrzejewska, E. Mercury levels in raccoons (Procyon lotor) from the Warta Mouth National Park, northwestern Poland. Biol. Trace Elem. Res. 2014, 159, 152–160. [Google Scholar] [CrossRef][Green Version]
- Kalisinska, E.; Lanocha-Arendarczyk, N.; Kosik-Bogacka, D.; Budis, H.; Podlasinska, J.; Popiolek, M.; Piróg, A.; Jedrzejewska, E. Brains of Native and Alien Mesocarnivores in Biomonitoring of Toxic Metals in Europe. PLoS ONE 2016, 11, e0159935. [Google Scholar] [CrossRef]
- Kalisinska, E.; Lisowski, P.; Kosik-Bogacka, D.I. Red fox Vulpes vulpes (L.; 1758) as a bioindicator of mercury contamination in terrestrial ecosystems of north-western Poland. Biol. Trace Elem. Res. 2012, 145, 172–180. [Google Scholar] [CrossRef]
- Felsmann, M.Z.; Szarek, J.; Felsmann, M.; Gulda, D. Lead in game bird meat as a risk to public health: New aspects in the light of physical phenomena generated by a projectile. J. Elem. 2016, 21, 2. [Google Scholar]
- Durkalec, M.; Kolenda, R.; Owczarek, T.; Szkoda, J.; Nawrocka, A.; Grzegrzółka, J.; Dzięgiel, P.; Sochae, P.; Kołacz, R.; Schierack, P.; et al. Expression of metallothionein in the liver and kidneys of the red deer (Cervus elaphus L.) from an industrial metal smelting area of Poland. Ecotoxicol. Environ. Saf. 2017, 137, 121–129. [Google Scholar] [CrossRef]
- Makarov, D.; Komarov, A.; Ovcharenko, V.; Nebera, E.; Kozhushkevich, A.; Kalantaenko, A.; Afanasieva, E.; Demidova, S. Dioxin and Heavy Metals Contamination of Reindeer Offal from Russian Far North Regions. Sel’skokhozyaistvennaya Biol. 2018, 53, 364–373. [Google Scholar] [CrossRef]
- Baloš, M.; Mihaljev, Ž.; Jakšić, S.; Prica, N.; Lazić, G.; Kapetanov, M.; Radulović, J.P. Incidence of Heavy Metals and Other Toxic Elements in Roe Deer (Capreolus capreolus) Tissues. Arch. Vet. Med. 2015, 2, 3–10. [Google Scholar] [CrossRef]
- Gašparík, J.; Binkowski, J.; Jahnátek, A.; Šmehýl, P.; Dobiaš, M.; Lukáč, N.; Błaszczyk, M.; Semla, M.; Massanyi, P. Levels of Metals in Kidney, Liver, and Muscle Tissue and their Influence on the Fitness for the Consumption of Wild Boar from Western Slovakia. Biol. Trace Elem. Res. 2017, 177, 258–266. [Google Scholar] [CrossRef]
- Rodríguez-Estival, J.; Martinez-Haro, M.; Monsalve-González, L.; Mateo, R. Interactions between endogenous and dietary antioxidants against Pb-induced oxidative stress in wild ungulates from a Pb polluted mining area. Sci. Total Environ. 2011, 409, 2725–2733. [Google Scholar] [CrossRef]
- Romero, D.; de José, A.; Theureau, J.M.; Ferrer, A.; Raigón, M.D.; Torregrosa, J.B. Lead in terrestrial game birds from Spain. Environ. Sci. Pollut. Res. 2020, 27, 1585–1597. [Google Scholar] [CrossRef]
- Wiklund, E.; Malmfors, G. Game Meat as a Resource in Sweden–with Particular Focus on Moose (Alces alces). In Trends in Game Meat Hygiene: From Forest to Fork; Wageningen Academic Publishers: Wageningen, The Netherlands, 2014; pp. 625–627. [Google Scholar]
- Oymak, T.; Ulusoy, H.I.; Hastaoglu, E.; Yılmaz, V.; Yıldırım, Ş. Some Heavy Metal Contents of Various Slaughtered Cattle Tissues in Sivas-Turkey. J. Turk. Chem. Soc. Sect. A Chem. 2017, 4, 721–728. [Google Scholar] [CrossRef]
- Demirbas, Y.; Erduran, N. Concentration of Selected Heavy Metals in Brown Hare (Lepus Europaeus) and Wild Boar (Sus Scrofa) From Central Turkey. Balk. J. Wildl. Res. 2017, 4, 26–33. [Google Scholar] [CrossRef]
- Pain, D.J.; Mateo, R.; Green, R.E. Effects of lead from ammunition on birds and other wildlife: A review and update. Ambio 2019, 48, 935–953. [Google Scholar] [CrossRef]
- Green, R.E.; Pain, D.J. Risks to human health from ammunition-derived lead in Europe. Ambio 2019, 48, 954–968. [Google Scholar] [CrossRef]
- Oldenkamp, R.E.; Bryan, A.L., Jr.; Kennamer, R.A.; Leaphart, J.C.; Webster, S.C.; Beasley, J.C. Trace elements and radiocesium in game species near contaminated sites. J. Wildl. Manag. 2017, 81, 1338–1350. [Google Scholar] [CrossRef]
- French, A.; Conway, W.C.; Cañas-Carrell, J.E.; Klein, D.M. Exposure, Effects and Absorption of Lead in American Woodcock (Scolopax minor): A Review. Bull. Environ. Contam. Toxicol. 2017, 99, 287–296. [Google Scholar] [CrossRef]
- Musilova, J.; Árvay, J.; Vollmannova, A.; Toth, T.; Tomas, J. Environmental Contamination by Heavy Metals in Region with Previous Mining Activity. Bull. Environ. Contam. Toxicol. 2016, 97, 569–575. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. Heavy Met. 2018. [Google Scholar] [CrossRef]
- Falandysz, J. Some toxic and trace metals in big game hunted in the northern part of Poland in 1987–1991. Sci. Total Environ. 1994, 141, 59–73. [Google Scholar] [CrossRef]
- Ertl, K.; Kitzer, R.; Goessler, W. Elemental composition of game meat from Austria. Food Addit. Contam. Part. B 2016, 9, 120–126. [Google Scholar] [CrossRef]
- Department of Health, Minister of Health, Government of South Africa. Regulations Relating to the Hazard Analysis and Critical Control Point System (HACCP System), Amendment; Department of Health, Minister of Health, Government of South Africa: Pretoria, South Africa, 2018.
- North, M.A.; Lane, E.P.; Marnewick, K.; Caldwell, P.; Carlisle, G.; Hoffman, L.C. Suspected lead poisoning in two captive cheetahs (Acinonyx jubatus jubatus) in South Africa, in 2008 and 2013: Case report. J. S. Afr. Vet. Assoc. 2015, 86, 1–5. [Google Scholar] [CrossRef]
- Wennberg, M.; Lundh, T.; Sommar, J.N.; Bergdahl, I.A. Time trends and exposure determinants of lead and cadmium in the adult population of northern Sweden 1990–2014. Environ. Res. 2017, 159, 111–117. [Google Scholar] [CrossRef]
- Eisler, R. Mercury hazards from gold mining to humans, plants, and animals. Reviews of Environmental Contam. Toxicol. 2004, 181, 139–198. [Google Scholar]
- Guynup, S.; Safina, B. Mercury: Sources in the environment, health effects, and politics. Blue Ocean. Inst. 2012, 1, 7–54. [Google Scholar]
- Solang, M.; Lamondo, D.; Kumaji, S.S. Zinc, calcium, protein, lead, mercury, and the sensorics quality of cireng snacks supplemented with blood cockle (Anadara granosa). Nusant. Biosci. 2017, 9, 385–391. [Google Scholar] [CrossRef]
- Evans, B.; Leighton, F.A. A history of One Health. Rev. Sci. Tech. Int. Off. Epizoot. 2014, 33, 413–420. [Google Scholar] [CrossRef]
- Monath, T.P.; Kahn, L.H.; Kaplan, B. One health perspective. ILAR J. 2010, 51, 193–198. [Google Scholar] [CrossRef]
- Shrivastava, S.; Shrivastava, P. Indispensable need to involve multiple stakeholders to ensure global food safety: World Health Organization. J. Integr. Health Sci. 2020, 8, 49. [Google Scholar] [CrossRef]
- Duc, P.P.; Toribio, J.-A.; Hai, N.H.T.; Dang-Xuan, S.; Luong, N.T.; Langley, S.J.; Dunham, J.G.; Thuy, D.T.; Hoa, D.V.; Nguyen-Viet, H.; et al. Food safety risk communication and training need of stakeholders and consumers regarding pork value chain in Vietnam. ILRI Res. Brief. 2020. Available online: https://cgspace.cgiar.org/handle/10568/108769 (accessed on 15 October 2021).
- Nkosi, D.; Bekker, J.; Hoffman, L. Impact of communal cattle farming practices on meat safety in central bushbuckridge, South Africa. Int. J. Vet. Sci. 2020, 9, 90–96. [Google Scholar]
- Das, A.K.; Nanda, P.; Das, A.; Biswas, S. Hazards and safety issues of meat and meat products. In Food Safety and Human Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 145–168. [Google Scholar]
- Nastasijevic, I.; Vesković, S.; Milijašević, M. Meat safety: Risk based assurance systems and novel technologies. Sci. J. Meat Technol. 2020, 61, 97–119. [Google Scholar] [CrossRef]
- Gerofke, A.; Ulbig, E.; Martin, A.; Müller-Graf, C.; Selhorst, T.; Gremse, C.; Spolders, M.; Schafft, H.; Heinemeyer, G.; Greiner, M.; et al. Lead content in wild game shot with lead or non-lead ammunition–does “state of the art consumer health protection” require non-lead ammunition? PLoS ONE 2018, 13, e0200792. [Google Scholar] [CrossRef]
- Liu, W.; Zafar, A.; Khan, Z.I.; Nadeem, M.; Ahmad, K.; Wajid, K.; Bashir, H.; Munir, M.; Malik, I.S.; Ashfaq, A. Bioaccumulation of lead in different varieties of wheat plant irrigated with wastewater in remote agricultural regions. Environ. Sci. Pollut. Res. 2020, 27, 27937–27951. [Google Scholar] [CrossRef]
- Mareri, B.; Kitur, E.; Obade, P. Bioaccumulation of zinc, lead, cadmium in water hyacinth, hippo grass and papyrus reed as water quality indicator in River Kisat in Kisumu County, Kenya. Afr. J. Pure Appl. Sci. 2021, 2, 100–107. [Google Scholar] [CrossRef]
- Czechowski, P.; Stevens, M.I.; Madden, C.; Weinstein, P. Steps towards a more efficient use of chironomids as bioindicators for freshwater bioassessment: Exploiting eDNA and other genetic tools. Ecol. Indic. 2020, 110, 105868. [Google Scholar] [CrossRef]
- Asif, N.; Malik, M.; Chaudhry, F. A Review of on Environmental Pollution Bioindicators. Pollution 2018, 4, 111–118. [Google Scholar] [CrossRef]
- Marescotti, M.E.; Caputo, V.; Demartini, E.; Gaviglio, A. Discovering market segments for hunted wild game meat. Meat Sci. 2018, 149, 163–176. [Google Scholar] [CrossRef]
- Laricheva, K.; Petrov, D. The Experience in Implementing the Food Safety Management System at a Meat Processing Enterprise; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Antic, D.; Houf, K.; Michalopoulou, E.; Blagojevic, B. Beef abattoir interventions in a risk-based meat safety assurance system. Meat Sci. 2021, 182, 108622. [Google Scholar] [CrossRef]
- ISO 22000:2018. Food Safety Management Systems—Requirements for Any Organization in the Food Chain; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- Europian Union Commission, EUC. Maximum Levels of Cadmium in Foodstuffs. J. Eur. Union. 2014. Available online: https://ec.europa.eu/ (accessed on 1 September 2021).
- Wang, X.; Zhang, Y.; Geng, Z.; Liu, Y.; Guo, L.; Xiao, G. Spatial analysis of heavy metals in meat products in China during 2015–2017. Food Control 2019, 104, 174–180. [Google Scholar] [CrossRef]
- Müller-Graf, C.; Gerofke, A.; Martin, A.; Bandick, N.; Lahrssen-Wiederholt, M.; Schafft, H.-A.; Selhorst, T.; Ulbig, E.; Hensel, A. Reduction of lead contents in game meat: Results of the ‘Food safety of game meat obtained through hunting’ research project. Game Meat Hyg. 2017, 201–212. [Google Scholar] [CrossRef]
- Boqvist, S.; Söderqvist, K.; Vågsholm, I. Food safety challenges and One Health within Europe. Acta Vet. Scand. 2018, 60, 1–13. [Google Scholar] [CrossRef]
| Country | Type | Arsenic (Semi-Metal) | Lead | Mercury | Cadmium | Source(s) |
|---|---|---|---|---|---|---|
| South Africa | Regulation | 1.0 | 0.10 | - | 0.050 | [35] |
| EU countries | Regulation | 0.3 | 0.10 | 1.0 | 0.050 | [36] |
| Republic of China | Regulation | 0.5 | 0.5 | 0.05 | 0.1 | [37] |
| Australia and New Zealand | Regulation | 0.01 | 0.10 | 0.002 | 0.05 | [11] |
| United States of America | Regulation | 0.01 | 0.01 | 0.01 | 0.01 | [38] |
| Countries without specified limits | Codex Alimentarius standards | 2.1 | 0.10 | - | 0.05 | [39] |
| Country | Aim | Species | Study Findings and Recommendations | Sources |
|---|---|---|---|---|
| Argentina | To investigate the presence and concentrations of toxic metals in waterfowl (Anseriformes) hunted. | Waterfowl (Anseriformes) |
| [40] |
| Australia | To analyse trace elements of Cd, Pb, Hg and As in the muscle, liver and kidney of sheep commercially farmed in Australia. | Sheep (Ovine aries) |
| [41] |
| Australia | To investigate the levels of Pb on carcasses shot as part of a culling program for wild animals’ control in Australia. | Game animals |
| [42] |
| Canada | To investigate trace elements of As, Cd, HG and Pb concentrations in the breast muscle of harvested auks from Newfoundland. | Birds (Uria spp. and Alca torda) |
| [43] |
| Canada | To measure the concentration and bio-accessibility of toxic metals in traditionally consumed foods | Wild game-Moose (Alcer alces), Deer (Cervidae) and Rabbit (Oryctolagus cuniculus) |
| [44] |
| Canada | To investigate the levels of Pb poisoning in Moose resulting from the consumption of ammunition residues in the tissues of big game killed by hunters. | Moose (Alces alces) |
| [45] |
| China | To investigate the role played by environmental pollution and its contamination of game animals | Wild game |
| [46] |
| EU Countries | To investigate the possible setting of maximum levels for lead in game meat in EC regulations. | Game meat animals species |
| [47] |
| Germany | To investigate the differences in the fragmentation patterns of Lead and Lead-free bullets in real hunting conditions | Game meat animals hunted. |
| [48] |
| Hungary | To evaluate the As, Cd, Pb and Hg concentration in different predator birds: Hen harrier and Marsh harrier around Hungary. | Hen harrier (Circus cyaneus) and Marsh harrier (C. aeruginosu) |
| [49] |
| Hungary | To investigate the concentration of Cd, Pb, Hg and As in the muscle tissue of Roe deer. | Roe deer (Capreolus capreolus) |
| [50] |
| Iran | To estimate the health risks of exposure to Cd, Cr, Fe, Pb, and Zn due to the consumption of pectoral muscle of mallard and pochard. | Mallard (Anas platyrhynchos) and pochard (Aythya ferina) |
| [51] |
| Iran | To determine the concentration of Cr, Pb, and Cd in chicken tissue from Mashhad, North-East of Iran. | Chicken (Gallus gallus domesticus) |
| [52] |
| Iran | To review the level of food contamination with toxic metals in Iran. | Chicken (Gallus gallus domesticus) |
| [53] |
| Kuwait | To investigate the presence of Hg, As, Pb, Cd, and Cr in slaughtered sheep (Ovine aries) at abattoirs in Kuwait. | Sheep (Ovine aries) |
| [54] |
| Mexico | To investigate the levels of Pb in Blue-Winged teals. | Birds (Teals) (Anas discors). |
| [55] |
| Mexico | To investigate the concentrations of As, Cd, Cr, Hg, Pb, and Se in pectoral muscle of Woodcock. | Woodcock (Scolopax) |
| [56] |
| Namibia | To assess toxic metal (Pb and Cd) values in meat and offal from harvested springbok. | Springbok (Antidorcas marsupialis) |
| [57] |
| New Zealand | To investigate the concentration of 22 elements (including Fe, Ca and Se) and selected organochlorines in Mutton birds over two years. | Mutton bird (Puffinus griseus) |
| [58] |
| Nigeria | To evaluate and compare the levels of toxic metal contamination in the muscle, liver and kidney of both fresh and smoke-dried porcupine meats sold in Edo State, Nigeria. | Porcupine (Hystricomorph Hystricidae) |
| [59] |
| Nigeria | To assess concentration levels of Cd, Pb, Cu and Mg in cattle slaughtered in Jos North and South of Plateau State, Northern Nigeria. | Cattle (Bos taurus) |
| [60] |
| Nigeria | To determine the concentrations of Cd, Fe, Mn, Pb and Zn in the kidney and liver of slaughtered cattle. | Cattle (Bos taurus) |
| [61] |
| Norway | To study the concentration of toxic elements in semi-domesticated reindeer in Norway. | Reindeer (Rangifer tarandus) |
| [62] |
| Pakistan | To detect the concentrations of various toxic metals in meat, liver, milk and fodder of buffaloes. | Buffalo (Bubalus bubalis) |
| [63] |
| Poland | To determine total Hg concentrations in the liver, kidney, semimembranosus muscle and brain of raccoons originating from the Warta Mouth National Park (WMNP) in north western Poland. | Raccoons (Procyon lotor) |
| [64] |
| Poland | The estimation of Hg, Pb and Cd concentrations and the determination of relationships between these elements in the brains of mesocarnivores (Wild badger and fox), road-killed or hunted animals. | Wild badger (T. taxus) and Fox (Vulpes vulpes) |
| [65] |
| Poland | To determine the concentrations of total Hg in samples of liver, kidney and skeletal muscle of red foxes. | Red foxes (Vulpes vulpes) |
| [66] |
| Poland | To assess the accumulation of Cd, Pb and Hg in the tissues of roe deer, red deer and wild boar from selected major industrial areas. | Roe deer (Capreolus capreolus), Red deer (Cervus elaphus) and Wild boar (Sus scrofa) |
| [30] |
| Poland | To investigate Pb in game bird meat as a risk to public health. | Game bird meat |
| [67] |
| Poland | To investigate the concentration levels of Pb, Cd and Hg in livers and kidneys of red deer harvested around industrial smelting plants. | Red deer (Cervus elaphus) |
| [68] |
| Russia | To investigate the levels of dioxins, dl-PCBs, Cd and Hg in reindeer meat, liver and kidneys samples. | Reindeer (Rangifer tarandus) |
| [69] |
| Serbian | To evaluate the concentration of environmental contaminants Pb, As, Hg, Cd and Cu in tissues of free-living roe deer in Serbia. | Roe deer (Capreolus capreolus) |
| [70] |
| Slovakia | To present the concentrations of Cd, Co, Cu, Hg, Pb and Zn in the kidneys, liver and muscles of wild boar | Wild boar (Sus scrofa) |
| [71] |
| Spain | To study the interactions between glutathione, superoxide dismutase and peroxidase antioxidants and to evaluate their role in fighting Pb-induced oxidative stress in wild ungulates. | Red deer (Cervus elaphus) and Wild boar (Sus scrofa) |
| [72] |
| Spain | To analyse the presence of Pb and its relationship with Pb-based ammunition in terrestrial game birds: Woodpigeons and Rock doves. | Woodpigeons (Columba palumbus) and rock doves (Columba livia) |
| [73] |
| Sweden | To investigate game meat hazards in moose killing, handling and processing in Sweden. | Moose (Alces alces) |
| [74] |
| Turkey | To evaluate the concentrations of As, Cd, Hg and Pb in the kidney, liver, lung, muscle and brain of slaughtered cattle from Sivas, Turkey. | Cattle (Bos taurus) |
| [75] |
| Turkey | To investigate concentrations of Mn, Cu, Zn, Cd, Hg, Pb and Se in Wild boars and brown hares. | Wild boars (Sus scrofa) and brown hares (Lepus europaeus) |
| [76] |
| United Kingdom | To investigate the effects of lead from ammunition on Wildfowl (Anatidae) birds. | Wildfowl (Anatidae) |
| [77] |
| United Kingdom | To estimate potential risks to human health in the UK from dietary exposure to Pb from wild game birds killed by shooting. | Game birds |
| [78] |
| United States of America | To determine the levels of Hg, Se and As in muscle and liver tissues of game meat animals. | Wild pigs (Sus scrofa), Waterfowl (Anseriformes) and grey squirrels (Sciurus carolinensis) |
| [79] |
| United States of America | This review discusses Pb exposure routes, effects of Pb toxicity and the distribution of Pb in American woodcock. | Woodcock (Scolopax minor) |
| [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkosi, D.V.; Bekker, J.L.; Hoffman, L.C. Toxic Metals in Wild Ungulates and Domestic Meat Animals Slaughtered for Food Purposes: A Systemic Review. Foods 2021, 10, 2853. https://doi.org/10.3390/foods10112853
Nkosi DV, Bekker JL, Hoffman LC. Toxic Metals in Wild Ungulates and Domestic Meat Animals Slaughtered for Food Purposes: A Systemic Review. Foods. 2021; 10(11):2853. https://doi.org/10.3390/foods10112853
Chicago/Turabian StyleNkosi, Davies Veli, Johan Leon Bekker, and Louwrens Christian Hoffman. 2021. "Toxic Metals in Wild Ungulates and Domestic Meat Animals Slaughtered for Food Purposes: A Systemic Review" Foods 10, no. 11: 2853. https://doi.org/10.3390/foods10112853
APA StyleNkosi, D. V., Bekker, J. L., & Hoffman, L. C. (2021). Toxic Metals in Wild Ungulates and Domestic Meat Animals Slaughtered for Food Purposes: A Systemic Review. Foods, 10(11), 2853. https://doi.org/10.3390/foods10112853






