Structural and Emulsifying Properties of Citric Acid Extracted Satsuma Mandarin Peel Pectin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
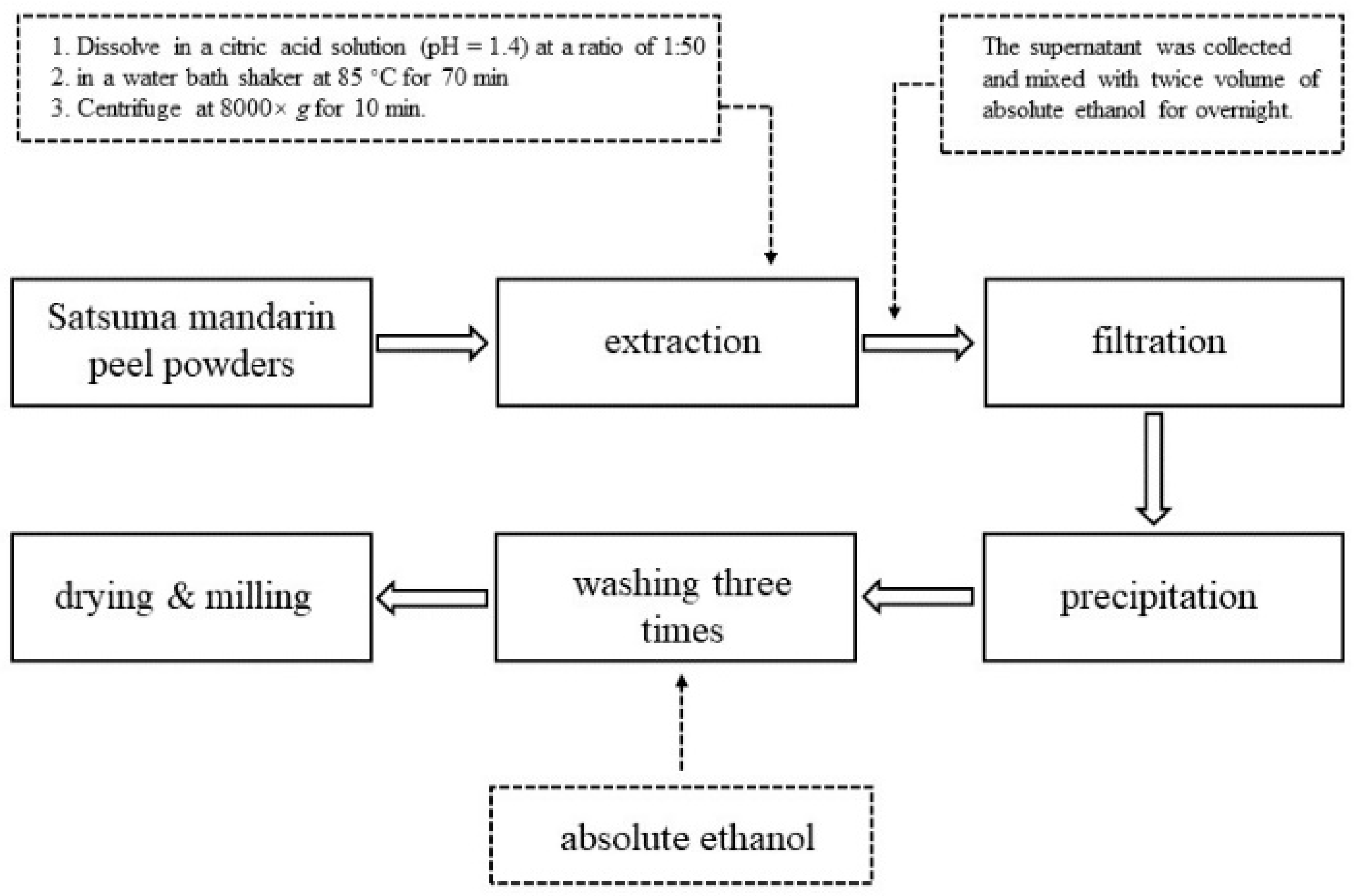
2.2. Preparation of the SAMPLE
2.3. Extraction of Pectin
2.4. Structural Characteristics Determination
2.4.1. Galacturonic acid (GalA) Content, Degree of Methoxylation (DM), and Monosaccharide Composition Analysis
2.4.2. Determination of Molecular Weight Distribution (MWD) and Average Molecular Weight (Mw)
2.4.3. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.4.4. Fourier Transforms-Infrared (FT-IR) Spectroscopy
2.5. Emulsifying Properties
2.5.1. Preparation of Emulsions
2.5.2. Droplet Size Determination of Emulsion
2.5.3. Rheological Properties of Emulsion
2.5.4. Determination of Emulsion Stability
2.5.5. Morphology of Emulsion
2.6. Statistical Analysis
3. Results and Discussion
3.1. The Monosaccharide Composition, DM, and Molecular Weight of MPP and CCP
3.2. Spectroscopy Analysis by NMR and FTIR
3.3. Emulsifying Properties
3.3.1. The Particle Size
3.3.2. The Stability of Emulsion
3.3.3. Viscosity of Emulsion
3.3.4. Microstructural Observations
3.4. Relation between Emulsion Properties and Pectin Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ozturk, B.; McClements, D.J. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Chivero, P.; Gohtani, S.; Yoshii, H.; Nakamura, A. Physical properties of oil-in-water emulsions as a function of oil and soy soluble polysaccharide types. Food Hydrocoll. 2014, 39, 34–40. [Google Scholar] [CrossRef]
- Ma, F.; Bell, A.E.; Davis, F.J. Effects of high-hydrostatic pressure and pH treatments on the emulsification properties of gum arabic. Food Chem. 2015, 184, 114–121. [Google Scholar] [CrossRef]
- Wang, M.; Huang, B.; Fan, C.; Zhao, K.; Hu, H.; Xu, X.; Pan, S.; Liu, F. Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. Int. J. Biol. Macromol. 2016, 91, 794–803. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Wan, L.; Chen, Q.; Huang, M.; Liu, F.; Pan, S. Physiochemical, rheological and emulsifying properties of low methoxyl pectin prepared by high hydrostatic pressure-assisted enzymatic, conventional enzymatic, and alkaline de-esterification: A comparison study. Food Hydrocoll. 2019, 93, 146–155. [Google Scholar] [CrossRef]
- Yang, X.; Nisar, T.; Hou, Y.; Gou, X.; Sun, L.; Guo, Y. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocoll. 2018, 85, 30–38. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Schmidt, K.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Pectins of different origin and their performance in forming and stabilizing oil-in-water-emulsions. Food Hydrocoll. 2015, 46, 59–66. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Schütz, L.; Schuchmann, H.P. Interfacial and emulsifying properties of citrus pectin: Interaction of pH, ionic strength and degree of esterification. Food Hydrocoll. 2017, 62, 288–298. [Google Scholar] [CrossRef]
- Funami, T.; Nakauma, M.; Ishihara, S.; Tanaka, R.; Inoue, T.; Phillips, G.O. Structural modifications of sugar beet pectin and the relationship of structure to functionality. Food Hydrocoll. 2011, 25, 221–229. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Li, Z.; McClements, D.J. Comparison of emulsifying properties of food-grade polysaccharides in oil-in-water emulsions: Gum arabic, beet pectin, and corn fiber gum. Food Hydrocoll. 2017, 66, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Alba, K.; Kontogiorgos, V. Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocoll. 2017, 68, 211–218. [Google Scholar] [CrossRef]
- Liu, Z.; Pi, F.; Guo, X.; Guo, X.; Yu, S. Characterization of the structural and emulsifying properties of sugar beet pectins obtained by sequential extraction. Food Hydrocoll. 2019, 88, 31–42. [Google Scholar] [CrossRef]
- Guo, J.; Gao, Z.; Xia, J.; Ritenour, M.A.; Li, G.; Shan, Y. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. Food Sci. Technol. 2018, 97, 825–839. [Google Scholar] [CrossRef]
- Li, Z.; Jin, R.; Yang, Z.; Wang, X.; You, G.; Guo, J.; Zhang, Y.; Liu, F.; Pan, S. Comparative study on physicochemical, nutritional and enzymatic properties of two Satsuma mandarin (Citrus unshiu Marc.) varieties from different regions. J. Food Compos. Anal. 2021, 95, 103614. [Google Scholar] [CrossRef]
- Yeoh, S.; Shi, J.; Langrish, T.A.G. Comparisons between different techniques for water-based extraction of pectin from orange peels. Desalination 2008, 218, 229–237. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Kaya, M.; Sousa, A.G.; Crépeau, M.-J.; Sørensen, S.O.; Ralet, M.-C. Characterization of citrus pectin samples extracted under different conditions: Influence of acid type and pH of extraction. Ann. Bot. 2014, 114, 1319–1326. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Wu, D.; Zhu, K.; Ye, X. Manosonication assisted extraction and characterization of pectin from different citrus peel wastes. Food Hydrocoll. 2021, 121, 106952. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, W.; Liao, X.; Hu, X.; Wu, J.; Wang, X. Extraction of pectin from the peels of pomelo by high-speed shearing homogenization and its characteristics. LWT-Food Sci. Technol. 2017, 79, 640–646. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Peng, X.Y.; Mu, T.-H.; Zhang, M.; Sun, H.-N.; Chen, J.-W.; Yu, M. Effects of pH and high hydrostatic pressure on the structural and rheological properties of sugar beet pectin. Food Hydrocoll. 2016, 60, 161–169. [Google Scholar] [CrossRef]
- Petkowicz, C.L.O.; Vriesmann, L.C.; Williams, P.A. Pectins from food waste: Extraction, characterization and properties of watermelon rind pectin. Food Hydrocoll. 2017, 65, 57–67. [Google Scholar] [CrossRef]
- Neckebroeck, B.; Verkempinck, S.H.E.; Van Audenhove, J.; Bernaerts, T.; de Wilde d’Estmael, H.; Hendrickx, M.E.; Van Loey, A.M. Structural and emulsion stabilizing properties of pectin rich extracts obtained from different botanical sources. Food Res. Int. 2021, 141, 110087. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, W.; Lan, X.; Gong, S.; Wu, J.; Wang, Z. Effects of high hydrostatic pressure and high pressure homogenization processing on characteristics of potato peel waste pectin. Carbohydr. Polym. 2018, 196, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhao, W.; Pang, X.; Liao, X.; Hu, X.; Wu, J. Emulsion stabilizing properties of pectins extracted by high hydrostatic pressure, high-speed shearing homogenization and traditional thermal methods: A comparative study. Food Hydrocoll. 2014, 35, 217–225. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, J.; Cao, Y.; Wang, J.; Xiao, J. Influence of microcrystalline cellulose on the microrheological property and freeze-thaw stability of soybean protein hydrolysate stabilized curcumin emulsion. LWT-Food Sci. Technol. 2016, 66, 590–597. [Google Scholar] [CrossRef]
- Kurita, O.; Fujiwara, T.; Yamazaki, E. Characterization of the pectin extracted from citrus peel in the presence of citric acid. Carbohydr. Polym. 2008, 74, 725–730. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Koch, L.; Rentschler, C.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Effect of Molecular Weight Reduction, Acetylation and Esterification on the Emulsification Properties of Citrus Pectin. Food Biophys. 2015, 10, 217–227. [Google Scholar] [CrossRef]
- Lutz, R.; Aserin, A.; Wicker, L.; Garti, N. Structure and physical properties of pectins with block-wise distribution of carboxylic acid groups. Food Hydrocoll. 2009, 23, 786–794. [Google Scholar] [CrossRef]
- Asgari, K.; Labbafi, M.; Khodaiyan, F.; Kazemi, M.; Hosseini, S.S. High-methylated pectin from walnut processing wastes as a potential resource: Ultrasound assisted extraction and physicochemical, structural and functional analysis. Int. J. Biol. Macromol. 2020, 152, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Han, D.; Xi, H.; Rao, L.; Liao, X.; Hu, X.; Wu, J. Extraction of pectin from navel orange peel assisted by ultra-high pressure, microwave or traditional heating: A comparison. Carbohydr. Polym. 2012, 88, 441–448. [Google Scholar] [CrossRef]
- Bagherian, H.; Zokaee Ashtiani, F.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. Process. Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Akhtar, M.; Dickinson, E.; Mazoyer, J.; Langendorff, V. Emulsion stabilizing properties of depolymerized pectin. Food Hydrocoll. 2002, 16, 249–256. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Cui, J.; Ren, W.; Zhao, C.; Gao, W.; Tian, G.; Bao, Y.; Lian, Y.; Zheng, J. The structure–property relationships of acid- and alkali-extracted grapefruit peel pectins. Carbohydr. Polym. 2020, 229, 115524. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Xue, S.J.; Zhang, X.; Liu, D.; Meng, R.; Chen, S. Effect of high-intensity ultrasound on the physicochemical properties and nanostructure of citrus pectin. J. Sci. Food Agric. 2013, 93, 2028–2036. [Google Scholar] [CrossRef]
- Ma, X.; Wang, D.; Chen, W.; Ismail, B.B.; Wang, W.; Lv, R.; Ding, T.; Ye, X.; Liu, D. Effects of ultrasound pretreatment on the enzymolysis of pectin: Kinetic study, structural characteristics and anti-cancer activity of the hydrolysates. Food Hydrocoll. 2018, 79, 90–99. [Google Scholar] [CrossRef]
- Yang, J.-S.; Mu, T.-H.; Ma, M.-M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef]
- Nakamura, A.; Maeda, H.; Corredig, M. Emulsifying properties of enzyme-digested soybean soluble polysaccharide. Food Hydrocoll. 2006, 20, 1029–1038. [Google Scholar] [CrossRef]
- Ninčević Grassino, A.; Ostojić, J.; Miletić, V.; Djaković, S.; Bosiljkov, T.; Zorić, Z.; Ježek, D.; Rimac Brnčić, S.; Brnčić, M. Application of high hydrostatic pressure and ultrasound-assisted extractions as a novel approach for pectin and polyphenols recovery from tomato peel waste. Innov. Food Sci. Emerg. Technol. 2020, 64, 102424. [Google Scholar] [CrossRef]
- Rahmani, Z.; Khodaiyan, F.; Kazemi, M.; Sharifan, A. Optimization of microwave-assisted extraction and structural characterization of pectin from sweet lemon peel. Int. J. Biol. Macromol. 2020, 147, 1107–1115. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Wu, D.; Zhu, K.; Ye, X. Physicochemical and macromolecule properties of RG-I enriched pectin from citrus wastes by manosonication extraction. Int. J. Biol. Macromol. 2021, 176, 332–341. [Google Scholar] [CrossRef]
- Hosseini, S.; Parastouei, K.; Khodaiyan, F. Simultaneous extraction optimization and characterization of pectin and phenolics from sour cherry pomace. Int. J. Biol. Macromol. 2020, 158, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019, 278, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Verkempinck, S.; Kyomugasho, C.; Salvia-Trujillo, L.; Denis, S.; Bourgeois, M.; Van, L.; Hendrickx, M.E.; Grauwet, T. Emulsion stabilizing properties of citrus pectin and its interactions with conventional emulsifiers in oil-in-water emulsions. Food Hydrocoll. 2018, 85, 144–157. [Google Scholar] [CrossRef]
- Piriyaprasarth, S.; Juttulapa, M.; Sriamornsak, P. Stability of rice bran oil-in-water emulsions stabilized by pectin–zein complexes: Effect of composition and order of mixing. Food Hydrocoll. 2016, 61, 589–598. [Google Scholar] [CrossRef]
- Pi, F.; Liu, Z.; Guo, X.; Guo, X.; Meng, H. Chicory root pulp pectin as an emulsifier as compared to sugar beet pectin. Part 1: Influence of structure, concentration, counterion concentration. Food Hydrocoll. 2019, 89, 792–801. [Google Scholar] [CrossRef]
- Jukkola, A.; Partanen, R.; Xiang, W.; Heino, A.; Rojas, O.J. Food emulsifiers based on milk fat globule membranes and their interactions with calcium and casein phosphoproteins. Food Hydrocoll. 2019, 94, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Jamsazzadeh Kermani, Z.; Shpigelman, A.; Pham, H.T.T.; Van Loey, A.M.; Hendrickx, M.E. Functional properties of citric acid extracted mango peel pectin as related to its chemical structure. Food Hydrocoll. 2015, 44, 424–434. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Y.; Li, F.; Li, D.; Huang, Q. Pectin extracted from persimmon peel: A physicochemical characterization and emulsifying properties evaluation. Food Hydrocoll. 2020, 101, 105561. [Google Scholar] [CrossRef]
- Zhao, S.; Ren, W.; Gao, W.; Tian, G.; Zhao, C.; Bao, Y.; Cui, J.; Lian, Y.; Zheng, J. Effect of mesoscopic structure of citrus pectin on its emulsifying properties: Compactness is more important than size. J. Colloid Interface Sci. 2020, 570, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Koocheki, A.; Kadkhodaee, R.; Mortazavi, S.A.; Shahidi, F.; Taherian, A.R. Influence of Alyssum homolocarpum seed gum on the stability and flow properties of O/W emulsion prepared by high intensity ultrasound. Food Hydrocoll. 2009, 23, 2416–2424. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll. 2008, 22, 1191–1202. [Google Scholar] [CrossRef]
- Alba, K.; Sagis, L.M.C.; Kontogiorgos, V. Engineering of acidic O/W emulsions with pectin. Colloids Surf. B Biointerfaces 2016, 145, 301–308. [Google Scholar] [CrossRef]
- Castellani, O.; Al-Assaf, S.; Axelos, M.; Phillips, G.O.; Anton, M. Hydrocolloids with emulsifying capacity. Part 2—Adsorption properties at the n-hexadecane–Water interface. Food Hydrocoll. 2010, 24, 121–130. [Google Scholar] [CrossRef]
- Dickinson, E. Microgels—An alternative colloidal ingredient for stabilization of food emulsions. Trends Food Sci. Technol. 2015, 43, 178–188. [Google Scholar] [CrossRef]
- Destribats, M.; Wolfs, M.; Pinaud, F.; Lapeyre, V.; Sellier, E.; Schmitt, V.; Ravaine, V. Pickering Emulsions Stabilized by Soft Microgels: Influence of the Emulsification Process on Particle Interfacial Organization and Emulsion Properties. Langmuir 2013, 29, 12367–12374. [Google Scholar] [CrossRef]
- Yapo, B.M.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Z.; Shangguan, W.; Fang, Y.; Nishinari, K.; Phillips, G.O.; Jiang, F. Emulsification properties of sugar beet pectin after modification with horseradish peroxidase. Food Hydrocoll. 2015, 43, 107–113. [Google Scholar] [CrossRef]
- Williams, P.A.; Sayers, C.; Viebke, C.; Senan, C.; Mazoyer, J.; Boulenguer, P. Elucidation of the Emulsification Properties of Sugar Beet Pectin. J. Agric. Food. Chem. 2005, 53, 3592–3597. [Google Scholar] [CrossRef] [PubMed]
- Siew, C.K.; Williams, P.A. Role of Protein and Ferulic Acid in the Emulsification Properties of Sugar Beet Pectin. J. Agric. Food Chem. 2008, 56, 4164–4171. [Google Scholar] [CrossRef] [PubMed]






| Time (min) | Flow Rate (min) | A Phase (%) | B Phase (%) | |
|---|---|---|---|---|
| Mobile phase gradient | 0.0 | 0.5 | 97.5 | 2.5 |
| 30.0 | 0.5 | 80.0 | 20.0 | |
| 30.1 | 0.5 | 60.0 | 40.0 | |
| 45.0 | 0.5 | 60.0 | 40.0 | |
| 45.1 | 0.5 | 97.5 | 2.5 | |
| 60.0 | 0.5 | 97.5 | 2.5 |
| Oil Ratios | The Amount of Oil Added (g) | CCP/MPP Solution (g) |
|---|---|---|
| 10% | 2 | 18 |
| 25% | 5 | 15 |
| 50% | 10 | 10 |
| MPP | CCP | ||
|---|---|---|---|
| GalA (%) | 72.0 ± 0.8 a | 70.1 ± 0.0 a | |
| DM (%) | 52.0 ± 0.8 b | 67.9 ± 1.6 a | |
| Relative monosaccharide content (%, w/w) | Fuc | 0.4 ± 0.0 a | 0.2 ± 0.0 b |
| Rha | 6.3 ± 0.2 a | 6.5 ± 0.0 a | |
| Ara | 7.5 ± 0.1 a | 3.2 ± 0.0 b | |
| Gal | 9.0 ± 0.3 b | 14.0 ± 0.1 a | |
| Glc | 1.6 ± 0.0 b | 4.3 ± 0.1 a | |
| Xyl | 1.3 ± 0.0 a | 0.6 ± 0.0 b | |
| Man | 0.7 ± 0.1 a | 0.2 ± 0.0 b | |
| Fru | 0.6 ± 0.1 a | 0.5 ± 0.1 a | |
| Monosaccharide ratio | Rha/GalA | 0.1 ± 0.0 a | 0.1 ± 0.0 a |
| (Gal + Ara)/Rha | 2.6 ± 0.0 a | 2.6 ± 0.0 a | |
| Molecular weight (kDa) | Mw (kDa) | 294.3 ± 2.5 a | 256.0 ± 4.7 b |
| Mn(kDa) | 125.1 ± 7.1 a | 97.6 ± 2.3 b | |
| Mw/Mn | 2.4 ± 0.1 a | 2.6 ± 0.0 b |
| 0 | 1-Week | 2-Week | 3-Week | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MPP | CCP | MPP | CCP | MPP | CCP | MPP | CCP | ||
| Pectin concentration | 0.50% | 4.2 ± 0.3 Ae | 19.7 ± 3.3 Ad | 6.4 ± 0.3 Ae | 50.2 ± 1.5 Ac | 6.3 ± 0.6 Ae | 68.1 ± 2.6 Ab | 8.3 ± 2.4 Ae | 88.3 ± 0.8 Aa |
| 1.0% | 2.3 ± 0.1 Be | 14.7 ± 0.4 >Bd | 2.8 ± 0.2 Be | 21.9 ± 1.3 Bc | 2.8 ± 0.4 Be | 23.0 ± 0.6 Bb | 2.8 ± 0.2 Be | 24.2 ± 0.3 Ba | |
| 1.50% | 1.7 ± 0.1 Cd | 12.3 ± 2.3 Bc | 1.8 ± 0.2 Cd | 14.6 ± 1.5 Ca | 1.8 ± 0.1 Cd | 17.5 ± 1.2 Cbc | 1.9 ± 0.1 Cd | 15.8 ± 3.3 Cab | |
| 2.00% | 1.5 ± 0.2 Cd | 12.9 ± 1.1 Ba | 1.5 ± 0.1 Cd | 12.6 ± 0.5 Da | 1.5 ± 0.1 Cd | 13.0 ± 1.0 Db | 1.5 ± 0.1 Cd | 10.3 ± 0.4 Dc |
| 0 | 1-Week | 2-Week | 3-Week | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MPP | CCP | MPP | CCP | MPP | CCP | MPP | CCP | ||
| pH | 3 | 2.1 ± 0.0 Ad | 13.9 ± 1.3 Ac | 2.3 ± 0.0 Ad | 23.8 ± 0.3 Aab | 2.8 ± 0.7 Ad | 23.3 ± 0.9 Ab | 2.2 ± 0.1 Ad | 24.3 ± 0.4 Aa |
| 7 | 8.61 ± 1.3 Be | 18.8 ± 0.9 Bc | 9.7 ± 0.4 Bed | 23.8 ± 0.2 Ab | 9.6 ± 0.4 Bed | 23.6 ± 0.0 Ab | 10.9 ± 1.2 Bd | 25.7 ± 0.9 Aa | |
| 8 | 8.4 ± 0.3 Bf | 19.4 ± 0.4 Bc | 10.1 ± 0.3 Bd | 23.9 ± 0.4 Ab | 9.4 ± 0.0 Bd | 24.2 ± 0.5 Ab | 10.3 ± 0.1 Bd | 26.3 ± 0.2 Aa |
| 0 | 1-Week | 2-Week | 3-Week | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MPP | CCP | MPP | CCP | MPP | CCP | MPP | CCP | ||
| Oil ratio | 10% | 1.0 ± 0.0 Ad | 2.0 ± 0.1 Ad | 1.4 ± 0.4 Ad | 3.2 ± 0.2 Aa | 1.5 ± 0.4 Ad | 4.2 ± 1.2 Ac | 1.3 ± 0.4 Ad | 5.5 ± 0.5 Ab |
| 25% | 2.2 ± 0.1 Bd | 14.8 ± 2.1 Bc | 2.6 ± 0.1 Bd | 23.4 ± 1.1 Bb | 2.7 ± 0.4 Bd | 27.4 ± 0.9 Ba | 2.4 ± 0.1 Bd | 26.1 ± 0.2 Ba | |
| 50% | 7.7 ± 0.3 Ce | 41.6 ± 0.9 Cc | 8.9 ± 0.1 Cde | 65.9 ± 2.3 Cb | 11.1 ± 0.2 Cde | 67.7 ± 2.8 Cab | 12.8 ± 0.1 Cd | 71.8 ± 6.9 Ca |
| Emulsion Stability (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Centrifugation Assay | Storage Assay (W) | ||||||||||
| EA10 | ES0 | ES1 | ES2 | ES3 | |||||||
| MPP | CCP | MPP | CCP | MPP | CCP | MPP | CCP | MPP | CCP | ||
| Pectin concentration | 0.5% | 46.43 | 40.71 | 100.0 | 81.67 | 86.67 | 53.33 | 83.33 | 50.00 | 54.67 | 48.33 |
| 1.0% | 95.00 | 39.64 | 100.0 | 88.33 | 95.83 | 53.00 | 95.00 | 51.17 | 94.50 | 50.67 | |
| 1.5% | 100 | 42.14 | 100.0 | 100.0 | 100.0 | 65.00 | 100.0 | 63.00 | 100.0 | 60.00 | |
| 2.0% | 100 | 41.79 | 100.0 | 100.0 | 100.0 | 71.67 | 100.0 | 66.50 | 100.0 | 65.50 | |
| pH | 3 | 93.21 | 41.43 | 100.0 | 96.83 | 100.0 | 62.67 | 97.97 | 59.57 | 96.58 | 55.65 |
| 7 | 44.64 | 45.00 | 100.0 | 70.00 | 66.80 | 61.50 | 57.47 | 60.23 | 56.43 | 58.52 | |
| 8 | 42.14 | 43.57 | 100.0 | 83.33 | 61.70 | 69.67 | 56.83 | 66.68 | 54.53 | 64.38 | |
| Oil ratio | 10% | 93.57 | 88.33 | 100.0 | 100.0 | 100.0 | 99.59 | 100.0 | 99.49 | 99.79 | 99.47 |
| 25% | 91.79 | 51.67 | 100.0 | 100.0 | 98.30 | 96.90 | 97.65 | 71.62 | 96.65 | 70.24 | |
| 50% | 82.86 | 71.67 | 100.0 | 100.0 | 96.67 | 98.62 | 94.52 | 98.33 | 92.97 | 98.24 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Yang, Z.; Yang, J.; Liu, F.; Xu, X.; Pan, S. Structural and Emulsifying Properties of Citric Acid Extracted Satsuma Mandarin Peel Pectin. Foods 2021, 10, 2459. https://doi.org/10.3390/foods10102459
Duan X, Yang Z, Yang J, Liu F, Xu X, Pan S. Structural and Emulsifying Properties of Citric Acid Extracted Satsuma Mandarin Peel Pectin. Foods. 2021; 10(10):2459. https://doi.org/10.3390/foods10102459
Chicago/Turabian StyleDuan, Xingke, Zhixuan Yang, Jinyan Yang, Fengxia Liu, Xiaoyun Xu, and Siyi Pan. 2021. "Structural and Emulsifying Properties of Citric Acid Extracted Satsuma Mandarin Peel Pectin" Foods 10, no. 10: 2459. https://doi.org/10.3390/foods10102459
APA StyleDuan, X., Yang, Z., Yang, J., Liu, F., Xu, X., & Pan, S. (2021). Structural and Emulsifying Properties of Citric Acid Extracted Satsuma Mandarin Peel Pectin. Foods, 10(10), 2459. https://doi.org/10.3390/foods10102459







