The Prevalence and Epidemiology of Salmonella in Retail Raw Poultry Meat in China: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
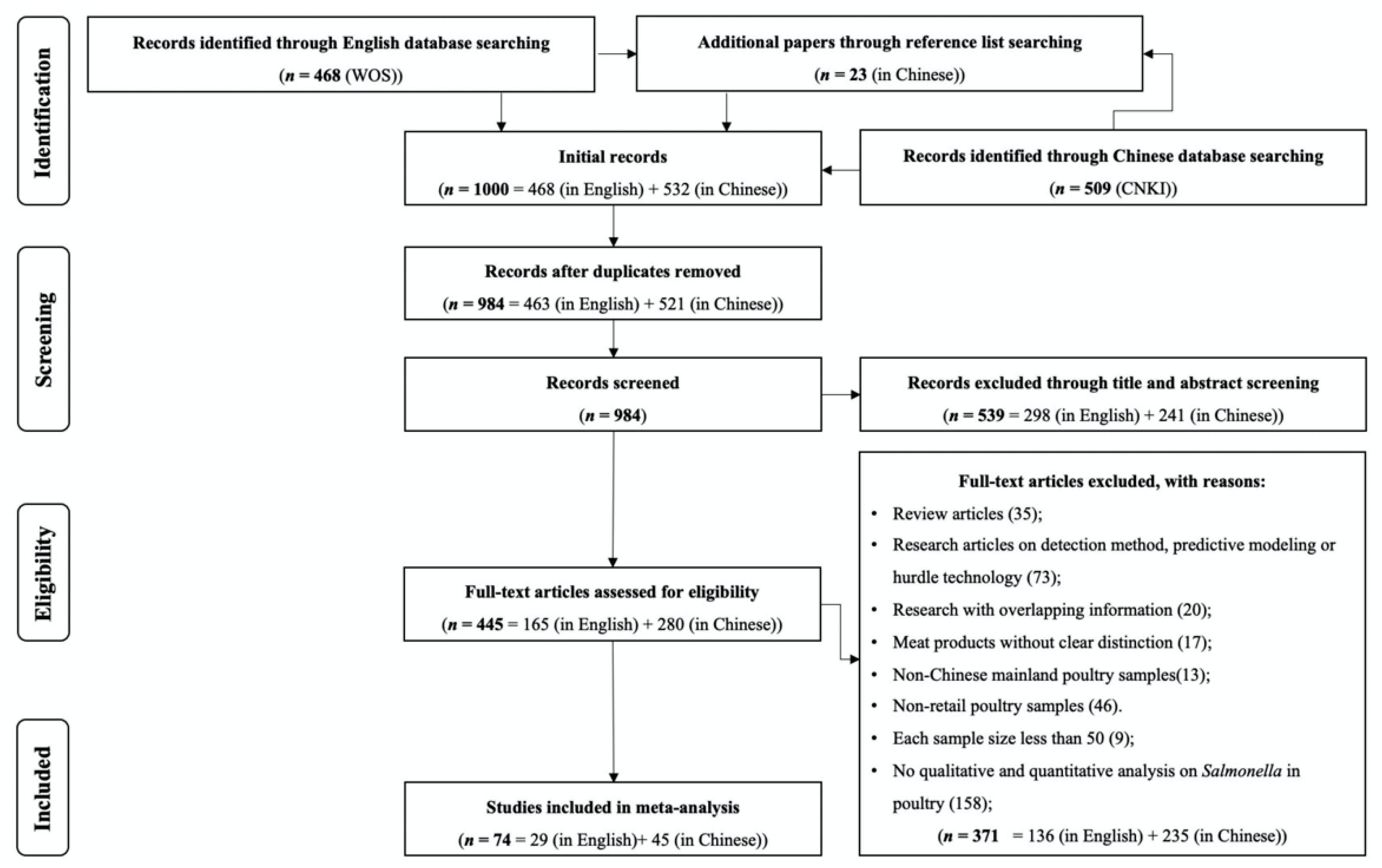
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction
2.3. Meta-Analysis and Statistical Analyses
3. Results
3.1. Characteristic of Literature and Datasets
3.2. Salmonella Prevalence in Different Poultry Meat Product Types
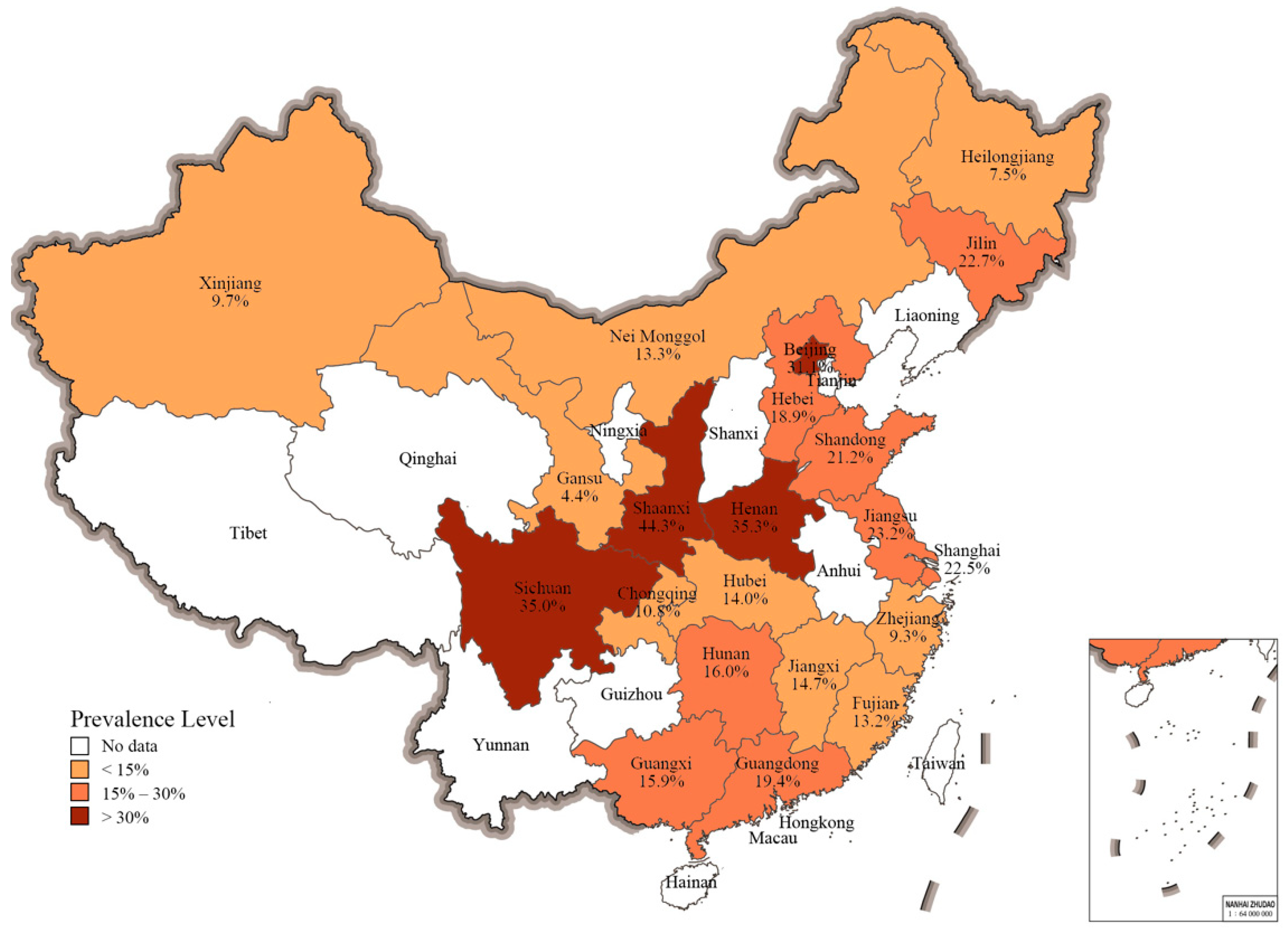
3.3. Salmonella Prevalence in Different Geographical Regions
3.4. Salmonella Prevalence under Different Preservation Types
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desai, P.T.; Porwollik, S.; Long, F.; Cheng, P.; Wollam, A.; Clifton, S.W.; Weinstock, G.M.; McClelland, M. Evolutionary genomics of Salmonella enterica subspecies. mBio 2013, 4, e00579-12:1–e00579-12:12. [Google Scholar] [CrossRef]
- Zhu, J.H.; Wang, Y.R.; Song, X.Y.; Cui, S.H.; Xu, H.B.; Yang, B.W.; Huang, J.L.; Liu, G.H.; Chen, Q.; Zhou, G.; et al. Prevalence and quantification of Salmonella contamination in raw chicken carcasses at the retail in China. Food Cont. 2014, 44, 198–202. [Google Scholar] [CrossRef]
- Cai, Y.Q.; Tao, J.; Jiao, Y.; Fei, X.; Zhou, L.; Wang, Y.; Zheng, H.J.; Pan, Z.M.; Jiao, X.N. Phenotypic characteristics and genotypic correlation between Salmonella isolates from a slaughterhouse and retail markets in Yangzhou, China. Int. J. Food Microbiol. 2016, 222, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Shi, Z.; Wei, J.; Ma, Z. A brief review of foodborne zoonoses in China. Epidemiol. Infect. 2011, 139, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Tenório, A.; Silva, B.N.; Rodrigues, V.; Cadavez, V.; Gonzales-Barron, U. Prevalence of pathogens in poultry meat: A meta-analysis of European published surveys. Foods 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Huang, J.H.; Zhang, Y.X.; Liu, S.R.; Chen, L.; Xiao, C.; Zeng, H.Y.; Wei, X.H.; Gu, Q.H.; Li, Y.; et al. Prevalence, abundance, serovars and antimicrobial resistance of Salmonella isolated from retail raw poultry meat in China. Sci. Total Environ. 2020, 713, 136385:1–136385:9. [Google Scholar] [CrossRef]
- Pires, S.M.; Knegt, L.D.; Hald, T. Estimation of the relative contribution of different food and animal sources to human Salmonella infections in the European Union. EFSA Supporting Publ. 2011, 8, 184E:1–184E:80. [Google Scholar] [CrossRef]
- USDA/FSIS. Salmonella and Campylobacter Verification Program for Raw Poultry Products. 2021. Available online: https://www.fsis.usda.gov/policy/fsis-directives/10250.1 (accessed on 3 February 2021).
- Yang, B.W.; Xi, M.L.; Wang, X.; Cui, S.H.; Yue, T.L.; Hao, H.S.; Wang, Y.; Cui, Y.; Alali, W.Q.; Meng, J.H.; et al. Prevalence of Salmonella on raw poultry at retail markets in China. J. Food Prot. 2011, 74, 1724–1728. [Google Scholar] [CrossRef]
- Wang, Y.R.; Chen, Q.; Cui, S.H.; Xu, X.; Zhu, J.H.; Luo, H.P.; Wang, D.; Li, F.Q. Enumeration and characterization of Salmonella isolates from retail chicken carcasses in Beijing, China. Foodborne Pathog. Dis. 2014, 11, 126–132. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses, in the EU, 2008-Part B: Analysis of factors associated with Salmonella contamination of broiler carcasses. EFSA J. 2011, 8, 1522. [Google Scholar] [CrossRef]
- Glass, G.V. Primary, secondary, and meta-analysis of research. Educ. Res. 1976, 5, 3–8. [Google Scholar] [CrossRef]
- Gonzales-Barron, U.; Butler, F. The use of meta-analytical tools in risk assessment for food safety. Food Microbiol. 2011, 28, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Barron, U.; Cadavez, V.; Sheridan, J.J.; Butler, F. Modelling the effect of chilling on the occurrence of Salmonella on pig carcasses at study, abattoir and batch levels by meta-analysis. Int. J. Food Microbiol. 2013, 163, 101–113. [Google Scholar] [CrossRef]
- Cufaoglu, G.; Ambarcioglu, P.; Ayaz, N.D. Meta-analysis of the prevalence of Listeria spp. and antibiotic resistant L. monocytogenes isolates from foods in Turkey. LWT-Food Sci. Technol. 2021, 144, 111210:1–111210:6. [Google Scholar] [CrossRef]
- FAO. Gateway to Poultry Production and Products. 2021. Available online: http://www.fao.org/poultry-production-products/production/en/ (accessed on 2 August 2021).
- Young, I.; Rajic, A.; Wilhelm, B.J.; Waddell, L.; Parker, S.; McEwen, S.A. Comparison of the prevalence of bacterial enteropathogens, potentially zoonotic bacteria and bacterial resistance to antimicrobials in organic and conventional poultry, swine and beef production: A systematic review and meta-analysis. Epidemiol. Infect. 2009, 137, 1217–1232. [Google Scholar] [CrossRef]
- Kerr, A.K.; Farrar, A.M.; Waddell, L.A.; Wilkins, W.; Wilhelm, B.J.; Bucher, O.; Wills, R.W.; Bailey, R.H.; Varga, C.; McEwen, S.A.; et al. A systematic review-meta-analysis and meta-regression on the effect of selected competitive exclusion products on Salmonella spp. prevalence and concentration in broiler chickens. Prev. Vet. Med. 2013, 111, 112–125. [Google Scholar] [CrossRef]
- Xavier, C.; Gonzales-Barron, U.; Paula, V.; Estevinho, L.; Cadavez, V. Meta-analysis of the incidence of foodborne pathogens in Portuguese meats and their products. Food Res. Int. 2014, 55, 311–323. [Google Scholar] [CrossRef]
- Golden, C.E.; Mishra, A. Prevalence of Salmonella and Campylobacter spp. in alternative and conventionally produced chicken in the United States: A systematic review and meta-analysis. J. Food Prot. 2020, 83, 1181–1197. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Martinez-Rios, V.; Dalgaard, P. Prevalence of Listeria monocytogenes in European cheeses: A systematic review and meta-analysis. Food Cont. 2018, 84, 205–214. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompsonm, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Dong, Q.L.; Barker, G.C.; Gorris, L.G.M.; Tian, M.S.; Song, X.Y.; Malakar, P.K. Status and future of quantitative microbiological risk assessment in China. Trends Food Sci. Technol. 2015, 42, 70–80. [Google Scholar] [CrossRef]
- Pei, X.Y.; Li, N.; Guo, Y.C.; Liu, X.M.; Yan, L.; Li, Y.; Yang, S.R.; Hu, J.; Zhu, J.H.; Yang, D.Y. Microbiological food safety surveillance in China. Int. J. Environ. Res. Public Health 2015, 12, 10662–10670. [Google Scholar] [CrossRef]
- Wu, Y.N.; Chen, J.S. Food safety monitoring and surveillance in China: Past, present and future. Food Cont. 2018, 90, 429–439. [Google Scholar] [CrossRef]
- Liu, Y.T.; Sun, W.X.; Sun, T.M.; Gorris, L.G.M.; Wang, X.; Liu, B.L.; Dong, Q.L. The prevalence of Listeria monocytogenes in meat products in China: A systematic literature review and novel meta-analysis approach. Int. J. Food Microbiol. 2020, 312, 108358:1–108358:9. [Google Scholar] [CrossRef]
- Luber, P. Cross-contamination versus undercooking of poultry meat or eggs-which risks need to be managed first? Int. J. food microbiol. 2009, 134, 21–28. [Google Scholar] [CrossRef]
- Moretro, T.; Nguyen-The, C.; Didier, P.; Maitre, I.; Izso, T.; Skuland, S.E.; Cardoso, M.J.; Ferreira, V.B.; Teixeira, P. Consumer practices and prevalence of Campylobacter, Salmonella and norovirus in kitchens from six European countries. Int. J. Food Microbiol. 2021, 347, 109172:1–109172:13. [Google Scholar] [CrossRef]
- Thomas, K.M.; de Glanville, M.A.; Barker, G.C.; Benschop, J.; Buza, J.J.; Cleaveland, S.; Davis, M.A.; French, N.P.; Mmbaga, B.T.; Prinsen, G.; et al. Prevalence of Campylobacter and Salmonella in African food animals and meat: A systematic review and meta-analysis. Int. J. Food Microbiol. 2020, 315, 108382:1–108382:22. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2020–2029; OECD Publishing: Paris, Italy; FAO: Rome, Italy, 2020; pp. 162–173. ISBN 978-92-64-58295-8. [Google Scholar]
- Wang, Y. Broiler production and consumption in China. Agric. Outlook 2013, 9, 68–74. (In Chinese) [Google Scholar]
- Prosser, D.J.; Wu, J.X.; Ellis, E.C.; Gale, F.; van Boeckel, T.P.; Wint, W.; Robinson, T.; Xiao, X.M.; Gilbert, M. Modelling the distribution of chickens, ducks, and geese in China. Agric. Ecosyst. Environ. 2011, 141, 381–389. [Google Scholar] [CrossRef]
- Hwang, D.; Rothrock, M.J.; Pang, H.; Kumar, G.D.; Mishra, A. Farm management practices that affect the prevalence of Salmonella in pastured poultry farms. LWT-Food Sci. Technol. 2020, 127, 109423:1–109423:8. [Google Scholar] [CrossRef]
- Ren, Q.Q. Research on cold chain distribution of fresh agricultural products under the environment of “Internet +”-taking Hainan as an example. Agric. Econ. 2017, 6, 124–126. (In Chinese) [Google Scholar]
- Lianou, A.; Sofos, J.N. A review of the incidence and transmission of Listeria monocytogenes in ready-to-eat products in retail and food service environments. J. Food Prot. 2007, 70, 2172–2198. [Google Scholar] [CrossRef]
- Wilcock, A.; Pun, M.; Khanona, J.; Aung, M. Consumer attitudes, knowledge and behaviour: A review of food safety issues. Trends Food Sci. Technol. 2004, 15, 56–66. [Google Scholar] [CrossRef]
- Liu, P. Tracing and periodizing China’s food safety regulation: A study on China’s food safety regime change. Regul. Gov. 2010, 4, 244–260. [Google Scholar] [CrossRef]
- Bucher, O.; Farrar, A.M.; Totton, S.C.; Wilkins, W.; Waddell, L.A.; Wilhelm, B.J.; McEwen, S.A.; Fazil, A.; Rajic, A. A systematic review-meta-analysis of chilling interventions and a meta-regression of various processing interventions for Salmonella contamination of chicken. Prev. Vet. Med. 2012, 103, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Regalado-Pineda, I.D.; Rodarte-Medina, R.; Resendiz-Nava, C.N.; Saenz-Garcia, C.E.; Castaneda-Serrano, P.; Nava, G.M. Three-Year Longitudinal Study: Prevalence of Salmonella Enterica in Chicken Meat is Higher in Supermarkets than Wet Markets from Mexico. Foods 2020, 9, 264. [Google Scholar] [CrossRef]
- Marmion, M.; Ferone, M.T.; Whyte, P.; Scannell, A.G.M. The changing microbiome of poultry meat; from farm to fridge. Food Microbiol. 2021, 99, 103823:1–103823:16. [Google Scholar] [CrossRef]
- Liu, R.; Xing, L.J.; Zhou, G.H.; Zhang, W.G. What is meat in China? Anim. Front. 2017, 7, 53–56. [Google Scholar] [CrossRef]
- Capita, R.; Alvarez-Astorga, M.; Alonso-Calleja, C.; Moreno, B.; Garcia-Fernandez, M.D. Occurrence of salmonellae in retail chicken carcasses and their products in Spain. Int. J. Food Microbiol. 2003, 81, 169–173. [Google Scholar] [CrossRef]
- Danyluk, M.D.; Schaffner, D.W. Quantitative assessment of the microbial risk of leafy greens from farm to consumption: Preliminary framework, data, and risk estimates. J. Food Prot. 2011, 74, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Pouillot, R.; Hoelzer, K.; Chen, Y.H.; Dennis, S. Estimating probability distributions of bacterial concentrations in food based on data generated using the most probable number (MPN) method for use in risk assessment. Food Cont. 2013, 29, 350–357. [Google Scholar] [CrossRef]
- Yang, B.W.; Cui, Y.; Shi, C.; Wang, J.Q.; Xia, X.D.; Xi, M.L.; Wang, X.; Meng, J.H.; Alali, W.Q.; Walls, I.; et al. Counts, serotypes, and antimicrobial resistance of Salmonella isolates on retail raw poultry in the People’s Republic of China. J. Food Prot. 2014, 77, 894–902. [Google Scholar] [CrossRef]
- Huang, J.L.; Zong, Q.; Zhao, F.; Zhu, J.Q.; Jiao, X.A. Quantitative surveys of Salmonella and Campylobacter on retail raw chicken in Yangzhou, China. Food Cont. 2016, 59, 68–73. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, B.W.; Cui, Y.; Alali, W.Q.; Xia, X.D.; Xi, M.L.; Wang, X.; Shi, X.M.; Wang, D.P.; Meng, J.H. Subtyping of Salmonella isolates on retail raw chicken in China by pulsed-field gel electrophoresis and plasmid analysis. Food Cont. 2015, 47, 420–426. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte, C.A. Worldwide epidemiology of Salmonella serovars in animal-based foods: A meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19:1–e00591-19:21. [Google Scholar] [CrossRef]
- Gong, J.S.; Wang, C.M.; Shi, S.R.; Bao, H.D.; Zhu, C.H.; Kelly, P.; Zhuang, L.L.; Lu, G.W.; Dou, X.H.; Wang, R. Highly drug-resistant Salmonella enterica serovar Indiana clinical isolates recovered from broilers and poultry workers with diarrhea in China. Antimicrob. Agents Chemother. 2016, 60, 1943–1947. [Google Scholar] [CrossRef]
- Foley, S.L.; Johnson, T.J.; Ricke, S.C.; Nayak, R.; Danzeisen, J. Salmonella pathogenicity and host adaptation in chicken-associated serovars. Microbiol. Mol. Biol. Rev. 2013, 77, 582–607. [Google Scholar] [CrossRef]
- Xia, S.L.; Hendriksen, R.S.; Xie, Z.Q.; Huang, L.L.; Zhang, J.; Guo, W.S.; Xu, B.L.; Ran, L.; Aarestrup, F.W. Molecular Characterization and Antimicrobial Susceptibility of Salmonella Isolates from Infections in Humans in Henan Province, China. J. Clin. Microbiol. 2009, 47, 401–409. [Google Scholar] [CrossRef][Green Version]
- Hendriksen, R.S.; Vieira, A.R.; Karlsmose, S.; Wong, D.M.A.L.F.; Jensen, A.B.; Wegener, H.C.; Aarestrup, F.M. Global monitoring of Salmonella serovar distribution from the world health organization global foodborne infections network country data bank: Results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 2011, 8, 887–900. [Google Scholar] [CrossRef]
- Foley, S.L.; Lynne, A.M.; Nayak, R. Salmonella challenges: Prevalence in swine and poultry and potential pathogenicity of such isolates. J. Anim. Sci. 2008, 86, E149–E162. [Google Scholar] [CrossRef] [PubMed]
- Augulo, F.J.; Johnson, K.R.; Tauxe, R.V.; Cohen, M.L. Origins and Consequences of Antimicrobial-Resistant Nontyphoidal Salmonella: Implications for the Use of Fluoroquinolones in Food Animals. Microb. Drug Resist. 2000, 6, 77–83. [Google Scholar] [CrossRef]
- Dallal, M.M.S.; Doyle, M.P.; Rezadehbashi, M.; Dabiri, H.; Sanaei, M.; Modarresi, S.; Bakhtiari, R.; Sharifiy, K.; Taremi, M.; Zali, M.R.; et al. Prevalence and antimicrobial resistance profiles of Salmonella serotypes, Campylobacter and Yersinia spp. isolated from retail chicken and beef, Tehran, Iran. Food Cont. 2010, 21, 388–392. [Google Scholar] [CrossRef]
- Li, R.C.; Lai, J.; Wang, Y.; Liu, S.L.; Li, Y.; Liu, K.Y.; Shen, J.Z.; Wu, C.M. Prevalence and characterization of Salmonella species isolated from pigs, ducks and chickens in Sichuan province, China. Int. J. Food Microbiol. 2013, 163, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhao, J.Y.; Gan, X.; Wang, J.; Zhang, X.L.; Cui, S.H.; Xia, S.L.; Hu, Y.J.; Yan, S.F.; Wang, J.H.; et al. Emergence and diversity of Salmonella enterica serovar Indiana isolates with concurrent resistance to ciprofloxacin and cefotaxime from patients and food-producing animals in China. Antimicrob. Agents Chemother. 2016, 60, 3365–3371. [Google Scholar] [CrossRef]

| Meat Category | Total | Positive | Pooled Prevalence (95% CI) a | τ2 b | I2 c |
|---|---|---|---|---|---|
| Raw poultry overall (random effects) | 21,824 | 5837 | 23.0% (19.8–26.6%) | 0.8953 | 97.0% |
| Chicken | 15,246 | 4716 | 26.4% (22.4–30.8%) | 0.8821 | 96.9% |
| Duck | 794 | 83 | 10.1% (5.3–18.2%) | 0.7475 | 87.9% |
| Pigeon | 292 | 66 | 22.6% (18.2–27.8%) | 0.0000 | 0.0% |
| Other | 5492 | 972 | 15.4% (12.0–19.5%) | 0.2419 | 93.1% |
| Preservation Type | Total | Positive | Pooled Prevalence (95% CI) a | τ2b | I2c |
|---|---|---|---|---|---|
| Raw poultry overall (random-effects) | 21,824 | 5837 | 23.0% (19.8%–26.6%) | 0.8953 | 97.0% |
| Ambient | 2825 | 649 | 17.2% (6.6%–37.8%) | 2.3137 | 99.1% |
| Chilled | 2066 | 974 | 42.1% (33.7%–51.0%) | 0.2596 | 92.1% |
| Frozen | 2173 | 535 | 25.3% (17.3%–35.4%) | 0.6518 | 94.8% |
| Unknown | 14,760 | 3679 | 21.3% (17.9%–25.1%) | 0.7795 | 96.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Liu, Y.; Qin, X.; Aspridou, Z.; Zheng, J.; Wang, X.; Li, Z.; Dong, Q. The Prevalence and Epidemiology of Salmonella in Retail Raw Poultry Meat in China: A Systematic Review and Meta-Analysis. Foods 2021, 10, 2757. https://doi.org/10.3390/foods10112757
Sun T, Liu Y, Qin X, Aspridou Z, Zheng J, Wang X, Li Z, Dong Q. The Prevalence and Epidemiology of Salmonella in Retail Raw Poultry Meat in China: A Systematic Review and Meta-Analysis. Foods. 2021; 10(11):2757. https://doi.org/10.3390/foods10112757
Chicago/Turabian StyleSun, Tianmei, Yangtai Liu, Xiaojie Qin, Zafeiro Aspridou, Jiaming Zheng, Xiang Wang, Zhuosi Li, and Qingli Dong. 2021. "The Prevalence and Epidemiology of Salmonella in Retail Raw Poultry Meat in China: A Systematic Review and Meta-Analysis" Foods 10, no. 11: 2757. https://doi.org/10.3390/foods10112757
APA StyleSun, T., Liu, Y., Qin, X., Aspridou, Z., Zheng, J., Wang, X., Li, Z., & Dong, Q. (2021). The Prevalence and Epidemiology of Salmonella in Retail Raw Poultry Meat in China: A Systematic Review and Meta-Analysis. Foods, 10(11), 2757. https://doi.org/10.3390/foods10112757







