Effects of Enzymatic Hydrolysis on Physicochemical Properties and Solubility and Bitterness of Milk Protein Hydrolysates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzyme Hydrolysates
2.3. Degree of Hydrolysis (DH)
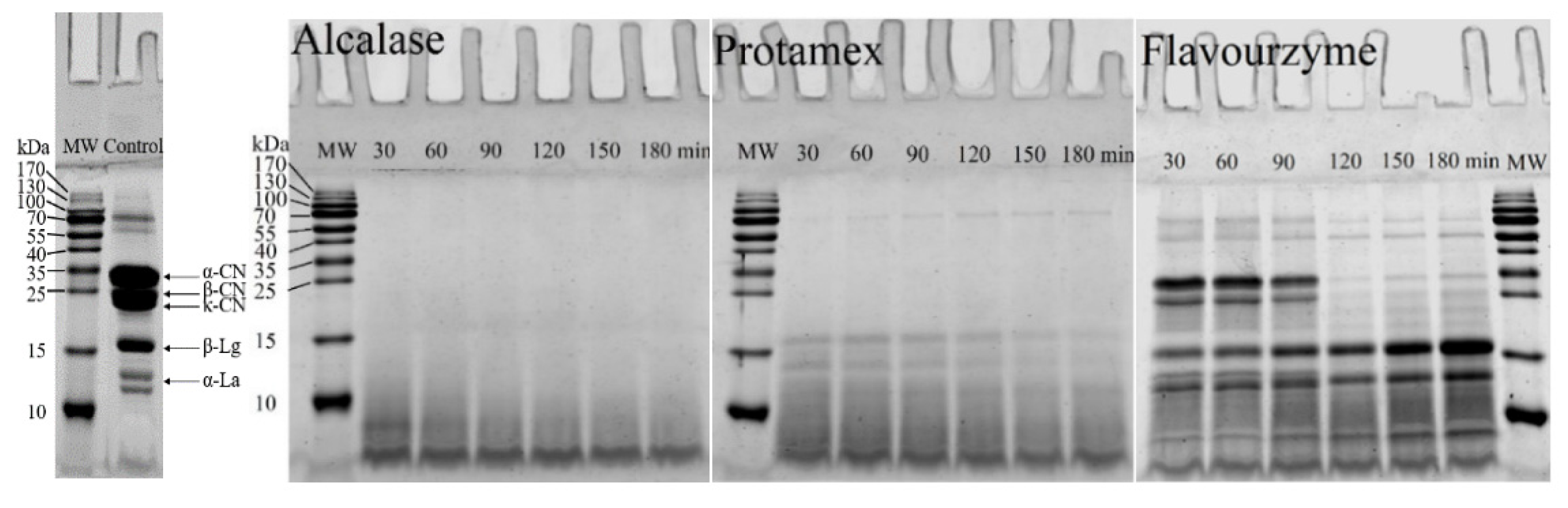
2.4. SDS-PAGE
2.5. Ultraviolet (UV) Spectra
2.6. Intrinsic Fluorescence Spectroscopy
2.7. Surface Hydrophobicity (H0) Measurements
2.8. Fourier Infrared Spectrum (FTIR)
2.9. Static Laser Light Scattering for Particle Size
2.10. Electronic Tongue Measurements
2.11. Soluble Protein Determination
2.12. Statistical Analysis
3. Results
3.1. Degree of Hydrolysis and Analysis of Soluble Protein
3.2. SDS-PAGE
3.3. Surface Hydrophobicity
3.4. Particle Size Distribution
3.5. Relative Fluorescence Intensity and Ultraviolet Absorption Spectroscopy
3.6. FTIR
3.7. Representation of Sensor Response of the Electronic Tongue
3.8. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McGregor, R.A.; Poppitt, S.D. Milk protein for improved metabolic health: A review of the evidence. Nutr. Metab. 2013, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Kameswaran, S.; Smith, D.E. Rennet clotting times of skim milk based rennet gels supplemented with an ultrafiltered milk protein concentrate. Milchwiss. Milk Sci. Int. 1999, 54, 546–549. [Google Scholar]
- O’Donnell, S.; Butler, F. Viscosity of Reconstituted Milk Protein Concentrate Solutions as a Function of Shear, Temperature and Concentration. Dev. Chem. Eng. Miner. Process. 2008, 7, 131–139. [Google Scholar] [CrossRef]
- Banach, J.; Lin, Z.; Lamsal, B. Enzymatic modification of milk protein concentrate and characterization of resulting functional properties. LWT Food Sci. Technol. 2013, 54, 397–403. [Google Scholar] [CrossRef]
- Tari, N.R.; Gaygadzhiev, Z.; Guri, A.; Wright, A. Effect of pH and heat treatment conditions on physicochemical and acid gelation properties of liquid milk protein concentrate. J. Dairy Sci. 2021, 104, 6609–6619. [Google Scholar] [CrossRef]
- Agarwal, S.; Beausire, R.L.W.; Patel, S.; Patel, H. Innovative Uses of Milk Protein Concentrates in Product Development. J. Food Sci. 2015, 80, A23–A29. [Google Scholar] [CrossRef]
- McKenna, A.B. Effect of Processing and Storage on the Reconstitution Properties of Whole Milk and Ultrafiltered Skim Milk Powders; Massey University: Palmerston North, New Zealand, 2002. [Google Scholar]
- Havea, P. Protein interactions in milk protein concentrate powders. Int. Dairy J. 2006, 16, 415–422. [Google Scholar] [CrossRef]
- Anema, S.; Pinder, D.; Hunter, R.; Hemar, Y. Effects of storage temperature on the solubility of milk protein concentrate (MPC85). Food Hydrocoll. 2006, 20, 386–393. [Google Scholar] [CrossRef]
- Kelly, P. Milk protein products/milk protein concentrate. In Encyclopedia of Diary Sciences, 2nd ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 848–854. [Google Scholar]
- Wouters, A.G.; Rombouts, I.; Fierens, E.; Brijs, K.; Delcour, J. Relevance of the Functional Properties of Enzymatic Plant Protein Hydrolysates in Food Systems. Compr. Rev. Food Sci. Food Saf. 2016, 15, 786–800. [Google Scholar] [CrossRef] [Green Version]
- Walstra, P. Physical Chemistry of Foods; Marcel Dekker, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Le, T.T.; Bhandari, B.; Deeth, H.C. Chemical and Physical Changes in Milk Protein Concentrate (MPC80) Powder during Storage. J. Agric. Food Chem. 2011, 59, 5465–5473. [Google Scholar] [CrossRef]
- Ye, A. Functional properties of milk protein concentrates: Emulsifying properties, adsorption and stability of emulsions. Int. Dairy J. 2011, 21, 14–20. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, J.; Zhang, S.; Li, H.; Lu, J.; Lu, L.; Lv, J. Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate. J. Food Eng. 2014, 124, 11–18. [Google Scholar]
- Guo, M.R.; Fox, P.F.; Flynn, A.; Kindstedt, P.S. Susceptibility of beta-lactoglobulin and sodium caseinate to proteolysis by pepsin and trypsin. J. Dairy Sci. 1995, 78, 2336–2344. [Google Scholar] [CrossRef]
- Evangelho, J.A.D.; Vanier, N.; Pinto, V.Z.; De Berrios, J.J.; Dias, A.R.G.; Zavareze, E.D.R. Black bean (Phaseolus vulgaris L.) protein hydrolysates: Physicochemical and functional properties. Food Chem. 2017, 214, 460–467. [Google Scholar] [CrossRef]
- Shen, P.; Zhou, F.; Zhang, Y.; Yuan, D.; Zhao, Q.; Zhao, M. Formation and characterization of soy protein nanoparticles by controlled partial enzymatic hydrolysis. Food Hydrocoll. 2020, 105, 105844. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Li, J.; Sun, H.; Liu, Y. Physicochemical and antioxidative characteristics of black bean protein hydrolysates obtained from different enzymes. Food Hydrocoll. 2019, 97, 105222. [Google Scholar] [CrossRef]
- Severin, S.; Xia, W. Enzymatic hydrolysis of whey proteins by two different proteases and their effect on the functional properties of resulting protein hydrolysates. J. Food Biochem. 2006, 30, 77–97. [Google Scholar] [CrossRef]
- Urista, C.R.M.; Fernández, R.; Rodríguez, F.A.R.; Arana-Cuenca, A.; Tellez-Jurado, A. Review: Production and functionality of active peptides from milk. Food Sci. Technol. Int. 2011, 17, 293–317. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Avramenko, N.A.; Low, N.H.; Nickerson, M.T. The effects of limited enzymatic hydrolysis on the physicochemical and emulsifying properties of a lentil protein isolate. Food Res. Int. 2013, 51, 162–169. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Zhao, M.; Ren, J.; Yang, B. Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chem. 2012, 131, 901–906. [Google Scholar] [CrossRef]
- Hu, H.; Cheung, I.W.; Pan, S.; Li-Chan, E.C. Effect of high intensity ultrasound on physicochemical and functional properties of aggregated soybean β-conglycinin and glycinin. Food Hydrocoll. 2014, 45, 102–110. [Google Scholar] [CrossRef]
- Silva, M.; Zisu, B.; Chandrapala, J. Influence of low-frequency ultrasound on the physico-chemical and structural characteristics of milk systems with varying casein to whey protein ratios. Ultrason. Sonochem. 2018, 49, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Mazloomi, S.N.; Mahoonak, A.S.; Ghorbani, M.; Houshmand, G. Physicochemical properties of chitosan-coated nanoliposome loaded with orange seed protein hydrolysate. J. Food Eng. 2020, 280, 109976. [Google Scholar] [CrossRef]
- Liu, B.-Y.; Zhu, K.-X.; Peng, W.; Guo, X.-N.; Zhou, H.-M. Effect of sequential hydrolysis with endo- and exo-peptidase on bitterness properties of wheat gluten hydrolysates. RSC Adv. 2016, 6, 27659–27668. [Google Scholar] [CrossRef]
- Liang, G.; Chen, W.; Qie, X.; Zeng, M.; Qin, F.; He, Z.; Chen, J. Modification of soy protein isolates using combined pre-heat treatment and controlled enzymatic hydrolysis for improving foaming properties. Food Hydrocoll. 2020, 105, 105764. [Google Scholar] [CrossRef]
- Al-Ruwaih, N.; Ahmed, J.; Mulla, M.F.; Arfat, Y.A. High-Pressure assisted enzymatic proteolysis of kidney beans protein isolates and characterization of hydrolysates by functional, structural, rheological and antioxidant properties. LWT Food Sci. Technol. 2018, 100, 231–236. [Google Scholar] [CrossRef]
- Ai, M.; Tang, T.; Zhou, L.; Ling, Z.; Guo, S.; Jiang, A. Effects of different proteases on the emulsifying capacity, rheological and structure characteristics of preserved egg white hydrolysates. Food Hydrocoll. 2018, 87, 933–942. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.; Meng, S. Comparing the kinetics of the hydrolysis of by-product from channel catfish (Ictalurus punctatus) fillet processing by eight proteases. LWT Food Sci. Technol. 2019, 111, 809–820. [Google Scholar] [CrossRef]
- Haque, E.; Bhandari, B.R.; Gidley, M.J.; Deeth, H.C.; Møller, S.M.; Whittaker, A.K. Protein Conformational Modifications and Kinetics of Water−Protein Interactions in Milk Protein Concentrate Powder upon Aging: Effect on Solubility. J. Agric. Food Chem. 2010, 58, 7748–7755. [Google Scholar] [CrossRef]
- Doucet, D.; Otter, D.E.; Gauthier, S.F.; Foegeding, E.A. Enzyme-Induced Gelation of Extensively Hydrolyzed Whey Proteins by Alcalase: Peptide Identification and Determination of Enzyme Specificity. J. Agric. Food Chem. 2003, 51, 6300–6308. [Google Scholar] [CrossRef] [PubMed]
- Tavano, L.O. Protein hydrolysis using proteases: An important tool for food biotechnology. J. Mol. Cayal B Enzym. 2013, 90, 1–11. [Google Scholar] [CrossRef]
- Zang, X.; Yue, C.; Wang, Y.; Shao, M.; Yu, G. Effect of limited enzymatic hydrolysis on the structure and emulsifying properties of rice bran protein. J. Cereal Sci. 2018, 85, 168–174. [Google Scholar] [CrossRef]
- Jung, S.; Murphy, P.A.; Johnson, L.A. Physicochemical and Functional Properties of Soy Protein Substrates Modified by Low Levels of Protease Hydrolysis. J. Food Sci. 2005, 70, C180–C187. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, A.; Li, R.; Wang, X.; Sun, L.; Jiang, L. Ultrasonic treatment affects emulsifying properties and molecular flexibility of soybean protein isolate-glucose conjugates. Food Biosci. 2020, 38, 100747. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, P.X.; Ren, D.; Xiao, Y.; Tomasula, P.M. Effect of homogenization and pasteurization on the structure and stability of whey protein in milk. J. Dairy Sci. 2015, 98, 2884–2897. [Google Scholar] [CrossRef] [Green Version]
- Podrażka, M.; Bączyńska, E.; Kundys, M.; Jeleń, P.; Witkowska, N.E. Electronic Tongue—A tool for all tastes? Biosensors 2017, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Humiski, L.; Aluko, R. Physicochemical and Bitterness Properties of Enzymatic Pea Protein Hydrolysates. J. Food Sci. 2007, 72, S605–S611. [Google Scholar] [CrossRef]
- Tong, X.; Lian, Z.; Miao, L.; Qi, B.; Zhang, S.; Li, Y.; Wang, H.; Jiang, L. An innovative two-step enzyme-assisted aqueous extraction for the production of reduced bitterness soybean protein hydrolysates with high nutritional value. LWT Food Sci. Technol. 2020, 134, 110151. [Google Scholar] [CrossRef]




| Sample (min) | DH | Solubility | ||||
|---|---|---|---|---|---|---|
| Alcalase | Protamex | Flavourzyme | Alcalase | Protamex | Flavourzyme | |
| control | - | - | - | 48.2 ± 0.51a | 48.2 ± 0.51a | 48.2 ± 0.51a |
| 30 | 12.9 ± 0.51a | 10.1 ± 0.97a | 9.9 ± 0.79a | 72.1 ± 2.3b | 68.2 ± 1.1d | 64.3 ± 0.19b |
| 60 | 13.2 ± 0.42a | 12.60 ± 1.2ab | 10.1 ± 1.1a | 74.1 ± 1.1c | 61.6 ± 2.1b | 64.9 ± 1.2b |
| 90 | 14.7 ± 1.1ab | 13.3 ± 0.76b | 10.4 ± 1.1a | 78.0 ± 3.20d | 67.3 ± 1.2d | 67.6 ± 0.87c |
| 120 | 15.3 ± 0.95b | 13.3 ± 0.83b | 11.5 ± 1.1a | 86.3 ± 2.1e | 64.1 ± 2.2c | 70.8 ± 0.94d |
| 150 | 15.6 ± 0.12b | 13.4 ± 1.2b | 11.6 ± 0.21a | 90.2 ± 1.1f | 81.1 ± 1.1e | 72.6 ± 1.2d |
| 180 | 15.7 ± 1.3b | 13.6 ± 0.93b | 11.9 ± 0.13a | 91.1 ± 0.91f | 85.1 ± 1.2f | 75.2 ± 2.1e |
| Sample (min) | Particle Size (nm) | ||
|---|---|---|---|
| Alcalase | Protamex | Flavourzyme | |
| control | 34.87 ± 1.21 (μm) | ||
| 30 | 245.8 ± 2.9a | 226.4 ± 4.6bc | 220.1 ± 4.3c |
| 60 | 261.7 ± 1.5b | 221.3 ± 1.7ab | 213.3 ± 4.9c |
| 90 | 258.8 ± 41b | 218.9 ± 3.9a | 221.7 ± 16.8c |
| 120 | 261.9 ± 4.20b | 229.1 ± 4.3cd | 221.1 ± 3.4c |
| 150 | 275.8 ± 3.2c | 243.9 ± 2.6e | 199.8 ± 1.1b |
| 180 | 286.9 ± 3.3d | 234.7 ± 3.3d | 183.3 ± 2.3a |
| Sample (min) | Alcalase | Protamex | Flavourzyme | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bitterness | Aftertaste−B | Astringency | Aftertaste−A | Bitterness | Aftertaste−B | Astringency | Aftertaste−A | Bitterness | Aftertaste−B | Astringency | Aftertaste−A | |
| control | 4.88 ± 0.46a | 2.76 ± 0.87a | −3.29 ± 0.06c | −1.65 ± 0.25a | 4.88 ± 0.46a | 2.76 ± 0.87a | −3.29 ± 0.06d | −1.65 ± 0.25a | 4.88 ± 0.46a | 2.76 ± 0.87ab | −3.29 ± 0.06b | −1.65 ± 0.25a |
| 30 | 8.22 ± 0.05b | 3.8 ± 0.06ab | −6.81 ± 0.03b | −1.14 ± 0.17ab | 6.48 ± 0.5b | 1.81 ± 1.2a | −3.88 ± 0.09c | −2.08 ± 1.18a | 7.71 ± 0.08c | 2.43 ± 0.17a | −2.96 ± 0.09b | −1.5 ± 0.11a |
| 60 | 8.40 ± 0.04b | 4.16 ± 0.18ab | −6.8 ± 0.05b | −1.06 ± 0.18ab | 6.42 ± 0.51b | 1.61 ± 1.3a | −4.01 ± 0.11bc | −2.1 ± 1.3a | 7.57 ± 0.03ab | 2.59 ± 0.17ab | −3.47 ± 0.16a | −1.55 ± 0.16a |
| 90 | 8.25 ± 0.08b | 3.71 ± 0.12ab | −6.87 ± 0.09b | −1.03 ± 0.31bab | 6.32 ± 0.68b | 1.04 ± 0.12a | −4.13 ± 0.12abc | −2.17 ± 1.4a | 7.47 ± 0.28ab | 2.86 ± 0.37ab | −3.67 ± 0.25a | −1.64 ± 0.40a |
| 120 | 8.14 ± 0.12b | 3.96 ± 0.64ab | −6.92 ± 0.14b | −1.07 ± 0.64ab | 6.18 ± 0.74b | 0.99 ± 0.12a | −4.19 ± 0.12abc | −2.29 ± 1.7a | 7.18 ± 0.14b | 3.06 ± 0.12ab | −4.15 ± 0.11a | −1.68 ± 0.24a |
| 150 | 8.41 ± 0.29b | 4.30 ± 0.75b | −6.91 ± 0.04b | −0.87 ± 0.75b | 6.36 ± 0.12b | 2.63 ± 0.21a | −4.32 ± 0.04ab | −1.99 ± 0.04a | 7.39 ± 0.12ab | 3.95 ± 0.30c | −4.24 ± 0.21a | −1.45 ± 0.43a |
| 180 | 8.11 ± 0.38b | 3.95 ± 0.96ab | −7.09 ± 0.07a | −0.99 ± 0.96b | 6.50 ± 0.04b | 2.78 ± 0.34a | −4.41 ± 0.08a | −2.01 ± 0.10a | 7.14 ± 0.36b | 3.49 ± 0.58bc | −4.46 ± 0.25a | −1.68 ± 0.50a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Q.; Sun, Y.; Zhou, Z.; Cheng, J.; Guo, M. Effects of Enzymatic Hydrolysis on Physicochemical Properties and Solubility and Bitterness of Milk Protein Hydrolysates. Foods 2021, 10, 2462. https://doi.org/10.3390/foods10102462
Cui Q, Sun Y, Zhou Z, Cheng J, Guo M. Effects of Enzymatic Hydrolysis on Physicochemical Properties and Solubility and Bitterness of Milk Protein Hydrolysates. Foods. 2021; 10(10):2462. https://doi.org/10.3390/foods10102462
Chicago/Turabian StyleCui, Qiang, Yuxue Sun, Zengjia Zhou, Jianjun Cheng, and Mingruo Guo. 2021. "Effects of Enzymatic Hydrolysis on Physicochemical Properties and Solubility and Bitterness of Milk Protein Hydrolysates" Foods 10, no. 10: 2462. https://doi.org/10.3390/foods10102462






