From Plantation to Cup: Changes in Bioactive Compounds during Coffee Processing
Abstract
:1. Introduction
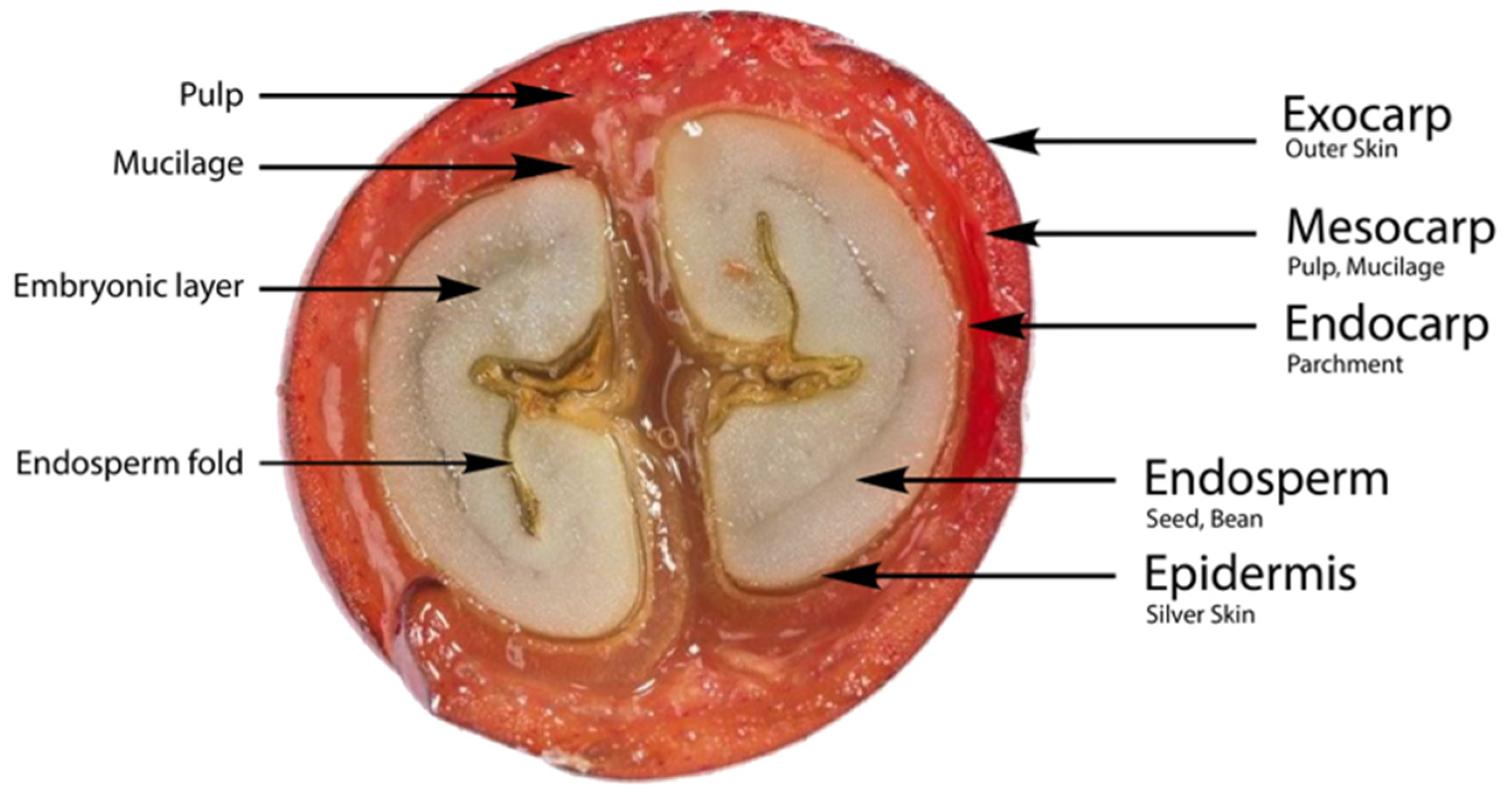
2. Parts and Chemical Compounds of Coffee Cherry Bean
2.1. Maturity of Coffee Bean
2.2. Bioactive Compound in Coffee Cherry Bean
2.3. Lipids, Proteins, and Carbohydrates in Cherry Coffee Bean
3. Harvesting of Coffee Beans
4. Mode of Processing Affects Coffee Quality
4.1. Dry Process/Natural Process/Sun-Dried
4.2. Wet Process/Washed/Fully-Washed
4.3. Semi-Dry Processing/Honey-Coffee
| Component | Coffee Sample | Processing Type | Value | References |
|---|---|---|---|---|
| Caffeine | Arabica, Brazil | Wet process | 1.05–1.53% | [56] |
| Arabica, Brazil | Wet process | 1.32–1.42% | [79] | |
| Arabica, Thailand | Wet process | 1.20–1.26% | [55] | |
| Robusta, Indonesia | Wet process | 1.82% | [80] | |
| Arabica, Brazil | Dry process | 1.24–1.35% | [79] | |
| Robusta, Indonesia | Dry process | 1.81% | [80] | |
| Robusta, China | Dry process | 1.88–2.61% | [81] | |
| Arabica, Brazil | Semi-dry process | 1.12–1.54% | [56] | |
| Trigonelline | Arabica, Brazil | Wet process | 0.80–1.40% | [56] |
| Arabica, Brazil | Wet process | 1.01–1.18% | [79] | |
| Arabica, Brazil | Dry process | 0.96–1.01% | [79] | |
| Robusta, China | Dry process | 0.75–0.87% | [81] | |
| Arabica, Brazil | Semi-dry process | 0.64–0.92 | [56] | |
| Chlorogenic acid | Arabica, Brazil | Wet process | 6.08% | [56] |
| Arabica, Brazil | Wet process | 7.53–7.58% | [79] | |
| Robusta, Indonesia | Wet process | 5.84% | [80] | |
| Arabica, Colombia | Wet process | 4.89% | [82] | |
| Arabica, Brazil | Dry process | 7.34–7.60% | [79] | |
| Robusta, Indonesia | Dry process | 9.57% | [80] | |
| Arabica, Brazil | Semi-dry process | 5.8% | [56] | |
| Arabica, Colombia | Semi-dry process | 5.04% | [82] | |
| Total Protein | Robusta, China | Dry process | 15.3–16.4% | [81] |
| Phenolic compounds | Arabica, Brazil | Dry Process | 6.9–7.6% | [83] |
| Arabica, Brazil | Semi-dry process | 7.67% | [84] | |
| Free amino acid | Arabica, Brazil | Dry process | 0.36% | [85] |
| Arabica, Brazil | Wet Process | 0.43% | [85] | |
| Arabica, Columbia | Dry process | 0.27% | [85] | |
| Arabica, Columbia | Wet process | 0.31% | [85] | |
| Arabica, Germany | Dry process | 0.51% | [85] | |
| Arabica, Germany | Wet process | 0.54% | [85] | |
| Arabica, Germany | Wet process | 0.27–0.48% | [86] | |
| Robusta, Germany | Wet process | 0.35–0.60% | [86] | |
| GABA | Arabica, Brazil | Wet Process | 93 nmol/seed | [58] |
| Arabica, Tanzania | Wet Process | 140 nmol/seed | [58] | |
| Arabica, Brazil | Dry Process | 1009 nmol/seed | [58] | |
| Arabica, Tanzania | Dry Process | 1860 nmol/seed | [58] | |
| Carbohydrates | ||||
| Sucrose | Arabica, Brazil | Wet process | 9% | [56] |
| Arabica, Brazil | Wet process | ±7.90% | [87] | |
| Arabica, Brazil | Wet process | 5.89–7.31% | [79] | |
| Arabica, Kenya | Wet Process | 9.31% | [87] | |
| Arabica, Costa Rica | Wet process | 6% | [88] | |
| Arabica, Thailand | Wet process | 4.43–4.85% | [55] | |
| Arabica, Brazil | Dry process | 7.07% | [87] | |
| Arabica, Ethiopia | Dry process | 8.26% | [87] | |
| Arabica, Brazil | Dry process | 6.81–8.95% | [79] | |
| Robusta, Indonesia | Dry process | 4.85% | [87] | |
| Robusta, Vietnam | Dry process | 3.15% | [87] | |
| Robusta, Uganda | Dry process | 4.56% | [87] | |
| Arabica, Brazil | Semi-dry process | ±8.10% | [87] | |
| Arabica, Brazil | Semi-dry process | 12.3% | [56] | |
| Glucose | Arabica, Costa Rica | Wet process | 0.02% | [88] |
| Arabica, Brazil | Dry process | 0.23% | [87] | |
| Arabica, Brazil | Wet process | ±0.03% | [87] | |
| Arabica, Brazil | Semi-dry process | ±0.11% | [87] | |
| Fructose | Arabica, Costa Rica | Wet process | 0.03% | [88] |
| Arabica, Brazil | Dry process | ±0.33% | [87] | |
| Arabica, Brazil | Wet process | ±0.04% | [87] | |
| Arabica, Brazil | Semi-dry process | ±0.19% | [87] | |
| Total pectin | Arabica, Brazil | Dry process | 899.09 mg/100 g | [83] |
| Arabica, Brazil | Dry process | 1191.81 mg/100 g | [84] | |
| Lipid | Robusta, China | Dry process | 8.60–12.03% | [81] |
5. Microbiota Associated with Coffee Processing
5.1. Microbiota in Dry-Process
5.2. Microbiota in Wet Process
5.3. Microbiota in Semi-Dry Process
6. Green Bean Storage
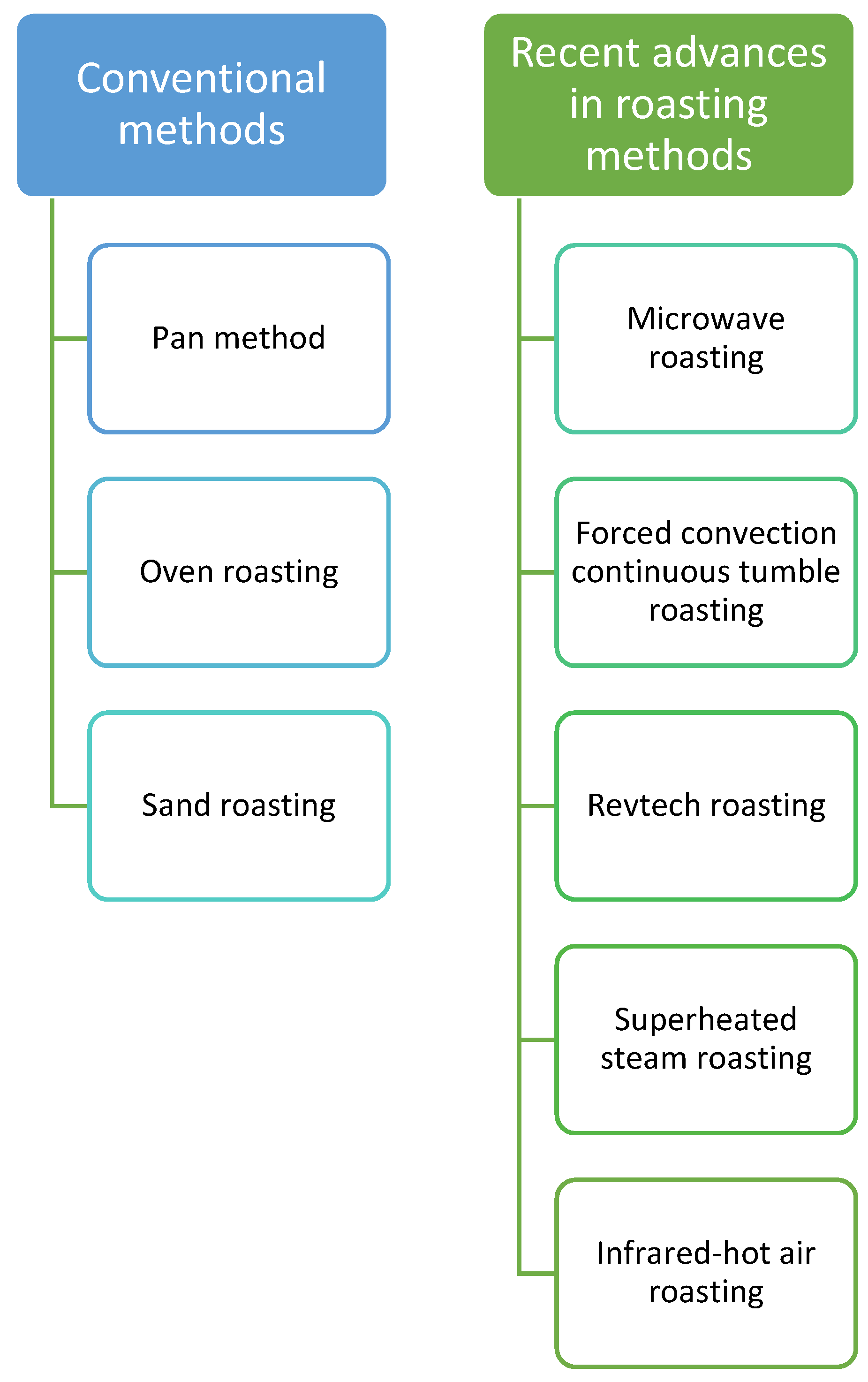
7. Roasting
7.1. Effect of Roasting on Caffeine
7.2. Effect of Roasting on Trigonelline
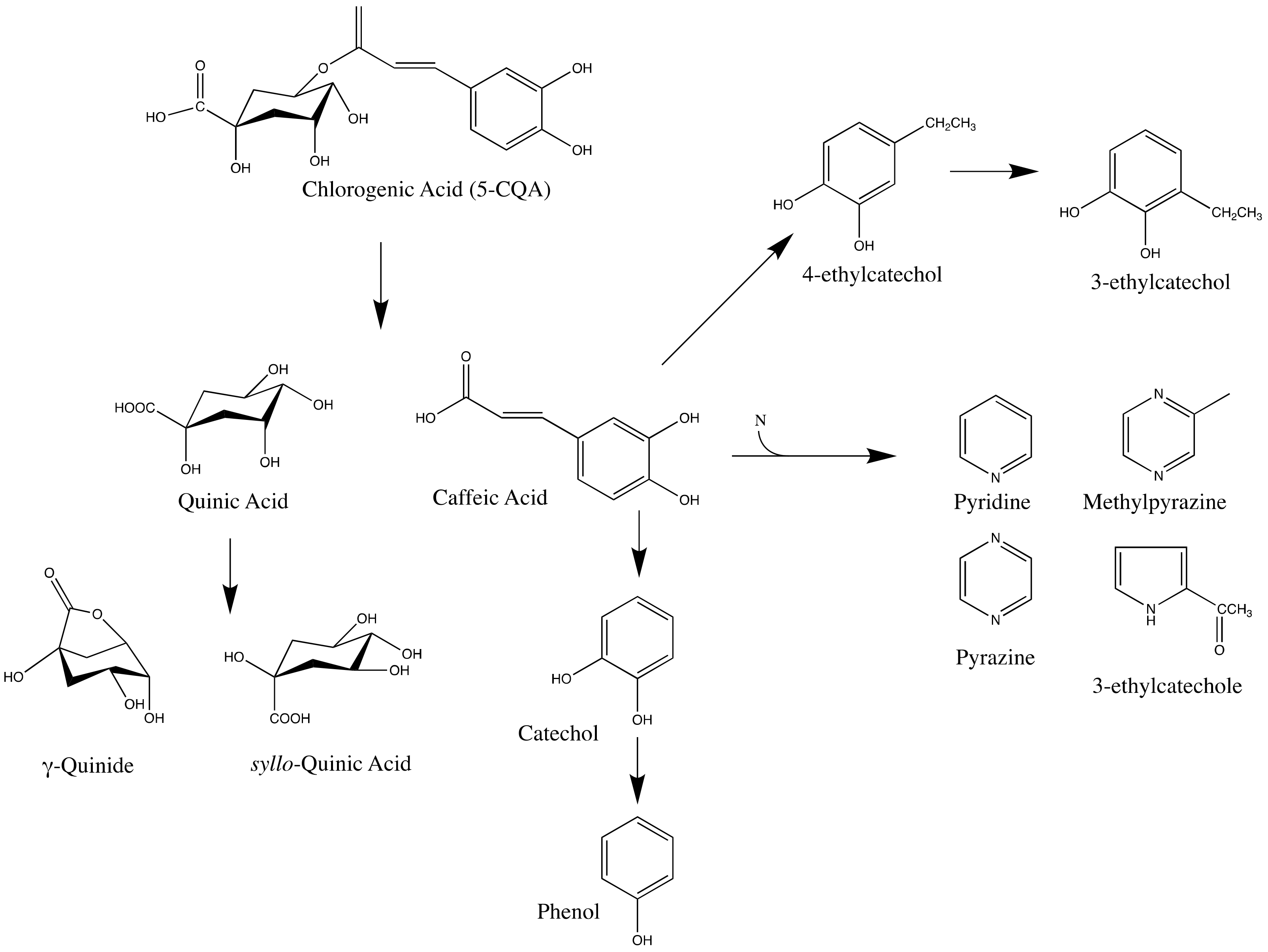
7.3. Effect of Roasting on Chlorogenic Acid
7.4. Effect of Roasting on Total Phenolic Content (TPC)
7.5. Effect of Roasting on Acrylamide Content
8. Storage of Roasted Coffee Bean
9. Brewing
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Stoffelen, P. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006, 152, 465–512. [Google Scholar] [CrossRef] [Green Version]
- Perrone, D.; Farah, A.; Donangelo, C.M.; de Paulis, T.; Martin, P.R. Comprehensive analysis of major and minor chlorogenic acids and lactones in economically relevant Brazilian coffee cultivars. Food Chem. 2008, 106, 859–867. [Google Scholar] [CrossRef]
- Zhang, S.J.; De Bruyn, F.; Pothakos, V.; Torres, J.; Falconi, C.; Moccand, C.; Weckx, S.; de Vuyst, L. Following Coffee Production from Cherries to Cup: Microbiological and Metabolomic Analysis of Wet Processing of Coffea arabica. Appl. Environ. Microbiol. 2019, 85, e02635-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haile, M.; Bae, H.M.; Kang, W.H. Comparison of the Antioxidant Activities and Volatile Compounds of Coffee Beans Obtained Using Digestive Bio-Processing (Elephant Dung Coffee) and Commonly Known Processing Methods. Antioxidants 2020, 9, 408. [Google Scholar] [CrossRef]
- Flament, I. Coffee Flavor Chemistry; Wiley: Hoboken, NJ, USA, 2002; p. 424. [Google Scholar]
- Dias, E.C.; Borém, F.M.; Pereira, R.G.F.A.; Guerreiro, M.C. Amino acid profiles in unripe Arabica coffee fruits processed using wet and dry methods. Eur. Food Res. Technol. 2012, 234, 25–32. [Google Scholar] [CrossRef]
- Dias, E.C.; Pereira, R.G.F.A.; Borém, F.M.; Mendes, E.; De Lima, R.R.; Fernandes, J.; Casal, S. Biogenic Amine Profile in Unripe Arabica Coffee Beans Processed According to Dry and Wet Methods. J. Agric. Food Chem. 2012, 60, 4120–4125. [Google Scholar] [CrossRef] [PubMed]
- Sinaga, H.L.R.; Bastian, F.; Syarifuddin, A. Effect of decaffeination and re-fermentation on level of caffeine, chlorogenic acid and total acid in green bean robusta coffee. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 807, p. 22069. [Google Scholar]
- De Castro, R.D.; Marraccini, P. Cytology, biochemistry and molecular changes during coffee fruit development. Braz. J. Plant Physiol. 2006, 18, 175–199. [Google Scholar] [CrossRef] [Green Version]
- Montavon, P.; Duruz, E.; Rumo, G.; Pratz, G. Evolution of Green Coffee Protein Profiles with Maturation and Relationship to Coffee Cup Quality. J. Agric. Food Chem. 2003, 51, 2328–2334. [Google Scholar] [CrossRef]
- Pereira, G.; Neto, D.P.D.C.; Júnior, A.I.M.; Vásquez, Z.S.; Medeiros, A.B.; Vandenberghe, L.; Soccol, C.R. Exploring the impacts of postharvest processing on the aroma formation of coffee beans—A review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef] [PubMed]
- De Mejia, E.G.; Ramirez-Mares, M.V. Impact of caffeine and coffee on our health. Trends Endocrinol. Metab. 2014, 25, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, M.C. The Impact of Caffeine and Coffee on Human Health. Nutrition 2019, 11, 416. [Google Scholar] [CrossRef] [Green Version]
- Leroy, T.; Marraccini, P.; Dufour, M.; Montagnon, C.; Lashermes, P.; Sabau, X.; Ferreira, L.P.; Jourdan, I.; Pot, D.; Andrade, A.C.; et al. Construction and characterization of a Coffea canephora BAC library to study the organization of sucrose biosynthesis genes. Theor. Appl. Genet. 2005, 111, 1032–1041. [Google Scholar] [CrossRef]
- Joët, T.; Laffargue, A.; Descroix, F.; Doulbeau, S.; Bertrand, B.; de Kochko, A.; Dussert, S. Influence of environmental factors, wet processing and their interactions on the biochemical composition of green Arabica coffee beans. Food Chem. 2010, 118, 693–701. [Google Scholar] [CrossRef]
- Bertrand, B.; Boulanger, R.; Dussert, S.; Ribeyre, F.; Berthiot, L.; Descroix, F.; Joët, T. Climatic factors directly impact the volatile organic compound fingerprint in green Arabica coffee bean as well as coffee beverage quality. Food Chem. 2012, 135, 2575–2583. [Google Scholar] [CrossRef]
- Wintgens, J.N. Coffee: Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders, and Researchers. In Coffee: Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders, and Researchers; Wiley: Weinheim, Germany, 2008; pp. 1–603. [Google Scholar]
- Marín-López, S.M.; Arcila-Pulgarín, J.; Montoya-Restrepo, E.C.; Oliveros-Tascón, C.E. Cambios Físicos Y Químicos Durante La Maduracíon del Fruto de Café (Coffea arabica L. var. Colombia). Cenifcafé 2003, 54, 208–225. [Google Scholar]
- Geromel, C.; Ferreira, L.P.; Davrieux, F.; Guyot, B.; Ribeyre, F.; Scholz, M.B.D.S.; Pereira, L.F.P.; Vaast, P.; Pot, D.; Leroy, T.; et al. Effects of shade on the development and sugar metabolism of coffee (Coffea arabica L.) fruits. Plant Physiol. Biochem. 2008, 46, 569–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geromel, C.; Ferreira, L.P.; Guerreiro, S.M.C.; Cavalari, A.A.; Pot, D.; Pereira, L.F.P.; Leroy, T.; Vieira, L.G.E.; Mazzafera, P.; Marraccini, P. Biochemical and genomic analysis of sucrose metabolism during coffee (Coffea arabica) fruit development. J. Exp. Bot. 2006, 57, 3243–3258. [Google Scholar] [CrossRef] [Green Version]
- Rawel, H.M.; Huschek, G.; Sagu, S.T.; Homann, T. Cocoa Bean Proteins—Characterization, Changes and Modifications due to Ripening and Post-Harvest Processing. Nutrition 2019, 11, 428. [Google Scholar] [CrossRef] [Green Version]
- Setoyama, D.; Iwasa, K.; Seta, H.; Shimizu, H.; Fujimura, Y.; Miura, D.; Wariishi, H.; Nagai, C.; Nakahara, K. High-Throughput Metabolic Profiling of Diverse Green Coffea arabica Beans Identified Tryptophan as a Universal Discrimination Factor for Immature Beans. PLoS ONE 2013, 8, e70098. [Google Scholar] [CrossRef] [PubMed]
- Lee, D. Plant pigments and their manipulation. Ann. Bot. 2005, 96, 1332–1333. [Google Scholar] [CrossRef] [Green Version]
- Koshiro, Y.; Jackson, M.C.; Nagai, C.; Ashihara, H. Changes in the Content of Sugars and Organic Acids During Ripening of Coffea arabica and Coffea Canephora Fruits. Eur. Chem. Bull. 2015, 4, 378–383. [Google Scholar]
- Buckeridge, M.S. Seed Cell Wall Storage Polysaccharides: Models to Understand Cell Wall Biosynthesis and Degradation. Plant Physiol. 2010, 154, 1017–1023. [Google Scholar] [CrossRef] [Green Version]
- Cheng, B.; Furtado, A.; Henry, R.J. The coffee bean transcriptome explains the accumulation of the major bean components through ripening. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; Monteiro, M.; Calado, V.; Franca, A.; Trugo, L. Correlation between cup quality and chemical attributes of Brazilian coffee. Food Chem. 2006, 98, 373–380. [Google Scholar] [CrossRef]
- Monteiro, M.C.; Farah, A. Chlorogenic acids in Brazilian Coffea arabica cultivars from various consecutive crops. Food Chem. 2012, 134, 611–614. [Google Scholar] [CrossRef]
- Belay, A. Some biochemical compounds in coffee beans and methods developed for their analysis. Int. J. Phys. Sci. 2011, 6, 6373–6378. [Google Scholar] [CrossRef]
- Hind, G.; Marshak, D.R.; Coughlan, S.J. Spinach Thylakoid Polyphenol Oxidase: Cloning, Characterization, and Relation to a Putative Protein Kinase. Biochemistry 1995, 34, 8157–8164. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]
- Guyot, S.; Serrand, S.; Le Quéré, J.M.; Sanoner, P.; Renard, C.M. Enzymatic synthesis and physicochemical characterisation of phloridzin oxidation products (POP), a new water-soluble yellow dye deriving from apple. Innov. Food Sci. Emerg. Technol. 2007, 8, 443–450. [Google Scholar] [CrossRef]
- Halbwirth, H. The Creation and Physiological Relevance of Divergent Hydroxylation Patterns in the Flavonoid Pathway. Int. J. Mol. Sci. 2010, 11, 595–621. [Google Scholar] [CrossRef]
- Mazzafera, P.; Robinson, S.P. Characterization of polyphenol oxidase in coffee. Phytochemistry 2000, 55, 285–296. [Google Scholar] [CrossRef]
- Bittner, S. When quinones meet amino acids: Chemical, physical and biological consequences. Amino Acids 2006, 30, 205–224. [Google Scholar] [CrossRef]
- Chen, X.-M.; Ma, Z.; Kitts, D.D. Effects of processing method and age of leaves on phytochemical profiles and bioactivity of coffee leaves. Food Chem. 2018, 249, 143–153. [Google Scholar] [CrossRef]
- Clifford, M.N. Chemical and Physical Aspects of Green Coffee and Coffee Products. In Coffee; Springer: Boston, MA, USA, 1985; pp. 305–374. [Google Scholar] [CrossRef]
- Teng, J.; Gong, Z.; Deng, Y.; Chen, L.; Li, Q.; Shao, Y.; Lin, L.; Xiao, W. Purification, characterization and enzymatic synthesis of theaflavins of polyphenol oxidase isozymes from tea leaf (Camellia sinensis). LWT 2017, 84, 263–270. [Google Scholar] [CrossRef]
- Bot, F.; Calligaris, S.; Cortella, G.; Plazzotta, S.; Nocera, F.; Anese, M. Study on high pressure homogenization and high power ultrasound effectiveness in inhibiting polyphenoloxidase activity in apple juice. J. Food Eng. 2018, 221, 70–76. [Google Scholar] [CrossRef]
- Ashihara, H.; Crozier, A. Caffeine: A well-known but little mentioned compound in plant science. Trends Plant Sci. 2001, 6, 407–413. [Google Scholar] [CrossRef]
- Ashihara, H.; Sano, H.; Crozier, A. Caffeine and related purine alkaloids: Biosynthesis, catabolism, function and genetic engineering. Phytochemistry 2008, 69, 841–856. [Google Scholar] [CrossRef]
- Perrois, C.; Strickler, S.R.; Mathieu, G.; Lepelley, M.; Bedon, L.; Michaux, S.; Husson, J.; Mueller, L.; Privat, I. Differential regulation of caffeine metabolism in Coffea arabica (Arabica) and Coffea canephora (Robusta). Planta 2014, 241, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Comai, S.; Bertazzo, A.; Bailoni, L.; Zancato, M.; Costa, C.V.L.; Allegri, G. The content of proteic and nonproteic (free and pro-tein-bound) tryptophan in quinoa and cereal flours. Food Chem. 2007, 100, 1350–1355. [Google Scholar] [CrossRef]
- Martins, A.C.C.; Glória, M.B.A. Changes on the levels of serotonin precursors—Tryptophan and 5-hydroxytryptophan—During roasting of Arabica and Robusta coffee. Food Chem. 2010, 118, 529–533. [Google Scholar] [CrossRef]
- Young, S.N. The effect of raising and lowering tryptophan levels on human mood and social behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20110375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gostner, J.; Schroecksnadel, S.; Jenny, M.; Klein, A.; Ueberall, F.; Schennach, H.; Fuchs, D. Coffee Extracts Suppress Tryptophan Breakdown in Mitogen-Stimulated Peripheral Blood Mononuclear Cells. J. Am. Coll. Nutr. 2015, 34, 212–223. [Google Scholar] [CrossRef]
- Servillo, L.; Giovane, A.; Casale, R.; Cautela, D.; D’Onofrio, N.; Balestrieri, M.L.; Castaldo, D. Glucosylated forms of serotonin and tryptophan in green coffee beans. LWT 2016, 73, 117–122. [Google Scholar] [CrossRef]
- Wintgens, J.N. Factors Influencing the Quality of Green Coffee. In Coffee: Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders, and Researchers; Wiley: Weinheim, Germany, 2008; pp. 789–809. [Google Scholar] [CrossRef]
- Aparecido, L.E.D.O.; Rolim, G.D.S.; Moraes, J.R.D.S.C.D.; Valeriano, T.T.B.; Lense, G.H.E. Maturation periods for Coffea arabica cultivars and their implications for yield and quality in Brazil. J. Sci. Food Agric. 2018, 98, 3880–3891. [Google Scholar] [CrossRef]
- Farah, A.; dos Santos, T.F. The Coffee Plant and Beans. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 5–10. [Google Scholar]
- Illy, E.; Illy, A. The complexity of Coffee. Sci. Am. 2014, 24, 10–15. [Google Scholar] [CrossRef]
- Poltronieri, P.; Rossi, F. Challenges in Specialty Coffee Processing and Quality Assurance. Challenges 2016, 7, 19. [Google Scholar] [CrossRef]
- Brando, C.H.J. Harvesting and Green Coffee Processing. In Coffee: Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders, and Researchers; Wiley: Weinheim, Germany, 2008; pp. 604–715. [Google Scholar]
- Kleinwächter, M.; Bytof, G.; Selmar, D. Coffee Beans and Processing. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015; pp. 73–81. [Google Scholar]
- Kulapichitr, F.; Borompichaichartkul, C.; Suppavorasatit, I.; Cadwallader, K.R. Impact of drying process on chemical composition and key aroma components of Arabica coffee. Food Chem. 2019, 291, 49–58. [Google Scholar] [CrossRef]
- Duarte, G.S.; Pereira, A.A.; Farah, A. Chlorogenic acids and other relevant compounds in Brazilian coffees processed by semi-dry and wet post-harvesting methods. Food Chem. 2010, 118, 851–855. [Google Scholar] [CrossRef]
- Valio, I.F.M. Inhibition of Germination of Coffee Seeds (Coffea arabica L. cv Mundo Novo) by the Endocarp. J. Seed Technol. 1980, 5, 32–39. [Google Scholar]
- Bytof, G.; Knopp, S.E.; Schieberle, P.; Teutsch, I.; Selmar, D. Influence of processing on the generation of γ-aminobutyric acid in green coffee beans. Eur. Food Res. Technol. 2005, 220, 245–250. [Google Scholar] [CrossRef]
- Selmar, D.; Bytof, G.; Knopp, S.E.; Bradbury, A.; Wilkens, J. Biochemical Insights into Coffee Processing: Quality and Nature of Green Coffees are Interconnected with an Active Seed Metabolism. In Proceedings of the ASIC 2004. 20th International Conference on Coffee Science, Bangalore, India, 11–15 October 2004; pp. 111–119. [Google Scholar]
- Koubaa, M.; Delbecq, F.; Roohinejad, S.; Mallikarjunan, K. Metabolism and functions of gamma-aminobutyric acid. Encycl. Food Chem. 2018, 1385, 528–534. [Google Scholar]
- Rashmi, D.; Zanan, R.; John, S.; Khandagale, K.; Nadaf, A. γ-Aminobutyric Acid (GABA): Biosynthesis, Role, Commercial Pro-duction, and Applications. Stud. Nat. Prod. Chem. 2018, 57, 413–452. [Google Scholar]
- Sahab, N.R.; Subroto, E.; Balia, R.L.; Utama, G.L. γ-Aminobutyric acid found in fermented foods and beverages: Current trends. Heliyon 2020, 6, e05526. [Google Scholar] [CrossRef]
- Kramer, D.; Breitenstein, B.; Kleinwächter, M.; Selmar, D. Stress Metabolism in Green Coffee Beans (Coffea arabica L.): Expression of Dehydrins and Accumulation of GABA during Drying. Plant Cell Physiol. 2010, 51, 546–553. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed Germination and Dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Lopes, A.C.; Andrade, R.P.; de Oliveira, L.C.C.; Lima, L.M.Z.; Santiago, W.D.; de Resende, M.L.V.; Cardoso, M.D.G.; Duarte, W.F. Production and characterization of a new distillate obtained from fermentation of wet processing coffee by-products. J. Food Sci. Technol. 2020, 57, 4481–4491. [Google Scholar] [CrossRef]
- Pereira, L.L.; Guarconi, R.; Pinheiro, P.F.; Osório, V.M.; Pinheiro, C.A.; Moreira, T.R.; Caten, C.S.T. New propositions about coffee wet processing: Chemical and sensory perspectives. Food Chem. 2020, 310, 125943. [Google Scholar] [CrossRef] [PubMed]
- Salengke, S.; Hasizah, A.; Reta, A.; Mochtar, A. Technology innovation for production of specialty coffee. IOP Conf. Ser. Earth Environ. Sci. 2019, 355, 012105. [Google Scholar] [CrossRef]
- Rühl, G.; Dambroth, M.; Biehl, B. Investigations in the causes of sensitivity to cold and water stress of tropical seeds, represented by cocoa seeds. I. Influence of developmental stage. Landbauforsch. Völkenrode 1988, 38, 220–234. [Google Scholar]
- Bewley, J.D.; Black, M. Seeds. In Physiology of Development and Germination, 2nd ed.; Plenum Press: New York, NY, USA, 1995. [Google Scholar]
- Sembdner, G.; Schneider, G.; Schreiber, K. (Eds.) Methoden zur Pflanzenhormonanalyse; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Selmar, D.; Bytof, G.; Knopp, S.-E.; Breitenstein, B. Germination of Coffee Seeds and its Significance for Coffee Quality. Plant Biol. 2006, 8, 260–264. [Google Scholar] [CrossRef]
- Bytof, G.; Knopp, S.-E.; Kramer, D.; Breitenstein, B.; Bergervoet, J.H.W.; Groot, S.P.C.; Selmar, D. Transient Occurrence of Seed Germination Processes during Coffee Post-harvest Treatment. Ann. Bot. 2007, 100, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Selmar, D.; Bytof, G.; Knopp, S.-E. The Storage of Green Coffee (Coffea arabica): Decrease of Viability and Changes of Potential Aroma Precursors. Ann. Bot. 2008, 101, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinícius de Melo Pereira, G.; Soccol, V.T.; Brar, S.K.; Neto, E.; Soccol, C.R. Microbial ecology and starter culture technology in coffee processing. Crit. Rev. Food Sci. Nutr. 2017, 57, 2775–2788. [Google Scholar] [CrossRef]
- Karim, M.A.; Wijayanti, F.; Sudaryanto, A. Comparative studies of coffee processing methods for decision making in appropriate technology implementation. In Exploring Resources, Process and Design for Sustainable Urban Development, Proceedings of the 5th International Conference on Engineering, Technology, and Industrial Application (ICETIA); Surakarta, Indonesia, 10 January 2018, AIP Publishing: New York, NY, USA, 2019; Volume 2114, p. 20015. [Google Scholar]
- Avallone, S.; Guiraud, J.-P.; Guyot, B.; Olguin, E.; Brillouet, J.-M. Fate of Mucilage Cell Wall Polysaccharides during Coffee Fermentation. J. Agric. Food Chem. 2001, 49, 5556–5559. [Google Scholar] [CrossRef]
- Schwan, R.; Silva, C.; Batista, L. Coffee Fermentation. In Handbook of Plant-Based Fermented Food and Beverage Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 677–690. [Google Scholar]
- Diego, E.R.; Flavio, M.B.; Marcelo, A.C.; Mariele, V.B.P.; Vany, P.F.; Helena, M.R.A.; Jose, H.D.S.T. Interaction of genotype, environment and processing in the chemical composition expression and sensorial quality of Arabica coffee. Afr. J. Agric. Res. 2016, 11, 2412–2422. [Google Scholar] [CrossRef] [Green Version]
- Wulandari, S.; Ainuri, M.; Sukartiko, A.C. Biochemical content of Robusta coffees under fully-wash, honey, and natural processing methods. IOP Conf. Ser. Earth Environ. Sci. 2021, 819, 012067. [Google Scholar] [CrossRef]
- Dong, W.; Tan, L.; Zhao, J.; Hu, R.; Lu, M. Characterization of Fatty Acid, Amino Acid and Volatile Compound Compositions and Bioactive Components of Seven Coffee (Coffea robusta) Cultivars Grown in Hainan Province, China. Molecules 2015, 20, 16687–16708. [Google Scholar] [CrossRef]
- Rodriguez, Y.F.B.; Guzman, N.G.; Hernandez, J.G. Effect of The Postharvest Processing Method on the Bio-Chemical Composition and Sensory Analysis of Arabica Coffee. Eng. Agric. 2020, 40, 177–183. [Google Scholar] [CrossRef]
- Silva, C.F.; Batista, L.; Abreu, L.M.; Dias, E.S.; Schwan, R.F. Succession of bacterial and fungal communities during natural coffee (Coffea arabica) fermentation. Food Microbiol. 2008, 25, 951–957. [Google Scholar] [CrossRef]
- Vilela, D.M.; Pereira, G.V.D.M.; Silva, C.F.; Batista, L.R.; Schwan, R.F. Molecular ecology and polyphasic characterization of the microbiota associated with semi-dry processed coffee (Coffea arabica L.). Food Microbiol. 2010, 27, 1128–1135. [Google Scholar] [CrossRef]
- Selmar, D.; Bytof, G.; Knopp, S.-E. New Aspects of Coffee Processing: The Relation Between Seed Germination and Coffee Quality. Available online: https://library.sweetmarias.com/wp-content/uploads/2020/08/New-Aspects-of-Coffee-Processing-older-paper.pdf (accessed on 8 January 2020).
- Arnold, U.; Ludwig, E. Analysis of free amino acids in green coffee beans. Eur. Food Res. Technol. 1994, 199, 22–25. [Google Scholar] [CrossRef]
- Knopp, S.; Bytof, G.; Selmar, D. Influence of processing on the content of sugars in green Arabica coffee beans. Eur. Food Res. Technol. 2006, 223, 195–201. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Selmar, D. Influence of drying on the content of sugars in wet processed green Arabica coffees. Food Chem. 2010, 119, 500–504. [Google Scholar] [CrossRef]
- Pereira, P.V.; Bravim, D.G.; Grillo, R.P.; Bertoli, L.D.; Osório, V.M.; Oliveira, D.D.S.; Miguel, M.G.D.C.P.; Schwan, R.F.; Silva, S.D.A.; Coelho, J.M.; et al. Microbial diversity and chemical characteristics of Coffea canephora grown in different environments and processed by dry method. World J. Microbiol. Biotechnol. 2021, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; De Bruyn, F.; Pothakos, V.; Contreras, G.F.; Cai, Z.; Moccand, C.; Weckx, S.; De Vuyst, L. Influence of Various Processing Parameters on the Microbial Community Dynamics, Metabolomic Profiles, and Cup Quality During Wet Coffee Processing. Front. Microbiol. 2019, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Cox, J.; Zhao, J. Ecological diversity, evolution and metabolism of microbial communities in the wet fermentation of Australian coffee beans. Int. J. Food Microbiol. 2020, 321, 108544. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, S.R.; da Cruz Pedrozo Miguel, M.G.; de Souza Cordeiro, C.; Silva, C.F.; Marques Pinheiro, A.C.; Schwan, R.F. Inoculation of starter cultures in a semi-dry coffee (Coffea arabica) fermentation process. Food Microbiol. 2014, 44, 87–95. [Google Scholar] [CrossRef]
- Gonzalez-Rios, O.; Suarez-Quiroz, M.L.; Boulanger, R.; Barel, M.; Guyot, B.; Guiraud, J.-P.; Schorr-Galindo, S. Impact of “ecological” post-harvest processing on the volatile fraction of coffee beans: I. Green coffee. J. Food Compos. Anal. 2007, 20, 289–296. [Google Scholar] [CrossRef]
- Masoud, W.; Kaltoft, C.H. The effects of yeasts involved in the fermentation of Coffea arabica in East Africa on growth and ochratoxin A (OTA) production by Aspergillus ochraceus. Int. J. Food Microbiol. 2006, 106, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Tareq, A.M.; Nainu, F.; Cicia, D.; Dhama, K.; Emran, T.B.; Simal-Gandara, J.; Capasso, R. Bromelain a potential bioactive compound: A comprehensive overview from a pharmacological perspective. Life 2021, 11, 317. [Google Scholar] [CrossRef]
- Silva, C.F.; Schwan, R.F.; Dias Ëustáquio, S.; Wheals, A. Microbial diversity during maturation and natural processing of coffee cherries of Coffea arabica in Brazil. Int. J. Food Microbiol. 2000, 60, 251–260. [Google Scholar] [CrossRef]
- Masoud, W.; Jespersen, L. Pectin degrading enzymes in yeasts involved in fermentation of Coffea arabica in East Africa. Int. J. Food Microbiol. 2006, 110, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bressani, A.P.P.; Martinez, S.J.; Sarmento, A.B.I.; Borém, F.M.; Schwan, R.F. Organic acids produced during fermentation and sensory perception in specialty coffee using yeast starter culture. Food Res. Int. 2020, 128, 108773. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Soccol, V.T.; Pandey, A.; Medeiros, A.B.P.; Lara, J.M.R.A.; Gollo, A.L.; Soccol, C.R. Isolation, selection and evaluation of yeasts for use in fermentation of coffee beans by the wet process. Int. J. Food Microbiol. 2014, 188, 60–66. [Google Scholar] [CrossRef]
- Massawe, G.A.; Lifa, S.J. Yeasts and lactic acid bacteria coffee fermentation starter cultures. Int. J. Postharvest Technol. Innov. 2010, 2, 41. [Google Scholar] [CrossRef]
- Taniwaki, M.; Pitt, J.; Teixeira, A.; Iamanaka, B. The source of ochratoxin A in Brazilian coffee and its formation in relation to processing methods. Int. J. Food Microbiol. 2003, 82, 173–179. [Google Scholar] [CrossRef]
- Suarez-Quiroz, M.; Gonzalez-Rios, O.; Barel, M.; Guyot, B.; Schorr-Galindo, S.; Guiraud, J.-P. Study of ochratoxin A-producing strains in coffee processing. Int. J. Food Sci. Technol. 2004, 39, 501–507. [Google Scholar] [CrossRef]
- Batista, L.R.; Chalfoun, S.M.; Prado, G.; Schwan, R.F.; Wheals, A.E. Toxigenic fungi associated with processed (green) coffee beans (Coffea arabica L.). Int. J. Food Microbiol. 2003, 85, 293–300. [Google Scholar] [CrossRef]
- Silva, C.F. Microbial Activity during Coffee Fermentation. In Cocoa and Coffee Fermentation; CRC Press: Boca Raton, FL, USA, 2015; pp. 397–423. [Google Scholar]
- Ribeiro, L.S.; Evangelista, S.R.; da Cruz Pedrozo Miguel, M.G.; van Mullem, J.; Silva, C.F.; Schwan, R.F. Microbiological and chemical-sensory characteristics of three coffee varieties processed by wet fermentation. Ann. Microbiol. 2018, 68, 705–716. [Google Scholar] [CrossRef]
- Rauf, A.; Olatunde, A.; Imran, M.; Alhumaydhi, F.A.; Aljohani, A.S.; Khan, S.A.; Uddin, M.S.; Mitra, S.; Bin Emran, T.; Khayrullin, M.; et al. Honokiol: A review of its pharmacological potential and therapeutic insights. Phytomedicine 2021, 90, 153647. [Google Scholar] [CrossRef] [PubMed]
- Mwithiga, G.; Jindal, V.K. Physical changes during coffee roasting in rotary conduction-type heating units. J. Food Process Eng. 2003, 26, 543–558. [Google Scholar] [CrossRef]
- Somporn, C.; Kamtuo, A.; Theerakulpisut, P.; Siriamornpun, S. Effects of roasting degree on radical scavenging activity, phenolics and volatile compounds of Arabica coffee beans (Coffea arabica L. cv. Catimor). Int. J. Food Sci. Technol. 2011, 46, 2287–2296. [Google Scholar] [CrossRef]
- Sruthi, N.; Premjit, Y.; Pandiselvam, R.; Kothakota, A.; Ramesh, S. An overview of conventional and emerging techniques of roasting: Effect on food bioactive signatures. Food Chem. 2021, 348, 129088. [Google Scholar] [CrossRef] [PubMed]
- Bolka, M.; Emire, S. Effects of coffee roasting technologies on cup quality and bioactive compounds of specialty coffee beans. Food Sci. Nutr. 2020, 8, 6120–6130. [Google Scholar] [CrossRef]
- Soares, C.M.D.; Alves, R.C.; Oliveira, M.B.P.P. Factors Affecting Acrylamide Levels in Coffee Beverages, 1st ed.; Preedy, V.R., Ed.; Elsevier: New York, NY, USA, 2015; pp. 218–224. [Google Scholar]
- Mazzafera, P.; Silvarolla, M.B. Caffeine content variation in single green Arabica coffee seeds. Seed Sci. Res. 2010, 20, 163–167. [Google Scholar] [CrossRef]
- Hečimović, I.; Belščak-Cvitanović, A.; Horžić, D.; Komes, D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011, 129, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The effect of roasting, storage, origin on the bioactive compounds in organic and conventional coffee (Caffea arabica). Eur. Food Res. Technol. 2019, 246, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Górecki, M.; Hallmann, E. The Antioxidant Content of Coffee and Its In Vitro Activity as an Effect of Its Production Method and Roasting and Brewing Time. Antioxidants 2020, 9, 308. [Google Scholar] [CrossRef] [Green Version]
- Baggenstoss, J.; Poisson, L.; Kaegi, R.; Perren, R.; Escher, F. Coffee Roasting and Aroma Formation: Application of Different Time−Temperature Conditions. J. Agric. Food Chem. 2008, 56, 5836–5846. [Google Scholar] [CrossRef]
- Taguchi, H.; Sakaguchi, M.; Shimabayashi, Y. Trigonelline content in coffee beans and the thermal conversion of trigonelline into nicotinic acid during the roasting of coffee beans. Agric. Biol. Chem. 1985, 49, 3467–3471. [Google Scholar]
- Wei, F.; Furihata, K.; Hu, F.; Miyakawa, T.; Tanokura, M. Two-Dimensional 1H–13C Nuclear Magnetic Resonance (NMR)-Based Comprehensive Analysis of Roasted Coffee Bean Extract. J. Agric. Food Chem. 2011, 59, 9065–9073. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.H.; Varga, N.; Hau, J.; Vera, F.A.; Welti, D.H. Alkylpyridiniums. 1. Formation in Model Systems via Thermal Degradation of Trigonelline. J. Agric. Food Chem. 2002, 50, 1192–1199. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Wei, F.; Furihata, K.; Koda, M.; Hu, F.; Miyakawa, T.; Tanokura, M. Roasting Process of Coffee Beans as Studied by Nuclear Magnetic Resonance: Time Course of Changes in Composition. J. Agric. Food Chem. 2012, 60, 1005–1012. [Google Scholar] [CrossRef]
- Scholz-Böttcher, B.M.; Ernst, L.; Maier, H.G. New stereoisomers of quinic acid and their lactones. Eur. J. Org. Chem. 1991, 1991, 1029–1036. [Google Scholar] [CrossRef]
- Farah, A.; de Paulis, T.; Trugo, L.C.; Martin, P.R. Effect of Roasting on the Formation of Chlorogenic Acid Lactones in Coffee. J. Agric. Food Chem. 2005, 53, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-K.; Shibamoto, T. Formation of Volatile Chemicals from Thermal Degradation of Less Volatile Coffee Components: Quinic Acid, Caffeic Acid, and Chlorogenic Acid. J. Agric. Food Chem. 2010, 58, 5465–5470. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Bhinder, S.; Singh, B.; Kaur, A.; Singh, N.; Kaur, M.; Kumari, S.; Yadav, M.P. Effect of infrared roasting on antioxidant activity, phenolic composition and Maillard reaction products of Tartary buckwheat varieties. Food Chem. 2019, 285, 240–251. [Google Scholar] [CrossRef]
- Bobková, A.; Hudáček, M.; Jakabová, S.; Belej, Ľ.; Capcarová, M.; Čurlej, J.; Bobko, M.; Árvay, J.; Jakab, I.; Čapla, J.; et al. The effect of roasting on the total polyphenols and antioxidant activity of coffee. J. Environ. Sci. Health Part B 2020, 55, 495–500. [Google Scholar] [CrossRef]
- Odžaković, B.; Džinić, N.; Kukrić, Z.; Grujić, S. Effect of roasting degree on the antioxidant activity of different Arabica coffee quality classes. Acta Sci. Pol. Technol. Aliment. 2015, 15, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Romero, E.; Chavez-Quintana, S.G.; Siche, R.; Castro-Alayo, E.M.; Cardenas-Toro, F.P. The Kinetics of Total Phenolic Content and Monomeric Flavan-3-ols during the Roasting Process of Criollo Cocoa. Antioxidants 2020, 9, 146. [Google Scholar] [CrossRef] [Green Version]
- Taeymans, D.; Wood, J.; Ashby, P.; Blank, I.; Studer, A.; Stadler, R.H.; Gondé, P.; Van Eijck, P.; Lalljie, S.; Lingnert, H.; et al. A Review of Acrylamide: An Industry Perspective on Research, Analysis, Formation, and Control. Crit. Rev. Food Sci. Nutr. 2004, 44, 323–347. [Google Scholar] [CrossRef] [PubMed]
- Bagdonaite, K.; Derler, K.; Murkovic, M. Determination of Acrylamide during Roasting of Coffee. J. Agric. Food Chem. 2008, 56, 6081–6086. [Google Scholar] [CrossRef] [PubMed]
- Guenther, H.; Anklam, E.; Wenzl, T.; Stadler, R.H. Acrylamide in coffee: Review of progress in analysis, formation and level reduction. Food Addit. Contam. 2007, 24, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, V.; Murthy, C.; Ramalakshmi, K.; Rao, P.S. Studies on roasting of coffee beans in a spouted bed. J. Food Eng. 1997, 31, 263–270. [Google Scholar] [CrossRef]
- Hoenicke, K.; Gatermann, R. Studies on the stability of acrylamide in food during storage. J. Assoc. Off. Anal. Chem. 2005, 88, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Lantz, I.; Ternité, R.; Wilkens, J.; Hoenicke, K.; Guenther, H.; Van Der Stegen, G.H.D. Studies on acrylamide levels in roasting, storage and brewing of coffee. Mol. Nutr. Food Res. 2006, 50, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, D.; Roach, J.A.G.; Gay, M.L.; Musser, S.M. Analysis of Coffee for the Presence of Acrylamide by LC-MS/MS. J. Agric. Food Chem. 2004, 52, 1996–2002. [Google Scholar] [CrossRef]
- Delatour, T.; Périsset, A.; Goldmann, T.; Riediker, A.S.; Stadler, R.H. Improved Sample Preparation to Determine Acrylamide in Difficult Matrixes Such as Chocolate Powder, Cocoa, and Coffee by Liquid Chromatography Tandem Mass Spectroscopy. J. Agric. Food Chem. 2004, 52, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Janda, K.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Kapczuk, P.; Kochman, J.; Rębacz-Maron, E.; Gutowska, I. Mineral Composition and Antioxidant Potential of Coffee Beverages Depending on the Brewing Method. Foods 2020, 9, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, S.; Ristenpart, W.D.; Guinard, J. Effect of Basket Geometry on the Sensory Quality and Consumer Acceptance of Drip Brewed Coffee. J. Food Sci. 2019, 84, 2297–2312. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.Z.; Fuller, M.; Grim, M.D. Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction. Foods 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, N.; Pataquiva, L.; Osorio, C.; Moreno, F.L.M.; Ruiz, R.Y. Effect of grinding, extraction time and type of coffee on the physicochemical and flavour characteristics of cold brew coffee. Sci. Rep. 2019, 9, 8440. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Coffee Sample | Microbiota | References |
|---|---|---|
| C. Arabica, Dry process, 750–800 m above sea level Minas Gerais, Brazil | Bacteria: Acinetobacter sp.; Arthrobacter sp.; Bacillus (cereus, subtilis, macerans, polymyxa, megaterium); Enterobacter agglomerans; Yersinia sp. Yeast: Arxula adeninivorans; Candida (saitoana, fermentati, membranifaciens); Debaryomyces (polymorphus, Hansenii); Pichia (guilliermondii, guilliermondii, sydowiorum, subpelliculosa, burtonii, anomala, burtonii); Stephanoascus smithiae; Saccharomyces cerevisiae Fungi: Aspergillus (flavus, ochraceus, tamarii, niger, sydowii); Cladosporium cladosporioides; Penicillium (corylophilum, chrysogenum, brevicompactum, roqueforti, solitum); Fusarium solani | [83] |
| C. canephora, Dry process, Jerônimo Monteiro 300 m above sea level | Bacteria: Bacillus (cereus, licheniformis, pumilus, shackletonii, subtilis); Cellulosimicrobium cellulans; Citrobacter freundii; Enterobacter cloacae; Escherichia vulneris; Kosakonia cowanii; Lactobacillus (oligofermentans, oris, paracasei); Leuconostoc mesenteroides; Micrococcus (luteus, yunnanensis); Pantoea (agglomerans, ananatis); Staphylococcus (cohnii, epidermidis, haemolyticus, saprophyticus, xylosus); Stenotrophomonas maltophilia Yeast: Candida (glabrata, orthopsilosis, dubliniensis, parapsilosis); Meyerozyma guilliermondii; Pichia cecembensis | [89] |
| C. canephora, Dry process, Espírito Santo, Brazi l600 m above sea level | Bacteria: Acinetobacter pittii; Acinetobacter radioresistens; Bacillus (altitudinis, cereus, safensis, subtilis); Citrobacter (braakii, freundii); Dermacoccus nishinomiyaensis; Enterobacter (asburiae, hormaechei, ludwigii); Enterococcus pallens; Enterobacteriaceae bacterium; Escherichia vulneris; Leuconostoc mesenteroides; Pantoea agglomerans; Pectobacterium parmentieri; Pseudomonas putida; Raoultella ornithinolytica; Salmonella sp.; Staphylococcus epidermidis, warneri); Streptomyces variabilis Yeast: Candida tropicalis; Hanseniaspora opuntiae; Hanseniaspora uvarum; Meyerozyma caribbica; Meyerozyma guilliermondii; Pichia kluyveri | [89] |
| C. arabica, Wet Processing 1300 m above sea level Jinghong in Yunnan, China | Bacteria: Lactobacillus (coryniformis, plantarum); Lactococcus (hircilactis, lactis); Leuconostoc (citreum, holzapfelii, mesenteroides, pseudomesenteroides); Weissella soli Yeasts: Candida (humilis, quercitrusa, solani); Cordyceps brongniartii; Hanseniaspora (uvarum, vineae); Lachancea lanzarotensis; Papiliotrema terrestris; Pichia kluyveri; Saccharomyces cerevisiae; Starmerella bacillaris; Torulaspora delbrueckii; Wickerhamomyces anomalus. | [90] |
| C. arabica, Wet Processing 6.6 m above sea level Teven, NSW, Australia | Bacteria: Acinetobacter lwoffii; Enterobacter ludwigii; Citrobacter koseri; Pseudomonas fluorescens; Klebsiella pneumoniae; Erwinia soli; Serratia marcescens; Brevibacillus parabrevis Anabaena; Salmonella enterica; Asaia sp.; Serratia marcescen; Brevibacillus parabrevis; Acetobacter persici; Gluconobacter cerinus; Leuconostoc mesenteroides; Lactococcus lactis Yeasts: Hanseniaspora uvarum; Pichia (fermentans, kudriavzevii); Candida (xylopsoci, railenensis); Wickerhamomyces anomalus | [91] |
| C. arabica Semi-dry process, Minas Gerais, Brazi 750–800 m | Bacteria: Weissella soil; Leuconostoc mesenteroides; Gluconobacter oxydans; Enterobacter agglomerans; Leuconostoc mesenteroides; Erwinia (toletana, herbicola); Erwinia tasmaniensis; Klebsiella (oxytoca, pneumoniae); Pseudomonas aeruginosa; Morganella morganii; Acinetobacter spp.; Bacillus (cereus, macerans, megaterium, subtilis); Escherichia coli; Lactobacillus (brevis, plantarum); Lactococcus lactis; Serratia sp.; Pantoea eucrina; Yeast: Arxula sp.; Candida (parapsilosis ernobii, fukuyamaensis, membranifaciens, carpophila); Pichia (guilliermondii, anomala, caribbica); Saccharomyces (cerevisiae, bayanus); Debaryomyces hansenii; Mitchella repens; Trichosporon cavernicola; Rhodotorula mucilaginosa; Torulaspora delbrueckii. Fungi: Aspergillus (chevalieri, foetidius, niger, ochraceus, tubingensis, versicolor); Cladosporium (cladosporioides, macrocarpum); Cylindrocarpon sp.; Eurotium chevalieri; Fusariella sp.; Fusarium sp.; Fusarium (chlamydosporum, lateritium, nivale, solani, sporotrichioides); Geotrichum sp.; Mucor hiemalis; Penicillium (brevicompactum, commune, decumbens, fellutanum, implicatum, roqueforti); Phoma sp.; Ulocladium sp. | [84,92] |
| Fermentation Impact | Microbiota | References |
|---|---|---|
| Pulp and mucilage degradation | Bacteria: Bacillus, Aerobacter, Escherichia, Erwinia, Leuconostoc mesenteroides, Lactobacillus plantarum, Lactobacillus brevis, and Streptococcus faecalis Yeast: Candida sp., Pichia sp., Kluyveromyces sp., Schizosaccharomyces sp., Saccharomyces sp., Debaryomyces. | [78,84,95,96,97] |
| Correlation with floral, fruity, and sweet character | Yeast: Pichia sp., Saccharomyces cerevisiae. | [92,98,99] |
| Produce organic acid | Bacteria: Leuconostoc mesenteroides; Bacillus sp. Yeast: Candida parapsilosis, Saccharomyces cerevisiae. | [92,98] |
| Inhibit ochratoxigenic fungi growth | Yeast: Pichia kluyvery, P. anomala, Hanseniaspora uvarum, Leuconostoc sp., Weissella sp., Enterococcus. | [78,94,100] |
| Produce mycotoxins and off-flavor | Fungi: Aspergillus, Fusarium, and Penicillium | [101,102,103] |
| Roasting Method | Coffee Variety | Degree of Roast | Percentage (%) |
|---|---|---|---|
| Drum roaster | Yirgacheffe | Raw | 1.572 |
| Light | 0.722 | ||
| Medium | 1.065 | ||
| Dark | 0.887 | ||
| Harar | Raw | 1.503 | |
| Light | 0.688 | ||
| Medium | 0.876 | ||
| Dark | 0.452 | ||
| Sidama | Raw | 1.640 | |
| Light | 0.567 | ||
| Medium | 0.567 | ||
| Dark | 0.796 | ||
| Fluidized bed roaster | Yirgacheffe | Raw | 1.503 |
| Light | 0.889 | ||
| Medium | 0.885 | ||
| Dark | 0.472 | ||
| Harar | Raw | 1.640 | |
| Light | 0.842 | ||
| Medium | 0.653 | ||
| Dark | 0.470 | ||
| Sidama | Raw | 1.572 | |
| Light | 0.637 | ||
| Medium | 0.979 | ||
| Dark | 0.935 | ||
| Traditional roaster | Yirgacheffe | Raw | 1.503 |
| Light | 0.860 | ||
| Medium | 0.687 | ||
| Dark | 0.465 | ||
| Harar | Raw | 1.572 | |
| Light | 1.478 | ||
| Medium | 0.813 | ||
| Dark | 0.997 |
| Type of Roaster | Degree of Roast (mg/L) | ||
|---|---|---|---|
| Light | Medium | Dark | |
| Drum | 2.056 | 1.241 | 1.323 |
| Fluidized bed | 0.092 | 2.290 | 0.468 |
| Traditional | 2.351 | 1.068 | 0.702 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastian, F.; Hutabarat, O.S.; Dirpan, A.; Nainu, F.; Harapan, H.; Emran, T.B.; Simal-Gandara, J. From Plantation to Cup: Changes in Bioactive Compounds during Coffee Processing. Foods 2021, 10, 2827. https://doi.org/10.3390/foods10112827
Bastian F, Hutabarat OS, Dirpan A, Nainu F, Harapan H, Emran TB, Simal-Gandara J. From Plantation to Cup: Changes in Bioactive Compounds during Coffee Processing. Foods. 2021; 10(11):2827. https://doi.org/10.3390/foods10112827
Chicago/Turabian StyleBastian, Februadi, Olly Sanny Hutabarat, Andi Dirpan, Firzan Nainu, Harapan Harapan, Talha Bin Emran, and Jesus Simal-Gandara. 2021. "From Plantation to Cup: Changes in Bioactive Compounds during Coffee Processing" Foods 10, no. 11: 2827. https://doi.org/10.3390/foods10112827
APA StyleBastian, F., Hutabarat, O. S., Dirpan, A., Nainu, F., Harapan, H., Emran, T. B., & Simal-Gandara, J. (2021). From Plantation to Cup: Changes in Bioactive Compounds during Coffee Processing. Foods, 10(11), 2827. https://doi.org/10.3390/foods10112827