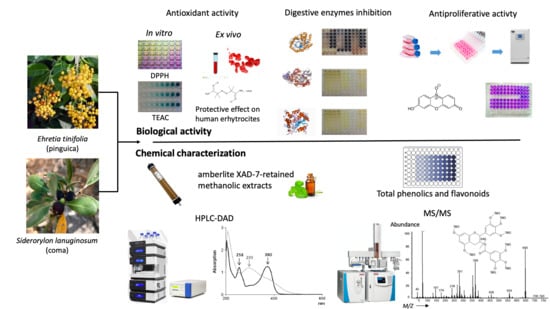
Phenolic Profiles and Biological Activities of Extracts from Edible Wild Fruits Ehretia tinifolia and Sideroxylon lanuginosum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phytochemicals Extraction
2.3. Determination of Total Phenolic Content (TPC)
2.4. Determination of Total Flavonoid Content (TFC)
2.5. Antiradical and Antioxidant Assays
2.5.1. 2,2′-Diphenyl-1-Picrylhydrazyl Radical (DPPH●) Assay
2.5.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.5.3. Protective Effect on Human Erythrocytes
2.6. Digestive Enzymes Inhibition
2.6.1. Inhibition of α-Glucosidase
2.6.2. Inhibition of α-Amylase
2.6.3. Inhibition of Pancreatic Lipase
2.7. Antiproliferative Activity
2.8. HPLC–DAD-ESI-MS/MS Analysis of Phenolics Profiles
2.9. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Content (TPC), Total Flavonoid Content (TFC), Antiradical, and Antioxidant Activity
3.2. HPLC-DAD-MS/MS Analysis of Phenolic Profiles
3.3. Digestive Enzymes Inhibition
3.3.1. Inhibition of α-Glucosidase
3.3.2. Inhibition of α-Amylase
3.3.3. Inhibition of Pancreatic Lipase
3.4. Antiproliferative Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiménez-Aspee, F.; Thomas-Valdés, S.; Schulz, A.; Ladio, A.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant activity and phenolic profiles of the wild currant Ribes magellanicum from Chilean and Argentinean Patagonia. Food Sci. Nutr. 2015, 4, 595–610. [Google Scholar] [CrossRef]
- Turner, N.J.; Łuczaj, L.J.; Migliorini, P.; Pieroni, A.; Dreon, A.L.; Sacchetti, L.E.; Paoletti, M.G. Edible and Tended Wild Plants, Traditional Ecological Knowledge and Agroecology. Crit. Rev. Plant Sci. 2011, 30, 198–225. [Google Scholar] [CrossRef]
- Bvenura, C.; Sivakumar, D. The role of wild fruits and vegetables in delivering a balanced and healthy diet. Food Res. Int. 2017, 99, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Hadjichambis, A.C.; Paraskeva-Hadjichambi, D.; Della, A.; Giusti, M.E.; De Pasquale, C.; Lenzarini, C.; Censorii, E.; Gonzales-Tejero, M.R.; Sanchez-Rojas, C.P.; Ramiro-Gutierrez, J.M.; et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2008, 59, 383–414. [Google Scholar] [CrossRef] [PubMed]
- Tardío, J.; Pardo-De-Santayana, M.; Morales, R. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Miller, J.S. A Revision of the New World Species of Ehretia (Boraginaceae). Ann. Mo. Bot. Gard. 1989, 76, 1050. [Google Scholar] [CrossRef]
- Monroy-Ortiz, C.; Monroy, R. Las Plantas Compañeras de Siempre. La Experiencia en Morelos; Universidad Autónoma del Estado de Morelos: Cuernavaca, Mexico, 2006. [Google Scholar]
- Martínez, M. Catálogo de Nombres Vulgares y Científicos de Plantas Mexicanas; Fondo de Cultura Económica: Ciudad de México, México, 1979; pp. 93–1071. [Google Scholar]
- Benítez-Badillo, G.; Pulido-Salas, M.T.P.; Equihua-Samora, M. Árboles Multiusos Nativos de Veracruz para Reforestación, Restauración y Plantaciones; Instituto de Ecología, A.C., Sigolfo, Conafor: Xalapa, México, 2008; 108p. [Google Scholar]
- Pío-León, J.F.; Díaz-Camacho, S.P.; López, M.G.; Montes-Avila, J.; López-Angulo, G.; Delgado-Vargas, F. Physicochemical, nutritional, and antioxidant characteristics of the fruit of Ehretia tinifolia. Rev. Mex. Biodivers. 2012, 83, 273–280. [Google Scholar] [CrossRef]
- Cowan, R.S.; Little, E.L. The Audubon Society Field Guide to North American Trees: Eastern Region. TAXON 1981, 30, 548. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Yang, C.S.; Landau, J.M.; Huang, M.-T.; Newmark, H.L. Inhibition of Carcinogenesis by Dietary Polyphenolic Compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef]
- Moein, S. Polyphenols and cancer: A review. Mol. Med. J. 2015, 1, 6–12. [Google Scholar]
- Wu, X. Rosa Roxburghii tratt Fruit and Green Jujube Beverage and Preparation Method Thereof. CN106578777A, 26 April 2017. [Google Scholar]
- Wang, C.; Li, J.; Li, Q.; Song, Q. A Kind of Stauntonvine Pulp Fruitcake and Preparation Method Thereof. CN108094661A, 1 June 2018. [Google Scholar]
- Yichun Zhongzhi Dashanwang Liquor Industry Co Ltd. Wild Indigo Naturalis-Cowberry Beer. CN105524756A, 27 April 2016.
- Foshan Huili Cosmetics Science and Technology Co Ltd. Post-Solar Restorative Composition. CN108272729A, 13 July 2018.
- Liu, J. Wild Cherry Berry Diet Therapy Drink and Preparation Method Thereof. CN106983058A, 28 July 2017. [Google Scholar]
- Derosa, G.; Romano, D.; D’Angelo, A.; Maffioli, P. Berberis aristata/Silybum marianum fixed combination (Berberol®) effects on lipid profile in dyslipidemic patients intolerant to statins at high dosages: A randomized, placebo-controlled, clinical trial. Phytomedicine 2015, 22, 231–237. [Google Scholar] [CrossRef]
- Viveros-Valdez, E.; Jaramillo-Mora, C.; Oranday-Cárdenas, A.; Morán-Martínez, J.; Carranza-Rosales, P. Antioxidant, cytotoxic and alpha-glucosidase inhibition activities from the Mexican berry Anacahuita (Cordia boissieri). Arch. Latinoam. Nutr. 2016, 66, 211–218. [Google Scholar]
- Singleton, V.; Rossi, J. Colorimetry of Total Phenolic Compounds with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Braca, A.; Sortino, C.; Politi, M.; Morelli, I.; Mendez, J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol. 2002, 79, 379–381. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, M.; Lakshmy, R.; Prabhakaran, D.; Reddy, K.S. Improved method of total antioxidant assay. Indian J. Biochem. Biophys. 2009, 46, 126–129. [Google Scholar]
- Silva-Beltrán, N.P.; Balderrama-Carmona, A.P.; López-Cuevas, O.; Portela-Márquez, M.A.; Umsza Guez, M.A.; López-Mata, M.A. Antioxidant and antimicrobial activity of Barchata (Zizhipus Obtusifolia). Revista Ciencias. 2019, 6, e523. [Google Scholar] [CrossRef]
- Kaskoos, R.A. In-vitro α-glucosidase inhibition and antioxidant activity of methanolic extract of Centaurea calcitrapa from Iraq. Am. J. Essent. Oil. Nat. Prod. 2013, 1, 122–125. [Google Scholar]
- Shuda, P.; Zinjarde, S.S.; Bhargava, S.Y.; Kumar, A.R. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement. Altern. Med. 2011, 11, 5. [Google Scholar] [CrossRef]
- Maqsood, M.; Ahmed, D.; Atique, I.; Malik, W. Lipase inhibitory activity of Lagenaria siceraria fruit as a strategy to treat obesity. Asian Pac. J. Trop. Med. 2017, 10, 305–310. [Google Scholar] [CrossRef]
- Viveros-Valdez, E.; Rivas-Morales, C.; Oranday-Cárdenas, A.; Castro-Garza, J.; Carranza-Rosales, P. Antiproliferative Effect from the Mexican Poleo (Hedeoma drummondii). J. Med. Food 2010, 13, 740–742. [Google Scholar] [CrossRef]
- Cittadini, M.C.; García-Estévez, I.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; Valentich, M.A.; Repossi, G.; Soria, E.A. Modulating activity of phenolic compounds from American plant infusions on fatty acid-related interleukin-6 release in glial cells. Nutr. Cancer 2018, 70, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Turker, G. Free radical scavenging activity and phenolic content of edible wild fruits from Kazdagi (Ida Mountains), Turkey. J. Med. Plants Res. 2012, 6. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Feresin, G.; Tapia, A.; Hilgert, N.; Theoduloz, C. Proximate composition and free radical scavenging activity of edible fruits from the Argentinian Yungas. J. Sci. Food Agric. 2005, 85, 1357–1364. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Quispe, C.; Soriano, M.D.P.C.; Gonzalez, J.F.; Hüneke, E.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant activity and characterization of constituents in copao fruits (Eulychnia acida Phil., Cactaceae) by HPLC–DAD–MS/MSn. Food Res. Int. 2014, 62, 286–298. [Google Scholar] [CrossRef]
- Ikram, E.H.K.; Eng, K.H.; Jalil, A.M.M.; Ismail, A.; Idris, S.; Azlan, A.; Nazri, H.S.M.; Diton, N.A.M.; Mokhtar, R.A.M. Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J. Food Compos. Anal. 2009, 22, 388–393. [Google Scholar] [CrossRef]
- Recuenco, M.; Lacsamana, M.; Hurtada, W.; Sabularse, V. Total Phenolic and Total Flavonoid Contents of Selected Fruits in the Philippines. Philipp. J. Sci. 2016, 145, 275–281. [Google Scholar]
- Goulas, V.; Georgiou, E. Utilization of Carob Fruit as Sources of Phenolic Compounds with Antioxidant Potential: Extraction Optimization and Application in Food Models. Foods 2019, 9, 20. [Google Scholar] [CrossRef]
- Magalhães, A.S.; Silva, B.M.; Pereira, J.A.; Andrade, P.; Valentão, P.; Carvalho, M. Protective effect of quince (Cydonia oblonga Miller) fruit against oxidative hemolysis of human erythrocytes. Food Chem. Toxicol. 2009, 47, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Aspee, F.; Theoduloz, C.; Pormetter, L.; Mettke, J.; Ávila, F.; Schmeda-Hirschmann, G. Andean Prumnopitys Andina (Podocarpacae) Fruit Extracts: Characterization of Secondary Metabolites and Potential Cytoprotective Effect. Molecules 2019, 24, 4028. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Rodriguez, B.M. Frutos Silvestres de uso Tradicional en la Alimentación: Evaluación de su Valor Nutricional, Compuestos Bioactivos y Capacidad Antioxidante. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2014. [Google Scholar]
- Mitjans, M.; Martínez, V.; del Campo, J.; Abajo, C.; Lozano, C.; Torres, J.; Vinardell, M.P. Novel epicatechin derivatives with antioxidant activity modulate interleukin-1β release in lipopolysaccharide-stimulated human blood. Bioorg. Med. Chem. Lett. 2004, 14, 5031–5034. [Google Scholar] [CrossRef]
- Prakash, M.; Basavaraj, B.V.; Murthy, K.C. Biological functions of epicatechin: Plant cell to human cell health. J. Funct. Foods 2018, 52, 14–24. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxidative Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Vega-Vega, V. Enriquecimento de la Capacidad Antioxidante y Proteción Antimicrobiana del Mango Fresco Cortado Aplicando Compuestos Fenólicos de sus Subproductos. Master’s Thesis, CIAD, Hermosillo, México, August 2011. [Google Scholar]
- Huang, G.; Liang, J.; Chen, X.; Lin, J.; Wei, J.; Huang, D.; Zhou, Y.; Sun, Z.; Zhao, L. Isolation and Identification of Chemical Constituents from Zhideke Granules by Ultra-Performance Liquid Chromatography Coupled with Mass Spectrometry. J. Anal. Methods Chem. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Feng, M.; Miao, Y.-L.; Li, Y.-Y.; Tong, L.-G.; He, P.; Ni, Y. Analysis of chemical components of Huanbei Zhike Prescription based on UPLC-Q-TOF-MS/MS technology. China J. Chin. Mater. Med. 2019, 44, 3022–3034. [Google Scholar]
- Girish, T.; Kumar, K.A.; Rao, U.P. C- Glycosylated flavonoids from black gram husk: Protection against DNA and erythrocytes from oxidative damage and their cytotoxic effect on HeLa cells. Toxicol. Rep. 2016, 3, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Inthongkaew, P.; Chatsumpun, N.; Supasuteekul, C.; Kitisripanya, T.; Putalun, W.; Likhitwitayawuid, K.; Sritularak, B. α-Glucosidase and pancreatic lipase inhibitory activities and glucose uptake stimulatory effect of phenolic compounds from Dendrobium formosum. Rev. Bras. Farm. 2017, 27, 480–487. [Google Scholar] [CrossRef]
- López-Martínez, L.X.; Aguilar Cisneros, L.M.; Dublán-García, O. Actividad antioxidante e inhibidora de α-glucosidasa y α-amilasa de tres variedades de cebolla (Allium cepa L.). Nova Sci. 2014, 6, 234–347. [Google Scholar] [CrossRef][Green Version]
- Hogan, S.; Zhang, L.; Li, J.; Sun, S.; Canning, C.; Zhou, K. Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutr. Metab. 2010, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.F.; Cazarolli, L.H.; Lavado, C.; Mengatto, V.; Figueiredo, M.S.R.B.; Guedes, A.; Pizzolatti, M.G.; Silva, F.R.M.B. Effects of flavonoids on α-glucosidase activity: Potential targets for glucose homeostasis. Nutrition 2011, 27, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dong, Y.; Zhao, H.; Wen, L.; Yang, B.; Zhao, M. Comparative evaluation of rosmarinic acid, methyl rosmarinate and pedalitin isolated from Rabdosia serra (MAXIM.) HARA as inhibitors of tyrosinase and α-glucosidase. Food Chem. 2011, 129, 884–889. [Google Scholar] [CrossRef]
- Meng, Y.; Su, A.; Yuan, S.; Zhao, H.; Tan, S.; Hu, C.Y.; Deng, H.; Guo, Y. Evaluation of Total Flavonoids, Myricetin, and Quercetin from Hovenia dulcis Thunb. As Inhibitors of α-Amylase and α-Glucosidase. Plant Foods Hum. Nutr. 2016, 71, 444–449. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Sari, S.; Barut, B.; Özel, A. Discovery of potent α-glucosidase inhibitor flavonols: Insights into mechanism of action through inhibition kinetics and docking simulations. Bioorg. Chem. 2018, 79, 257–264. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, L.; Xu, C.; Jiang, H.; Zhu, C.; Sun, L.; Sun, C.; Li, X. α-Glucosidase inhibitors from Chinese bayberry (Morella rubra Sieb. et Zucc.) fruit: Molecular docking and interaction mechanism of flavonols with different B-ring hydroxylations. RSC Adv. 2020, 10, 29347–29361. [Google Scholar] [CrossRef]
- Xngo, Y.L.; Chua, Y.L.N.A.L.S.; Ngo, Y.L. Anti-diabetic Activity of Rosmarinic Acid Rich Fractions from Orthosiphon stamineus. Curr. Enzym. Inhib. 2018, 14, 97–103. [Google Scholar] [CrossRef]
- Kotowaroo, M.I.; Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Screening of traditional antidiabetic medicinal plants of mauritius for possibleα-amylase inhibitory effectsin vitro. Phytother. Res. 2006, 20, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Grussu, D.; Stewart, D.; McDougall, G.J. Berry Polyphenols Inhibit α-Amylase In Vitro: Identifying Active Components in Rowanberry and Raspberry. J. Agric. Food Chem. 2011, 59, 2324–2331. [Google Scholar] [CrossRef]
- Subramaniam, S.; Selvaduray, K.R.; Radhakrishnan, A.K. Bioactive Compounds: Natural Defense against Cancer? Biomolecules 2019, 9, 758. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, M.M.G.; Mohan, C. Fruits as Prospective Reserves of bioactive Compounds: A Review. Nat. Prod. Bioprospect. 2018, 8, 335–346. [Google Scholar] [CrossRef]
- Al-Attabi, Z.H.; Al Hasani, S.; Waly, M.; Rahman, M.S.; Tamimi, Y. Antioxidant and Antitumor Properties of Wild Blueberry (Sideroxylon mascatense): Effects of Drying Methods. Int. J. Nutr. Pharmacol. Neurol. Dis. 2021, 11, 71. [Google Scholar] [CrossRef]
- Vahabi, L.; Monajemi, R.; Hosseini, S.A. The Cytotoxic Effect of Methanolic Extract of Pyracanthacoccinea M. Roemer Fruit on Hela Cell Line, Antioxidant Capacities and Total Phenol Contents of Methanolic and Aquatic Extract of this Fruit. Biomed. Pharmacol. J. 2015, 8, 99–103. [Google Scholar] [CrossRef]
- Razavi, S.M.; Ghasemiyan, A.; Salehi, S.; Zahri, F. Screening of biological activity of Zosima absinthifolia fruits extracts. EurAsian J. Biosci. 2009, 25–28. [Google Scholar] [CrossRef]
- Nie, J.; Li, R.; Wang, Y.; Tan, J.; Tang, S.; Jiang, Z.-T. Antioxidant activity evaluation of rosemary ethanol extract and their cellular antioxidant activity toward HeLa cells. J. Food Biochem. 2019, 43, e12851. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, R.H. Synergistic Effect of Apple Extracts and Quercetin 3-β-d-Glucoside Combination on Antiproliferative Activity in MCF-7 Human Breast Cancer Cells In Vitro. J. Agric. Food Chem. 2009, 57, 8581–8586. [Google Scholar] [CrossRef]
- Lyashenko, S.; Fabrikov, D.; González-Fernández, M.J.; Gómez-Mercado, F.; Ruiz, R.L.; Fedorov, A.; de Bélair, G.; Urrestarazu, M.; Rodríguez-García, I.; Álvarez-Corral, M.; et al. Phenolic composition and in vitro antiproliferative activity of Borago spp. seed extracts on HT-29 cancer cells. Food Biosci. 2021, 42, 101043. [Google Scholar] [CrossRef]
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic Compounds from Blueberries Can Inhibit Colon Cancer Cell Proliferation and Induce Apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef] [PubMed]
| Sample | TPC mg GAE/100g F.W. | TFC mg CE/100g F.W. | TEAC μmol TE/g | DPPH EC50 mg/mL | Hemolytic Inhibition IC50 (μg/mL) |
| S. lanuginosum | 21.4 ± 1.5 b | 6.42 ± 0.9 a | 4134 ± 97 a | 0.48 ± 0.05 a | 61.76 ± 7.9 b |
| E. tinifolia | 64.7 ± 2.6 a | 5.1 ± 0.4 b | 2454 ± 38 b | 0.32 ± 0.03 b | 58.55 ± 6.5 b |
| ** Control | — | — | — | 0.013 ± 2 c | 289 ± 20 a |
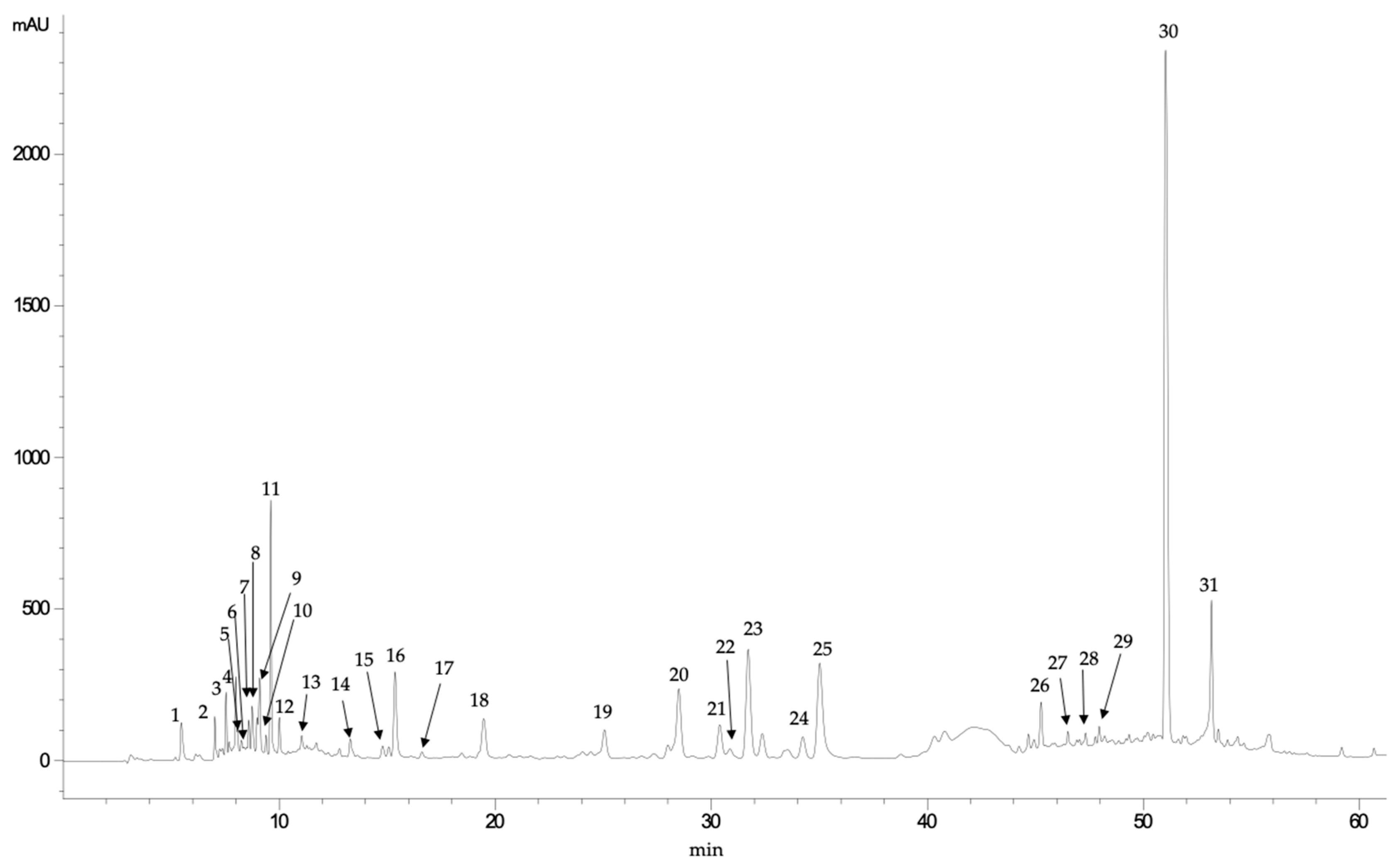
| Peak | Rt (min) | UV Max | [M+H]+ | MS/MS Fragments | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 5.46 | 261 | 297.4 | 135 | Unknown |
| 2 | 7.01 | 281 | 191.2 | 173, 129, 111 | Quinic acid |
| 3 | 7.53 | 262 | 373.0 | 311, 285, 267, 249, 241, 227, 196 | Unknown |
| 4 | 7.98 | 252, 275 (sh) | 282.5 | 150, 133 | Unknown |
| 5 | 8.07 | 301 | 331.3 | 169, 125 | Galloil-glucose |
| 6 | 8.58 | 282 | 507.2 | 489, 459, 293, 233, 195, 131, 125,113 | Caffeic acid derivative |
| 7 | 8.74 | 280, 314 (sh) | 165.0 | 137 | Unknown |
| 8 | 8.96 | 279 | 507.2 | 233, 165, 150, 125 | Unknown |
| 9 | 9.08 | 276 | 719.0 | 515, 359, 197, 179, 135 | Rosmarinic acid derivative |
| 10 | 9.38 | 272 | 515.0 | 269, 251, 225, 213, 179, 159, 135, 109 | Unknown |
| 11 | 9.60 | 278 | 359.0 | 197, 179, 135 | Syringic acid hexoside |
| 12 | 10.00 | 281 | 521.0 | 197, 179, 135 | Syringic acid dihexoside |
| 13 | 11.03 | 272 | 165.0 | 150, 121 | Unknown |
| 14 | 13.28 | 278, 320 (sh) | 401.0 | 359, 341, 297, 197, 179,135 | Unknown |
| 15 | 14.78 | 278, 314 | 365.4 | 323, 262, 250 | Unknown |
| 16 | 15.06 | 311, 292 (sh) | 373.0 | 211, 179, 123 | Methyl rosmarinate |
| 17 | 15.35 | 281, 330 (sh) | 567.0 | 179, 135 | Caffeic acid derivative |
| 18 | 19.45 | 291, 322 | 179.0 | 135 | Caffeic acid |
| 19 | 25.04 | 276 | 863.0 | 701, 521, 359, 315, 297, 197, 135 | Syringil-rosmarinic acid dihexoside |
| 20 | 28.48 | 276 | 695.5 | 579, 554, 537, 493, 312, 295, 277, 203, 135 | Lithospermic acid derivative |
| 21 | 30.38 | 276 | 695.5 | 579, 554, 537, 493, 312, 295, 277, 203, 135 | Lithospermic acid derivative |
| 22 | 31.69 | 276 | 695.5 | 579, 554, 537, 493, 312, 295, 277, 203, 135 | Lithospermic acid derivative |
| 23 | 32.35 | 276 | 695.5 | 579, 554, 537, 493, 312, 295, 277, 203, 135 | Lithospermic acid derivative |
| 24 | 34.22 | 276 | 695.5 | 579, 554, 537, 493, 312, 295, 277, 203, 135 | Lithospermic acid derivative |
| 25 | 35.01 | 276 | 695.5 | 579, 554, 537, 493, 312, 295, 277, 203, 135 | Lithospermic acid derivative |
| 26 | 45.25 | 275, 330 (sh) | 597.4 | 579, 509, 491, 355, 337, 329, 311, 293, 267, 239, 197, 179, 135, 109 | Unknown |
| 27 | 46.49 | 284, 320 | 861.5 | 843, 817, 655, 521, 501, 475, 457, 383, 359, 339, 323, 197, 179, 135 | Rosmarinic acid derivative |
| 28 | 47.31 | 285, 318 | 521.0 | 359, 197, 179, 161, 135 | Rosmarinic acid-hexoside |
| 29 | 47.94 | 283, 327 | 521.0 | 359, 197, 179, 161, 135 | Rosmarinic acid-hexoside |
| 30 | 51.02 | 330, 290 (sh) | 359.0 | 197, 179, 161, 135 | Rosmarinic acid |
| 31 | 53.13 | 278 | 537.0 | 493, 359, 295, 277, 203, 185, 159, 135, 109 | Lithospermic acid A |
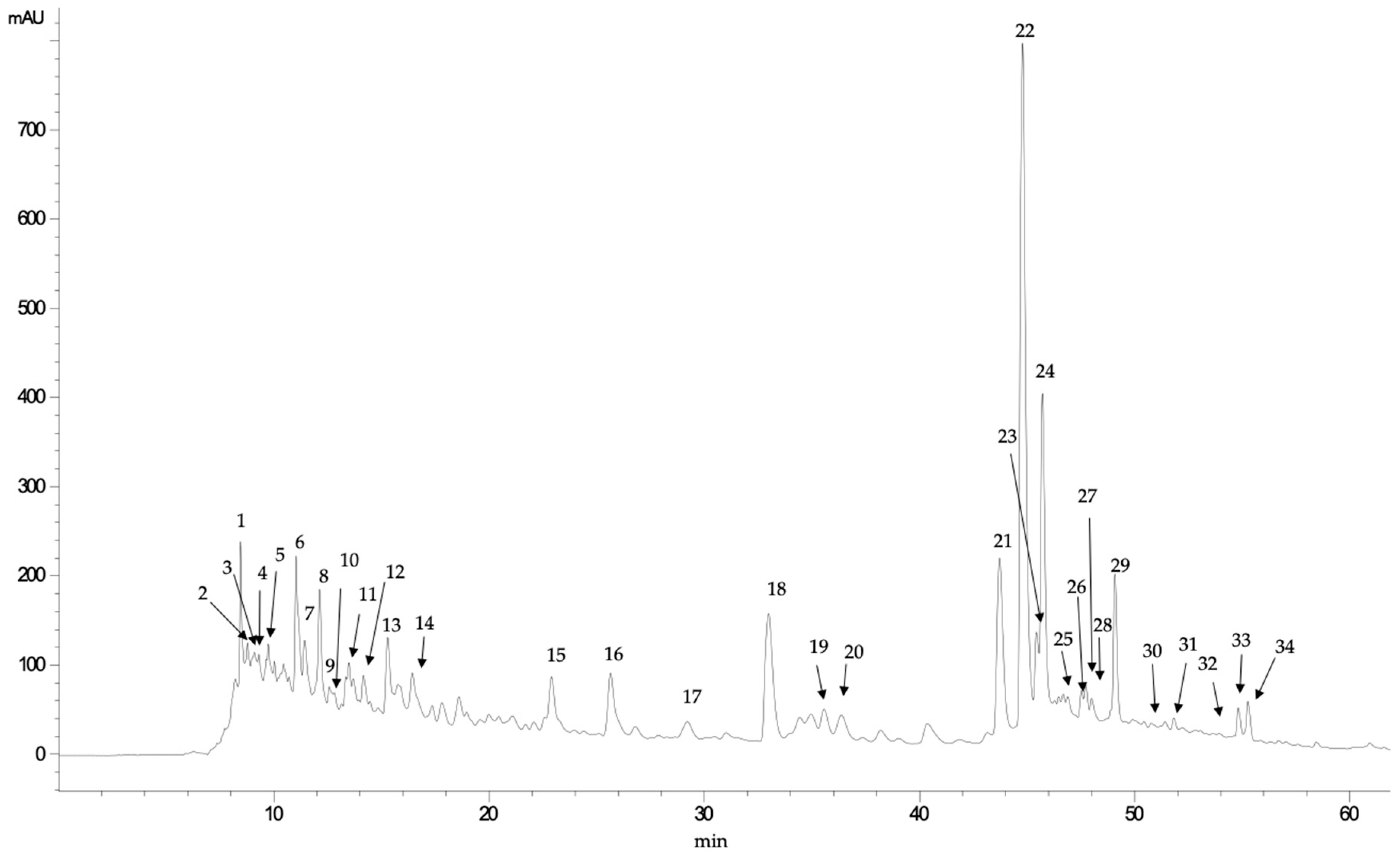
| Peak | Rt (Min) | UV Max | [M+H]+ | MS/MS Fragments | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 8.44 | 272 | 169.1 | 125, 113 | Gallic acid |
| 2 | 8.76 | 245 | 137.0 | p-hydroxybenzoic acid | |
| 3 | 8.89 | 278 | 329.3 | 167, 151, 109 | Unknown |
| 4 | 9.30 | 282 | 331.2 | 169, 125 | Unknown |
| 5 | 9.74 | 283 | 315.2 | 152, 108 | Unknown |
| 6 | 11.02 | 259-293 | 153.1 | 123, 109 | Protocatechuic acid |
| 7 | 11.43 | 286, 315 (sh) | 461.0 | 351, 323, 248, 233, 193 | Ferulic acid derivative |
| 8 | 12.11 | 285, 324 (sh) | 463.3 | 283, 272, 255, 175, 163 | Unknown |
| 9 | 13.48 | 295, 311 | 487.4 | 187, 163, 145, 119 | Coumaric dihexoside |
| 10 | 14.15 | 256, 311 (sh) | 435.2 | 241, 193, 153 | Ferulic acid derivative |
| 11 | 15.26 | 255, 335 | 311.3 | 249, 231, 205, 187, 161, 14 147, 135, 121 | Unknown |
| 12 | 15.82 | 303 | 421.2 | 241 | Unknown |
| 13 | 16.41 | 280 | 417.1 | 399, 227, 167, 153 | Unknown |
| 14 | 17.77 | 281, 310 (sh) | 387.0 | 163 | Coumaric acid derivative |
| 15 | 22.88 | 267, 327 (sh) | 241.2 | 197, 168, 141, 130 | Phenolic acid derivative |
| 16 | 25.63 | 262, 331 (sh) | 295.0 | 251, 189, 137, 121 | Phenolic acid derivative |
| 17 | 29.23 | 307, 290 (sh) | 163.0 | 119 | Coumaric acid |
| 18 | 32.95 | 255, 351, 301 (sh) | 755.5 | 609, 489, 355, 343, 325 301, 271, 179 | Quercetin glucoside dirhamnoside |
| 19 | 34.82 | 354, 300 (sh) | 479.0 | 317, 287, 271, 179, 151 | Myricetin glucoside |
| 20 | 35.54 | 254, 352, 302 (sh) | 771.0 | 301 | Quercetin diglucoside rhamnoside |
| 21 | 43.66 | 256, 353, 305 (sh) | 609.4 | 343, 301, 271, 255, 179, 151 | Quercetin neohesperidoside |
| 22 | 44.73 | 256, 355, 301 (sh) | 463.4 | 306, 301, 271, 255, 248 179, 151, 121 | Quercetin glucoside |
| 23 | 45.40 | 257, 342, 302 (sh) | 477.0 | 301, 151 | Quercetin glucuronide |
| 24 | 45.68 | 256, 354, 300 (sh) | 477.0 | 301, 179, 151 | Quercetin glucuronide |
| 25 | 46.68 | 267, 359 (sh) | 433.8 | 301, 271, 179, 151 | Quercetin pentoside |
| 26 | 47.52 | 255, 351 | 433.9 | 301, 271, 256, 180, 152 | Quercetin pentoside |
| 27 | 47.71 | 264, 349 | 447.9 | 285, 256, 227, 151 | Kaempferol glucoside |
| 28 | 47.99 | 284, 340 (sh) | 436.0 | 346, 316, 274, 167, 123 | Unknown |
| 29 | 49.06 | 257, 347 | 447.0 | 301, 273, 257, 179, 151 | Kaempferol glucoside |
| 30 | 50.85 | 371 | 317.0 | 179, 151, 138 | Myricetin |
| 31 | 51.18 | 327, 287 (sh) | 359.0 | 197, 179, 161, 135 | Rosmarinic acid |
| 32 | 54.79 | 264, 316, 356 (sh) | 609.8 | 463, 301, 151 | Quercetin rutinoside |
| 33 | 55.25 | 251, 330, 300 (sh) | 639.8 | 477, 463, 316, 300 | β-hydroverbascoside |
| 34 | 57.00 | 370, 300 (sh) | 301.2 | 273, 229, 179, 161, 151, 121 | Quercetin |
| Sample | Half Maximal Inhibitory Concentration [mg/mL] | ||||||
|---|---|---|---|---|---|---|---|
| α-Glu | α-Amy | Lipase | MCF-7 | HeLa | HT-29 | * RBCs | |
| S. lanuginosum | 0.21 ± 0.3 a | >5 | >5 | 1.99 ± 0.3 a | 3.22 ± 0.8 a | 1.97 ± 0.2 a | >5 |
| E. tinifolia | 0.17 ± 0.1 a,b | >5 | >5 | 0.99 ± 0.01b | 1.36 ± 0.2 b | 0.82 ± 0.09 b | >5 |
| ** Control | 0.13 ± 0.2 b | 0.97 ± 0.08 | 0.17 ± 0.20 | 0.013 ± 0.001 c | 0.011 ± 0.002 c | 0.015 ± 0.001 c | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monroy-García, I.N.; Carranza-Torres, I.E.; Carranza-Rosales, P.; Oyón-Ardoiz, M.; García-Estévez, I.; Ayala-Zavala, J.F.; Morán-Martínez, J.; Viveros-Valdez, E. Phenolic Profiles and Biological Activities of Extracts from Edible Wild Fruits Ehretia tinifolia and Sideroxylon lanuginosum. Foods 2021, 10, 2710. https://doi.org/10.3390/foods10112710
Monroy-García IN, Carranza-Torres IE, Carranza-Rosales P, Oyón-Ardoiz M, García-Estévez I, Ayala-Zavala JF, Morán-Martínez J, Viveros-Valdez E. Phenolic Profiles and Biological Activities of Extracts from Edible Wild Fruits Ehretia tinifolia and Sideroxylon lanuginosum. Foods. 2021; 10(11):2710. https://doi.org/10.3390/foods10112710
Chicago/Turabian StyleMonroy-García, Imelda N., Irma Edith Carranza-Torres, Pilar Carranza-Rosales, María Oyón-Ardoiz, Ignacio García-Estévez, Jesús Fernando Ayala-Zavala, Javier Morán-Martínez, and Ezequiel Viveros-Valdez. 2021. "Phenolic Profiles and Biological Activities of Extracts from Edible Wild Fruits Ehretia tinifolia and Sideroxylon lanuginosum" Foods 10, no. 11: 2710. https://doi.org/10.3390/foods10112710
APA StyleMonroy-García, I. N., Carranza-Torres, I. E., Carranza-Rosales, P., Oyón-Ardoiz, M., García-Estévez, I., Ayala-Zavala, J. F., Morán-Martínez, J., & Viveros-Valdez, E. (2021). Phenolic Profiles and Biological Activities of Extracts from Edible Wild Fruits Ehretia tinifolia and Sideroxylon lanuginosum. Foods, 10(11), 2710. https://doi.org/10.3390/foods10112710