Structure Characterization of Polysaccharide from Chinese Yam (Dioscorea opposite Thunb.) and Its Growth-Promoting Effects on Streptococcus thermophilus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strain and Culture Condition
2.3. Extraction of Crude Yam Polysaccharide (CYP)
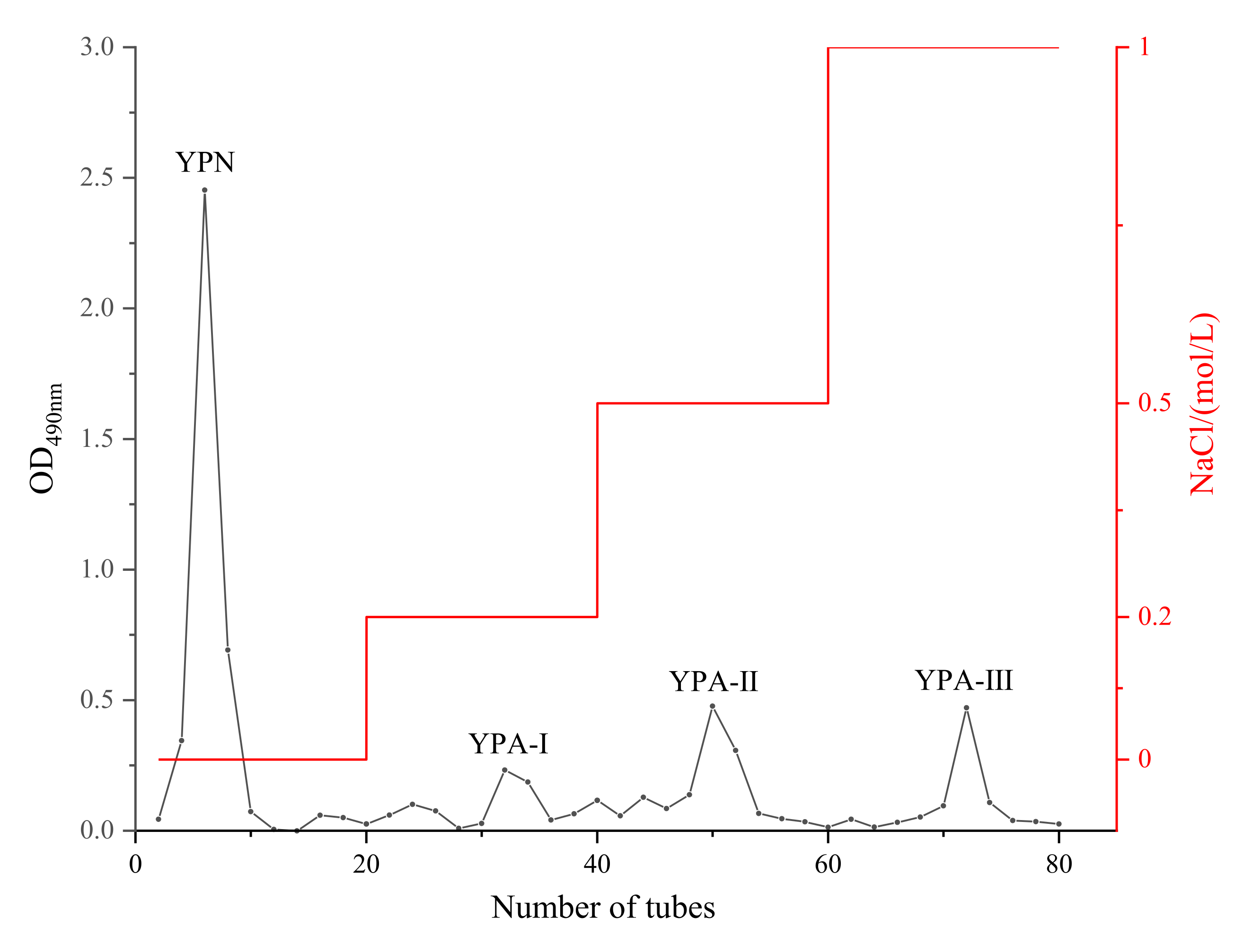
2.4. Separation of Yam Polysaccharide
2.5. Growth-Promoting Effects of Yam Polysaccharide on S. thermophilus
2.6. Determination of Molecular Weight
2.7. Determination of Monosaccharide Compositions
2.8. Scanning Electron Microscope (SEM) Analysis
2.9. Fourier Transform-Infrared Spectral (FT-IR) Analysis
2.10. Nuclear Magnetic Resonance (NMR) Analysis
2.11. Statistical Analysis
3. Results
3.1. Separation of Yam Polysaccharide
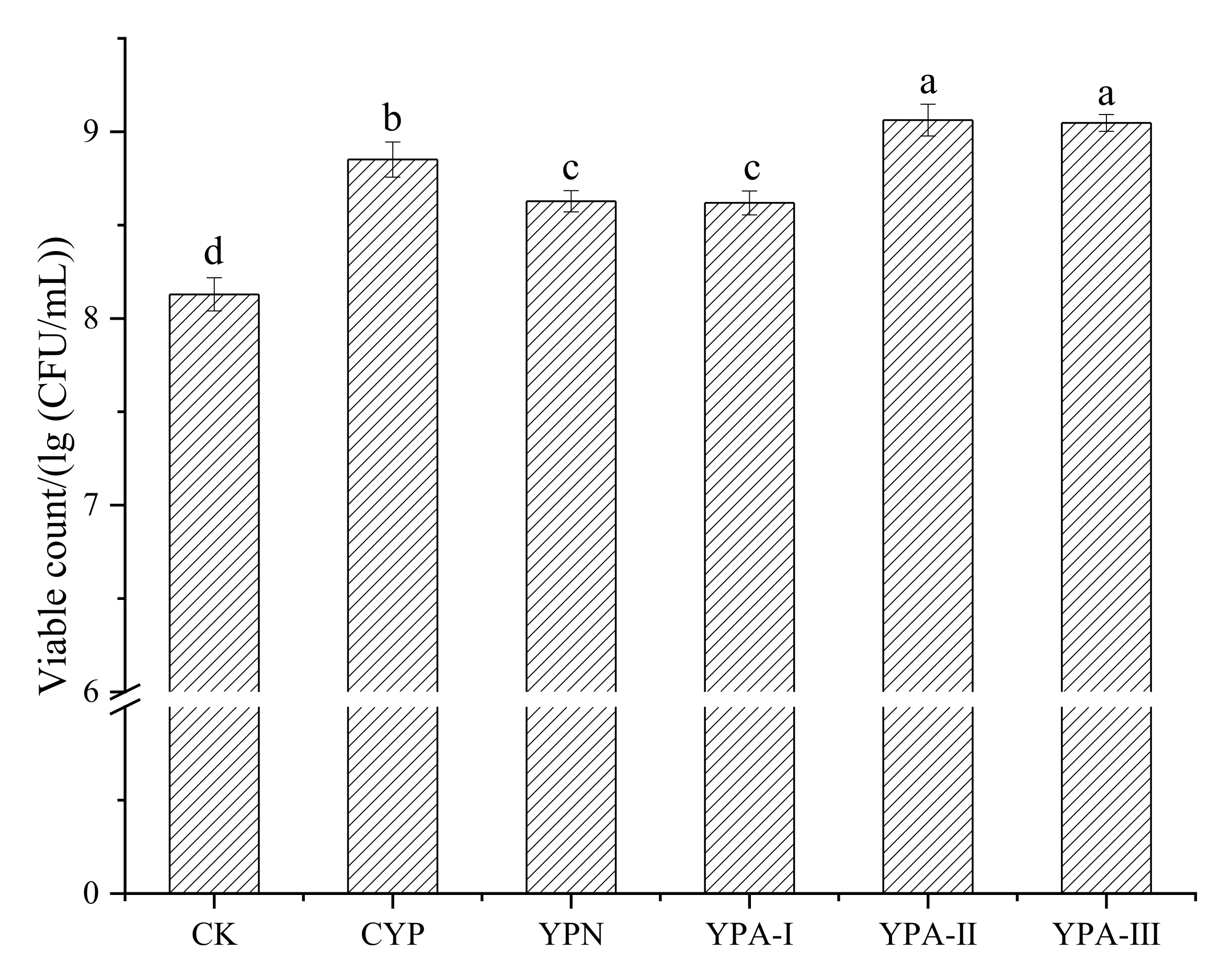
3.2. Effect of Yam Polysaccharide Fractions on the Growth of S. thermophilus
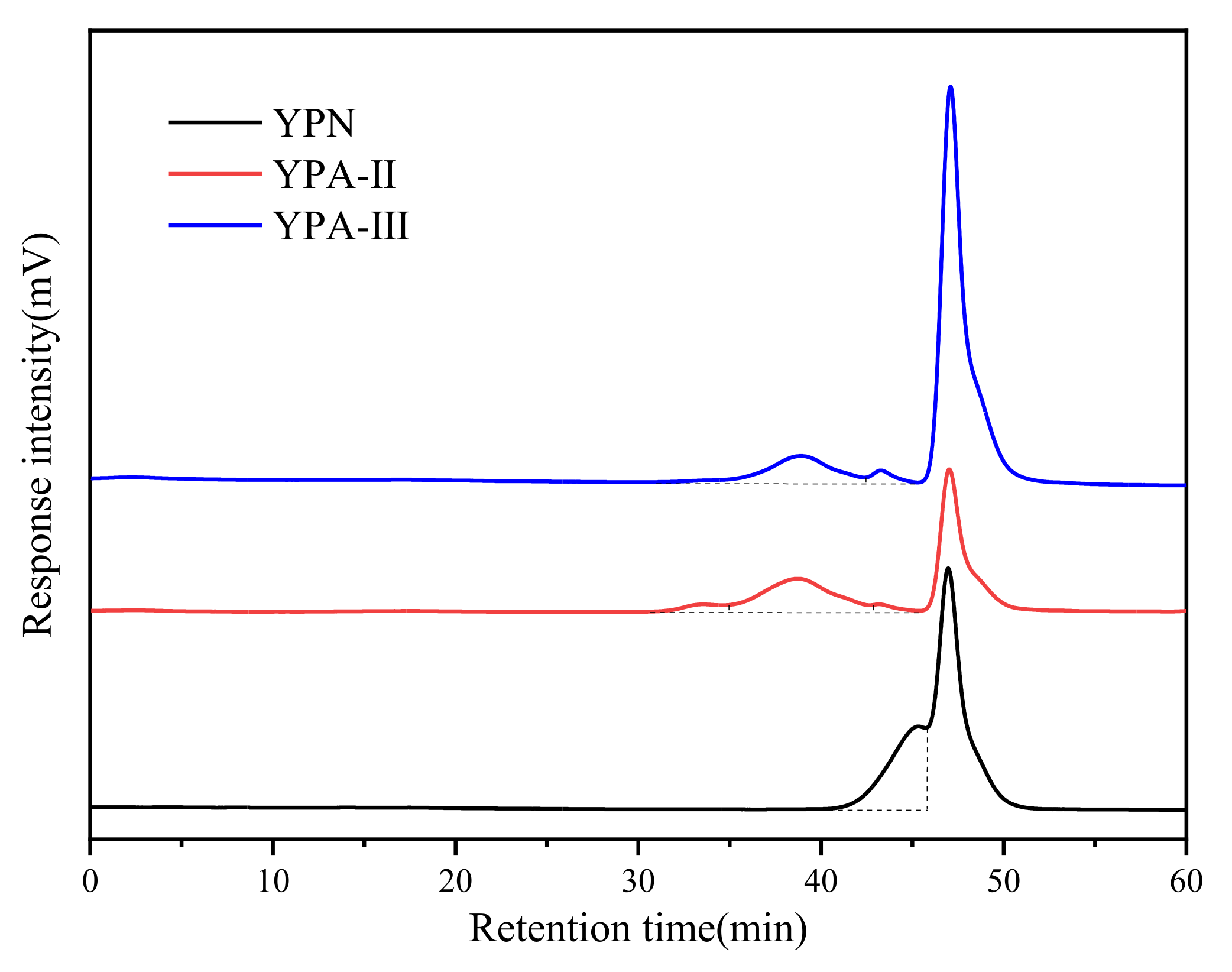
3.3. Analysis of Molecular Weight
3.4. Analysis of Monosaccharide Compositions
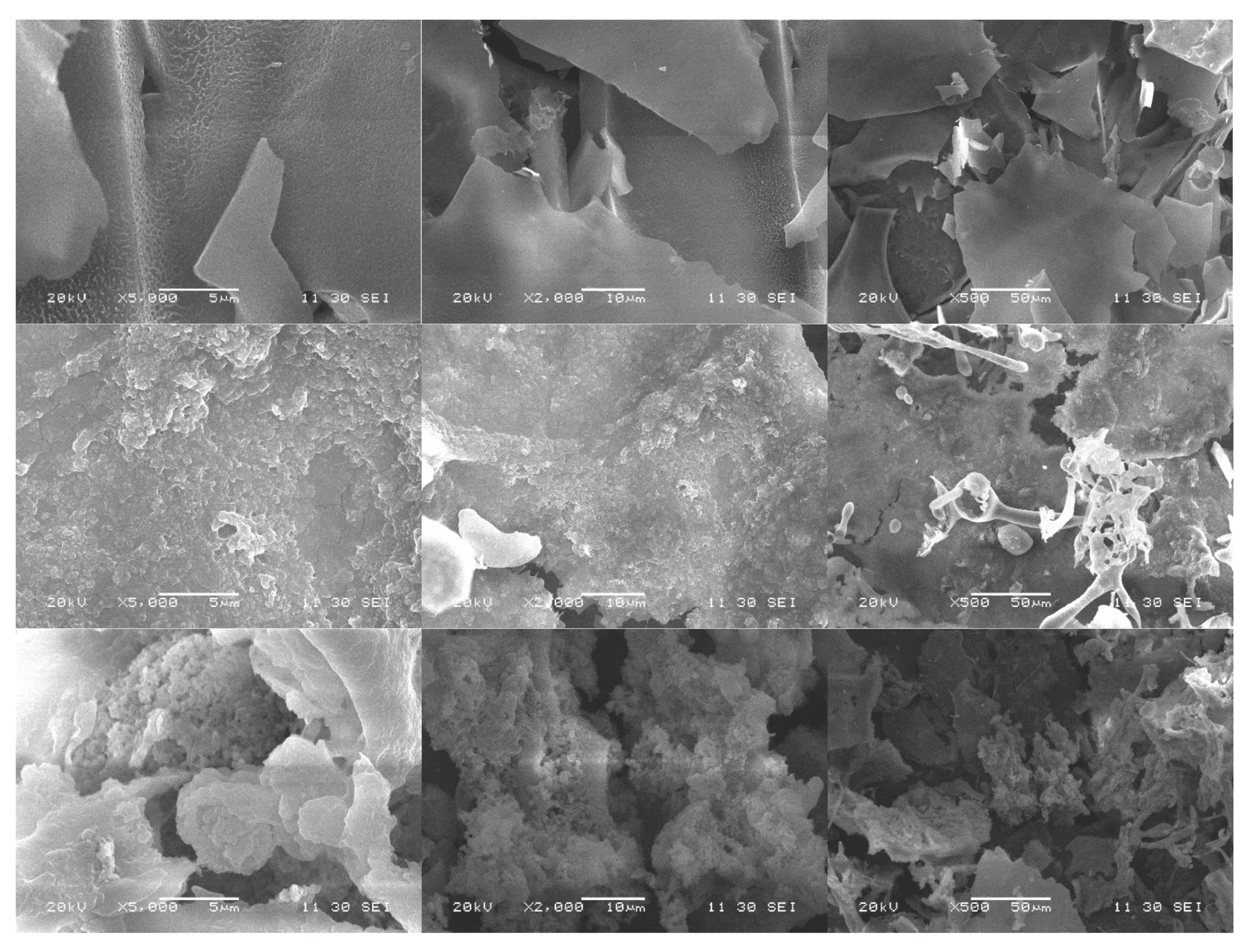
3.5. Analysis of SEM
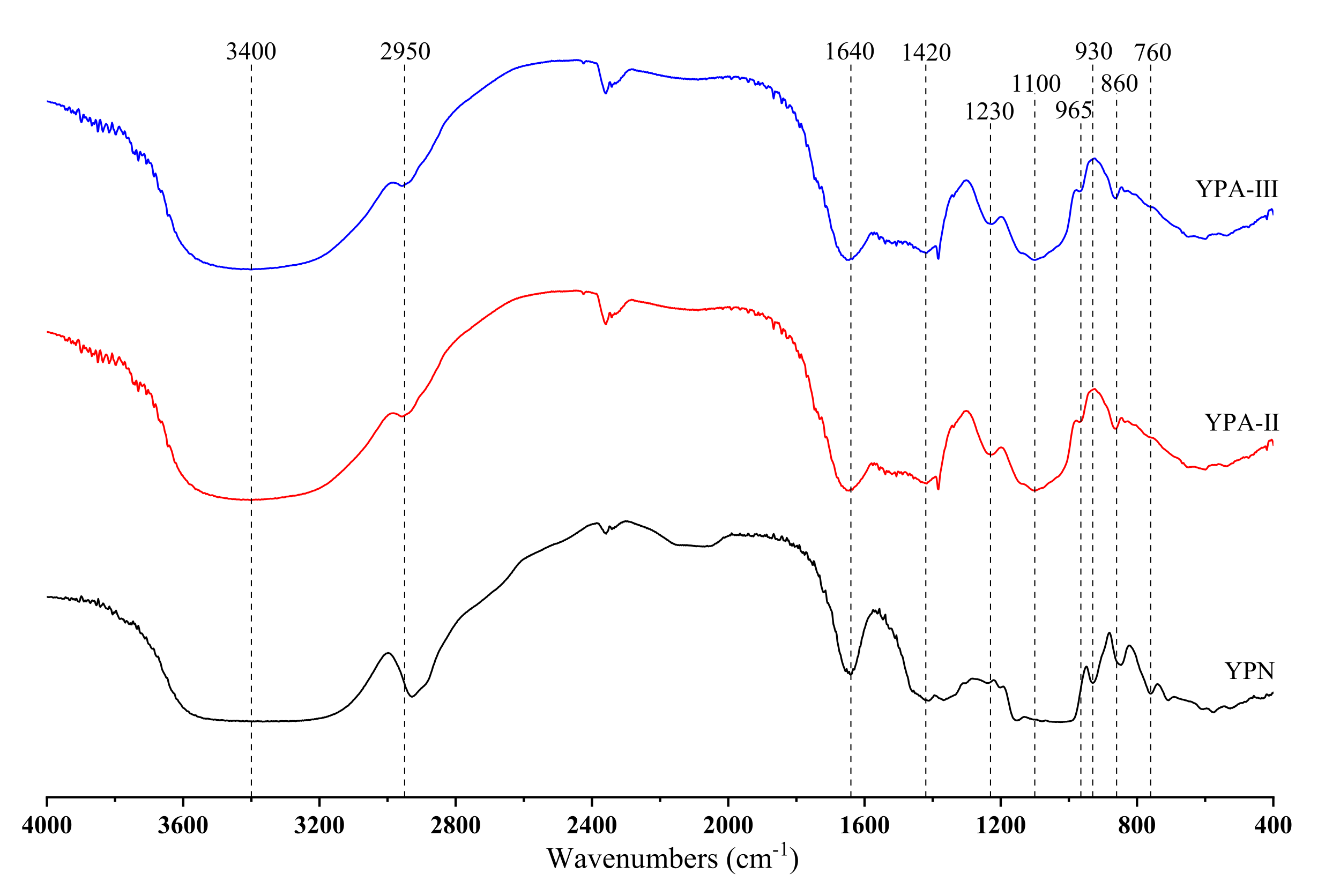
3.6. Analysis of FT-IR
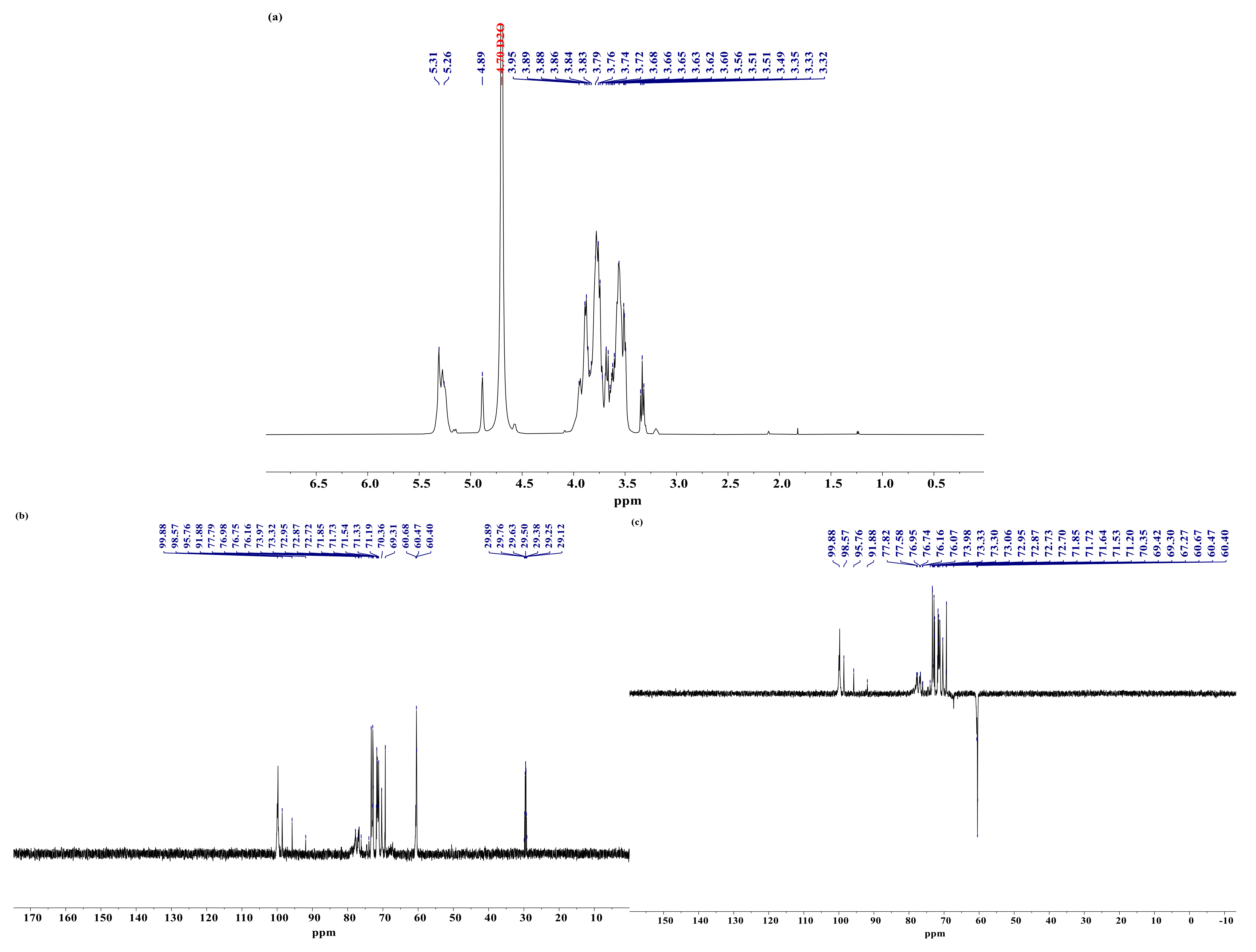
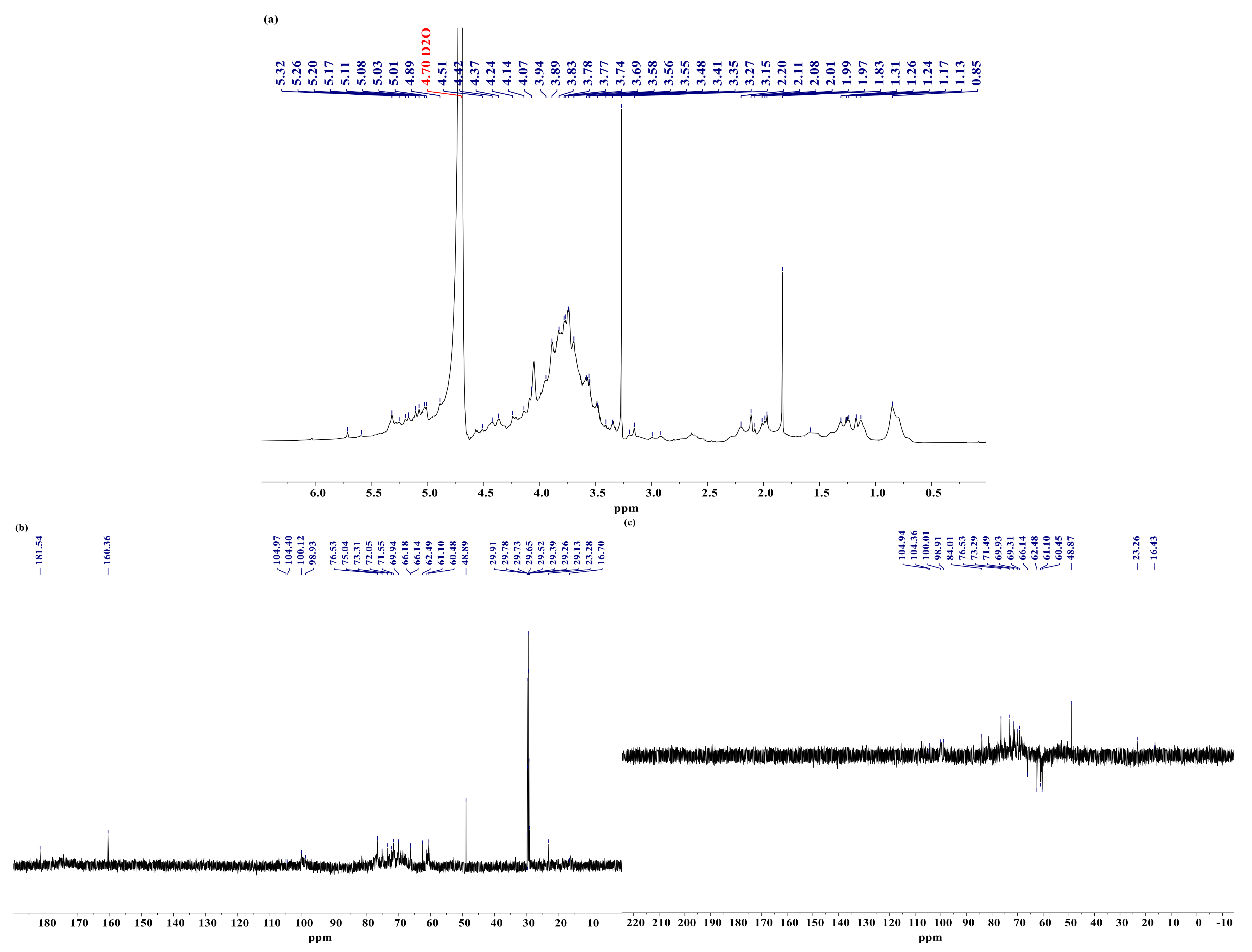
3.7. Analysis of NMR
3.8. Analysis of Growth-Promoting Mechanisms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.J.; Yu, J.L.; Yu, J.G.; Chen, H.X.; Pang, J.P.; Liu, H.Y. Partial characterization of starches from Dioscorea opposita Thunb. cultivars. J. Food Eng. 2008, 88, 287–293. [Google Scholar] [CrossRef]
- Huang, R.; Xie, J.; Yu, Y.; Shen, M. Recent progress in the research of yam mucilage polysaccharides: Isolation, structure and bioactivities. Int. J. Biol. Macromol. 2020, 155, 1262–1269. [Google Scholar] [CrossRef]
- Huang, H.H.; Jiang, Q.Q.; Chen, Y.L.; Li, X.; Mao, X.H.; Chen, X.T.; Huang, L.Q.; Gao, W.Y. Preparation, physico-chemical characterization and biological activities of two modified starches from yam (Dioscorea Opposita Thunb.). Food Hydrocoll. 2016, 55, 244–253. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, Y.; Wen, Y.; Yao, Y.; Zhu, J.; Liu, X.; Bell, A.; Tikkanen-Kaukanen, C. Emulsification properties of polysaccharides from Dioscorea opposita Thunb. Food Chem. 2017, 221, 919–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Huang, G.; Chen, G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Shen, M.; Yu, Y.; Liu, X.; Xie, J. Physicochemical characterization and immunomodulatory activity of sulfated Chinese yam polysaccharide. Int. J. Biol. Macromol. 2020, 165, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.X.; Zhao, X.H. Immunomodulatory potentials of the water-soluble yam (Dioscorea opposita Thunb) polysaccharides for the normal and cyclophosphamide-suppressed mice. Food Agric. Immunol. 2016, 27, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.Y.; Hu, M.; Tao, J.; Yang, H.; Yan, P.J.; An, G.P.; Wang, H.L. The protective effects of Chinese yam polysaccharide against obesity-induced insulin resistance. J. Funct. Foods 2019, 55, 238–247. [Google Scholar] [CrossRef]
- Huang, R.; Xie, J.; Liu, X.; Shen, M. Sulfated modification enhances the modulatory effect of yam polysaccharide on gut microbiota in cyclophosphamide-treated mice. Food Res. Int. 2021, 145, 110393. [Google Scholar] [CrossRef]
- Winarti, S.; Harmayani, E.; Marsono, Y.; Pranoto, Y. Effect of inulin isolated from lesser yam (Dioscorea esculenta) on the growth of probiotics bacteria and SCFA formation during fermentation. Int. Res. J. Microbiol. 2013, 4, 53–63. [Google Scholar]
- Zhao, C.; Li, X.; Miao, J.; Jing, S.; Li, X.; Huang, L.; Gao, W. The effect of different extraction techniques on property and bioactivity of polysaccharides from Dioscorea hemsleyi. Int. J. Biol. Macromol. 2017, 102, 847–856. [Google Scholar] [CrossRef]
- Ma, F.; Wang, R.; Zhu, J.; Zhang, Y.; Wang, Y.; Hu, W.; Bell, A.E.; Liu, X. Characterisation comparison of polysaccharides from Dioscorea opposita Thunb. growing in sandy soil, loessial soil and continuous cropping. Int. J. Biol. Macromol. 2019, 126, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, Q.; Zhang, H.; Xiong, Z.; Zhang, X.; Ai, L. Structural characterisation of EPS of Streptococcus thermophilus S-3 and its application in milk fermentation. Int. J. Biol. Macromol. 2021, 178, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chu, H.; Padmanabhan, A.; Shah, N.P. Functional Genomic Analyses of Exopolysaccharide-Producing Streptococcus thermophilus ASCC 1275 in Response to Milk Fermentation Conditions. Front Microbiol. 2019, 10, 1975. [Google Scholar] [CrossRef] [Green Version]
- Szotysik, M.; Kucharska, A.Z.; Sokó-towska, A.; Dbrowska, A.; Chrzanowska, J. The Effect of Rosa spinosissima Fruits Extract on Lactic Acid Bacteria Growth and Other Yoghurt Parameters. Foods 2020, 9, 1167. [Google Scholar] [CrossRef]
- Byakika, S.; Mukisa, I.M.; Byaruhanga, Y.B. Sorghum Malt Extract as a Growth Medium for Lactic Acid Bacteria Cultures: A Case of Lactobacillus plantarum MNC 21. Int. J. Microbiol. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Teixeira, L.S.; Martim, S.R.; Silva, L.S.C.; Kinupp, V.F.; Teixeira, M.F.S.; Porto, A.L.F. Efficiency of Amazonian tubers flours in modulating gut microbiota of male rats. Innov. Food Sci. Emerg. Technol. 2016, 38, 1–6. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Sun, Y.; Zheng, H.; Tang, Y.; Gao, X.; Song, C.; Liu, J.; Long, Y.; Liu, L.; et al. Apple polysaccharide could promote the growth of Bifidobacterium longum. Int. J. Biol. Macromol. 2020, 152, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Jayamanohar, J.; Devi, P.B.; Kavitake, D.; Priyadarisini, V.B.; Shetty, P.H. Prebiotic potential of water extractable polysaccharide from red kidney bean (Phaseolus vulgaris L.). LWT 2019, 101, 703–710. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, W.; Lin, Y.; Zhang, S.; Zou, B.; Xiao, D.; Lin, L.; Zhong, Y.; Zheng, H.; Liao, Q.; et al. Compound polysaccharides ameliorate experimental colitis by modulating gut microbiota composition and function. J. Gastroenterol. Hepatol. 2019, 34, 1554–1562. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sanchez-Alcoholado, L.; Perez-Martinez, P.; Andres-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuno, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.H.; Cheng, J.Y.; Deng, M.C.; Chou, C.H.; Jan, T.R. Prebiotic effect of diosgenin, an immunoactive steroidal sapogenin of the Chinese yam. Food Chem. 2012, 132, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, M. Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2017, 95, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, L.; Zhang, C.; Tian, Y.; Zhang, F.; Li, X. Structural and functional analyses of three purified polysaccharides isolated from Chinese Huaishan-yams. Int. J. Biol. Macromol. 2018, 120, 693–701. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Li, X.; Yu, P. Purification and structural characterization of Chinese yam polysaccharide and its activities. Carbohydr. Polym. 2015, 117, 1021–1027. [Google Scholar] [CrossRef]
- Akbari-Alavijeh, S.; Soleimanian-Zad, S.; Sheikh-Zeinoddin, M.; Hashmi, S. Pistachio hull water-soluble polysaccharides as a novel prebiotic agent. Int. J. Biol. Macromol. 2018, 107, 808–816. [Google Scholar] [CrossRef]
- Thambiraj, S.R.; Phillips, M.; Koyyalamudi, S.R.; Reddy, N. Yellow lupin (Lupinus luteus L.) polysaccharides: Antioxidant, immunomodulatory and prebiotic activities and their structural characterisation. Food Chem. 2018, 267, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nor, N.N.; Abbasiliasi, S.; Marikkar, M.N.; Ariff, A.; Amid, M.; Lamasudin, D.U.; Abdul Manap, M.Y.; Mustafa, S. Defatted coconut residue crude polysaccharides as potential prebiotics: Study of their effects on proliferation and acidifying activity of probiotics in vitro. J. Food. Sci. Technol. 2017, 54, 164–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, T.S.; Gibson, G.R. Microbial-gut interactions in health and disease. Prebiotics. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, M.B.; Haddar, A.; Ghazala, I.; Jeddou, K.B.; Helbert, C.B.; Ellouz-Chaabouni, S. Optimization of polysaccharides extraction from watermelon rinds: Structure, functional and biological activities. Food Chem. 2017, 216, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Hou, C.; Yan, Y.; Shi, M.; Liu, Y. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef]
- Xiao, W.; Huang, M.; Fan, Y.; Sun, H.; Zhou, X.; Ying, G.; Wang, X.; Zhang, M. Rapeseed polysaccharides as prebiotics on growth and acidifying activity of probiotics in vitro. Carbohydr. Polym. 2015, 125, 232–240. [Google Scholar] [CrossRef]
- Hu, B.; Gong, Q.; Wang, Y.; Ma, Y.; Li, J.; Yu, W. Prebiotic effects of neoagaro-oligosaccharides prepared by enzymatic hydrolysis of agarose. Anaerobe 2006, 12, 260–266. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Guo, Y.; Xie, Y.; Yu, H.; Yao, W.; Cheng, Y.; Qian, H. Extraction, characterization of aloe polysaccharides and the in-depth analysis of its prebiotic effects on mice gut microbiota. Carbohydr. Polym. 2021, 261, 117874. [Google Scholar] [CrossRef] [PubMed]
- Zhi, F.; Yang, T.L.; Wang, Q.; Jiang, B.; Wang, Z.P.; Zhang, J.; Chen, Y.Z. Isolation, structure and activity of a novel water-soluble polysaccharide from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2019, 133, 1201–1209. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, G.; Yu, Z.; Song, X.; Li, X.; Yang, Y.; Wang, L.; Liu, L.; Dai, J. Purification, characterization and antiglycation activity of a novel polysaccharide from black currant. Food Chem. 2016, 199, 694–701. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Wu, X.; Wang, Y.; Xu, K. 1H NMR-based metabolic profiling approach to identify the geo-authentic Chinese yam (Dioscorea polystachya Turczaninow cv. Tiegun). J. Food Compos. Anal. 2021, 98, 103805. [Google Scholar] [CrossRef]
- Li, N.; Mao, W.; Yan, M.; Liu, X.; Xia, Z.; Wang, S.; Xiao, B.; Chen, C.; Zhang, L.; Cao, S. Structural characterization and anticoagulant activity of a sulfated polysaccharide from the green alga Codium divaricatum. Carbohydr. Polym. 2015, 121, 175–182. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Liu, L. Novel polysaccharide from Chaenomeles speciosa seeds: Structural characterization, alpha-amylase and alpha-glucosidase inhibitory activity evaluation. Int. J. Biol. Macromol. 2020, 153, 755–766. [Google Scholar] [CrossRef]
- Lam, K.-L.; Keung, H.-Y.; Ko, K.-C.; Kwan, H.-S.; Cheung, P.C.-K. In vitro fermentation of beta-glucans and other selected carbohydrates by infant fecal inoculum: An evaluation of their potential as prebiotics in infant formula. Bioact. Carbohydr. Diet Fibre 2018, 14, 20–24. [Google Scholar] [CrossRef]
- Lam, K.L.; Ko, K.C.; Li, X.; Ke, X.; Cheng, W.Y.; Chen, T.; You, L.; Kwan, H.S.; Cheung, P.C. In Vitro Infant Faecal Fermentation of Low Viscosity Barley beta-Glucan and Its Acid Hydrolyzed Derivatives: Evaluation of Their Potential as Novel Prebiotics. Molecules 2019, 24, 828. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Jang, W.J.; Lee, E.-W.; Kong, I.-S. β-glucooligosaccharides derived from barley β-glucan promote growth of lactic acid bacteria and enhance nisin Z secretion by Lactococcus lactis. LWT 2020, 122, 109014. [Google Scholar] [CrossRef]

| Retention Time/Min | Mobile Phase (%) | ||
|---|---|---|---|
| A | B | C | |
| 0.00 | 98.8 | 1.2 | 0.0 |
| 20.0 | 98.8 | 1.2 | 0.0 |
| 20.1 | 50.0 | 50.0 | 0.0 |
| 30.0 | 50.0 | 50.0 | 0.0 |
| 30.1 | 0.0 | 0.0 | 100.0 |
| 46.0 | 0.0 | 0.0 | 100.0 |
| 46.1 | 0.0 | 100.0 | 0.0 |
| 50.0 | 0.0 | 100.0 | 0.0 |
| 50.1 | 98.8 | 1.2 | 0.0 |
| 80.0 | 98.8 | 1.2 | 0.0 |
| Polysaccharide Fractions | Retention Time (min) | Mw/Da | Mn/Da | Mw/Mn |
|---|---|---|---|---|
| YPN | 45.353 | 3209 | 2646 | 1.213 |
| YPA-Ⅱ | 33.469 | 733,506 | 405,556 | 1.809 |
| 38.667 | 68,171 | 44,889 | 1.519 | |
| 43.158 | 8753 | 6703 | 1.306 | |
| YPA-Ⅲ | 38.835 | 63,132 | 41,806 | 1.510 |
| 43.223 | 8496 | 6521 | 1.303 |
| Monosaccharides | Retention Time/Min | Molar Ratio | ||
|---|---|---|---|---|
| YPN | YPA-Ⅱ | YPA-Ⅲ | ||
| Fucose | 5.984 | ND | 1.8 | 1.7 |
| Galactosamine hydrochloride | 11.217 | ND | 0.3 | 2.3 |
| Rhamnose | 11.942 | ND | 2.5 | 6.7 |
| Arabinose | 12.767 | ND | 7.6 | 8.5 |
| Glucosamine hydrochloride | 14.167 | ND | 0.6 | 8.6 |
| Galactose | 16.075 | 0.8 | 12.4 | 1.5 |
| Glucose | 18.275 | 99.2 | ND | ND |
| Xylose | 21.484 | ND | 11.0 | ND |
| Mannose | 22.484 | ND | 15.4 | ND |
| Galacturonic acid | 45.534 | ND | 48.5 | 66.1 |
| Glucuronic acid | 48.959 | ND | ND | 4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, J.; Wang, F.; Li, W.; Li, Q.; Su, X. Structure Characterization of Polysaccharide from Chinese Yam (Dioscorea opposite Thunb.) and Its Growth-Promoting Effects on Streptococcus thermophilus. Foods 2021, 10, 2698. https://doi.org/10.3390/foods10112698
Ouyang J, Wang F, Li W, Li Q, Su X. Structure Characterization of Polysaccharide from Chinese Yam (Dioscorea opposite Thunb.) and Its Growth-Promoting Effects on Streptococcus thermophilus. Foods. 2021; 10(11):2698. https://doi.org/10.3390/foods10112698
Chicago/Turabian StyleOuyang, Jia, Feng Wang, Wenjia Li, Qingming Li, and Xiaojun Su. 2021. "Structure Characterization of Polysaccharide from Chinese Yam (Dioscorea opposite Thunb.) and Its Growth-Promoting Effects on Streptococcus thermophilus" Foods 10, no. 11: 2698. https://doi.org/10.3390/foods10112698
APA StyleOuyang, J., Wang, F., Li, W., Li, Q., & Su, X. (2021). Structure Characterization of Polysaccharide from Chinese Yam (Dioscorea opposite Thunb.) and Its Growth-Promoting Effects on Streptococcus thermophilus. Foods, 10(11), 2698. https://doi.org/10.3390/foods10112698






