Integrated Analysis of Metabolome and Volatile Profiles of Germinated Brown Rice from the Japonica and Indica Subspecies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Materials and Germination Process of Brown Rice
2.3. Sample Preparation and Metabolomics Analysis
2.4. Metabolomics Data Processing
2.5. Volatile Analysis by HS-SPME-GC-MS
2.6. Data Analysis
3. Results
3.1. Metabolic Changes in BR upon the Germination Process
3.2. Metabolic Differentiations between Japonica and Indica Rice Subspecies
3.3. Metabolic Pathway Analysis
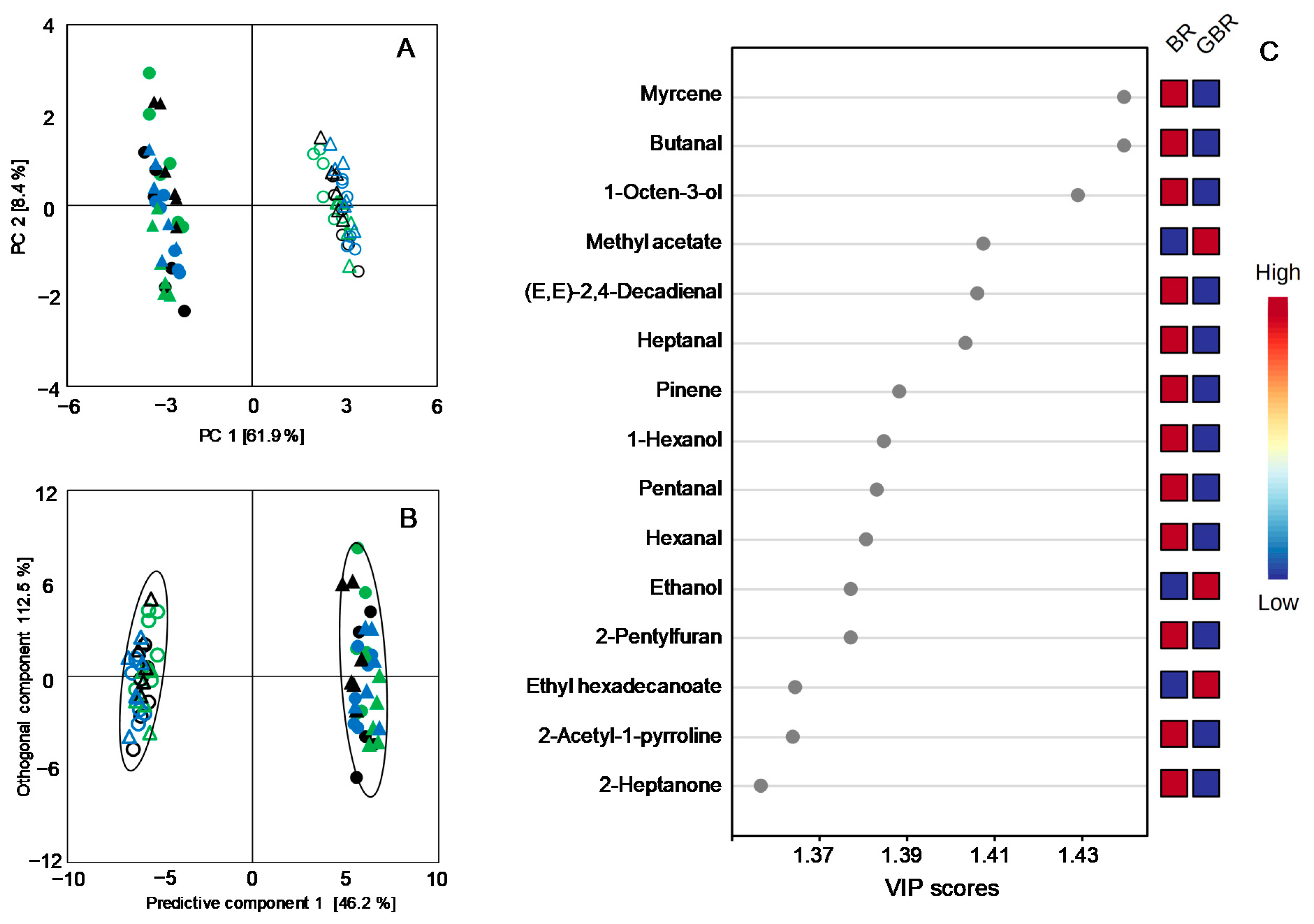
3.4. Changes in Volatile Profiles Induced by Germination
3.5. Comparisons between Volatile Profiles of the Japonica and the Indica Subspecies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jeng, T.L.; Shih, Y.J.; Ho, P.T.; Lai, C.C.; Lin, Y.W.; Wang, C.S.; Sung, J.M. gamma-Oryzanol, tocol and mineral compositions in different grain fractions of giant embryo rice mutants. J. Sci. Food Agric. 2012, 92, 1468–1474. [Google Scholar] [CrossRef]
- Wu, F.F.; Yang, N.; Toure, A.; Jin, Z.Y.; Xu, X.M. Germinated Brown Rice and Its Role in Human Health. Crit. Rev. Food Sci. Nutr. 2013, 53, 451–463. [Google Scholar] [CrossRef]
- Golozar, A.; Khalili, D.; Etemadi, A.; Poustchi, H.; Fazeltabar, A.; Hosseini, F.; Kamangar, F.; Khoshnia, M.; Islami, F.; Hadaegh, F.; et al. White rice intake and incidence of type-2 diabetes: Analysis of two prospective cohort studies from Iran. BMC Public Health 2017, 17, 133. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Spiegelman, D.; van Dam, R.M.; Holmes, M.D.; Malik, V.S.; Willett, W.C.; Hu, F.B. White Rice, Brown Rice, and Risk of Type 2 Diabetes in US Men and Women. Arch. Intern. Med. 2010, 170, 961–969. [Google Scholar] [CrossRef]
- Bornhorst, G.M.; Chang, L.Q.; Rutherfurd, S.M.; Moughan, P.J.; Singh, R.P. Gastric emptying rate and chyme characteristics for cooked brown and white rice meals in vivo. J. Sci. Food Agric. 2013, 93, 2900–2908. [Google Scholar] [CrossRef]
- Wang, H.; Hu, F.F.; Wang, C.F.; Ramaswamy, H.S.; Yu, Y.; Zhu, S.M.; Wu, J. Effect of germination and high pressure treatments on brown rice flour rheological, pasting, textural, and structural properties. J. Food Process. Preserv. 2020, 44, E14474. [Google Scholar] [CrossRef]
- Ti, H.H.; Zhang, R.F.; Zhang, M.W.; Li, Q.; Wei, Z.C.; Zhang, Y.; Tang, X.J.; Deng, Y.Y.; Liu, L.; Ma, Y.X. Dynamic changes in the free and bound phenolic compounds and antioxidant activity of brown rice at different germination stages. Food Chem. 2014, 161, 337–344. [Google Scholar] [CrossRef]
- Ng, L.T.; Huang, S.H.; Chen, Y.T.; Su, C.H. Changes of Tocopherols, Tocotrienols, gamma-Oryzanol, and gamma-Aminobutyric Acid Levels in the Germinated Brown Rice of Pigmented and Nonpigmented Cultivars. J. Agric. Food Chem. 2013, 61, 12604–12611. [Google Scholar] [CrossRef]
- Ruan, Y.F.; Cai, Z.D.; Deng, Y.; Pan, D.D.; Zhou, C.Y.; Cao, J.X.; Chen, X.J.; Xia, Q. An untargeted metabolomic insight into the high-pressure stress effect on the germination of wholegrain Oryza sativa L. Food Res. Int. 2021, 140, 109984. [Google Scholar] [CrossRef]
- Na Jom, K.; Lorjaroenphon, Y.; Udompijitkul, P. Differentiation of Four Varieties of Germinating Thai Colored Indica Rice (Oryza sativa L.) by Metabolite Profiling. Food Sci. Technol. Res. 2016, 22, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.Y.; Cui, D.; Zhou, J.; Li, W.Q.; Ma, X.D.; Han, B.; Guo, X.H.; Zhao, Z.W.; Han, L.Z. Comparative analysis of genetic diversity of rice (Oryza sativa L.) varieties cultivated in different periods in China. Genet. Resour. Crop Evol. 2021, 68, 1439–1451. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants (vol 1, pg 387, 2006). Nat. Protoc. 2015, 10, 1457. [Google Scholar] [CrossRef]
- Lai, Z.J.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.X.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, H.J.; Luo, J.; Liu, J.W.; Zhao, S.Q.; Hu, Q.H.; Ding, C. Effect of dielectric barrier discharge cold plasma treatments on flavor fingerprints of brown rice. Food Chem. 2021, 352, 129402. [Google Scholar] [CrossRef]
- Pang, Z.Q.; Chong, J.; Zhou, G.Y.; Morais, D.A.D.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.E.; Li, S.Z.; Xia, J.G. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Hu, C.Y.; Shi, J.X.; Quan, S.; Cui, B.; Kleessen, S.; Nikoloski, Z.; Tohge, T.; Alexander, D.; Guo, L.N.; Lin, H.; et al. Metabolic variation between japonica and indica rice cultivars as revealed by non-targeted metabolomics. Sci. Rep. 2014, 4, 5067. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Mei, J.; Yu, W.J.; Li, Y.F. High hydrostatic pressure treatments enhance volatile components of pre-germinated brown rice revealed by aromatic fingerprinting based on HS-SPME/GC-MS and chemometric methods. Food Res. Int. 2017, 91, 103–114. [Google Scholar] [CrossRef]
- Wu, F.F.; Yang, N.; Chen, H.Y.; Jin, Z.Y.; Xu, X.M. Effect of Germination on Flavor Volatiles of Cooked Brown Rice. Cereal Chem. 2011, 88, 497–503. [Google Scholar] [CrossRef]
- Hu, X.Q.; Lu, L.; Guo, Z.L.; Zhu, Z.W. Volatile compounds, affecting factors and evaluation methods for rice aroma: A review. Trends Food Sci. Technol. 2020, 97, 136–146. [Google Scholar] [CrossRef]
- Kim, H.; Kim, O.W.; Ahn, J.H.; Kim, B.M.; Oh, J.; Kim, H.J. Metabolomic Analysis of Germinated Brown Rice at Different Germination Stages. Foods 2020, 9, 1130. [Google Scholar] [CrossRef]
- Han, C.X.; Zhen, S.M.; Zhu, G.R.; Bian, Y.W.; Yan, Y.M. Comparative metabolome analysis of wheat embryo and endosperm reveals the dynamic changes of metabolites during seed germination. Plant Physiol. Biochem. 2017, 115, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Cai, S.G.; Ye, L.Z.; Hu, H.L.; Li, C.D.; Zhang, G.P. The effects of GA and ABA treatments on metabolite profile of germinating barley. Food Chem. 2016, 192, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Q.; Wang, P.; Elbaloula, M.F.; Gu, Z.X. Effect of Germination on Main Physiology and Biochemistry Metabolism of Sorghum Seeds. Biosci J 2016, 32, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.L.; Dong, R.; Hu, X.Z.; Ren, C.Z.; Li, Y.W. Oat-Based Foods: Chemical Constituents, Glycemic Index, and the Effect of Processing. Foods 2021, 10, 1304. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [Green Version]
- Hung, P.V.; Maeda, T.; Yamamoto, S.; Morita, N. Effects of germination on nutritional composition of waxy wheat. J. Sci. Food Agric. 2012, 92, 667–672. [Google Scholar] [CrossRef]
- Mohan, B.H.; Malleshi, N.G.; Koseki, T. Physico-chemical characteristics and non-starch polysaccharide contents of Indica and Japonica brown rice and their malts. Lwt-Food Sci Technol 2010, 43, 784–791. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Le, K.A.; Van den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr Rev Food Sci F 2019, 18, 305–328. [Google Scholar] [CrossRef] [Green Version]
- Gan, R.Y.; Lui, W.Y.; Wu, K.; Chan, C.L.; Dai, S.H.; Sui, Z.Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Koutroubas, S.D.; Ntanos, D.A. Genotypic differences for grain yield and nitrogen utilization in Indica and Japonica rice under Mediterranean conditions. Field Crop. Res. 2003, 83, 251–260. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Chu, C.C. Nitrogen-Use Divergence Between Indica and Japonica Rice: Variation at Nitrate Assimilation. Mol Plant 2020, 13, 6–7. [Google Scholar] [CrossRef]
- Okpala, N.E.; Mo, Z.W.; Duan, M.Y.; Tang, X.R. The genetics and biosynthesis of 2-acetyl-1-pyrroline in fragrant rice. Plant Physiol. Biochem. 2019, 135, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Sriseadka, T.; Wongpornchai, S.; Kitsawatpaiboon, P. Rapid method for quantitative analysis of the aroma impact compound, 2-acetyl-1-pyrroline, in fragrant rice using automated headspace gas chromatography. J. Agric. Food Chem. 2006, 54, 8183–8189. [Google Scholar] [CrossRef] [PubMed]
- Maraval, I.; Sen, K.; Agrebi, A.; Menut, C.; Morere, A.; Boulanger, R.; Gay, F.; Mestres, C.; Gunata, Z. Quantification of 2-acetyl-1-pyrroline in rice by stable isotope dilution assay through headspace solid-phase microextraction coupled to gas chromatography-tandem mass spectrometry. Anal. Chim. Acta 2010, 675, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Yang, Y.; Shi, W.W.; Ji, Q.; He, F.; Zhang, Z.D.; Cheng, Z.K.; Liu, X.N.; Xu, M.L. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell 2008, 20, 1850–1861. [Google Scholar] [CrossRef] [Green Version]
- Shan, Q.W.; Zhang, Y.; Chen, K.L.; Zhang, K.; Gao, C.X. Creation of fragrant rice by targeted knockout of the OsBADH2 gene using TALEN technology. Plant Biotechnol. J. 2015, 13, 791–800. [Google Scholar] [CrossRef] [PubMed]
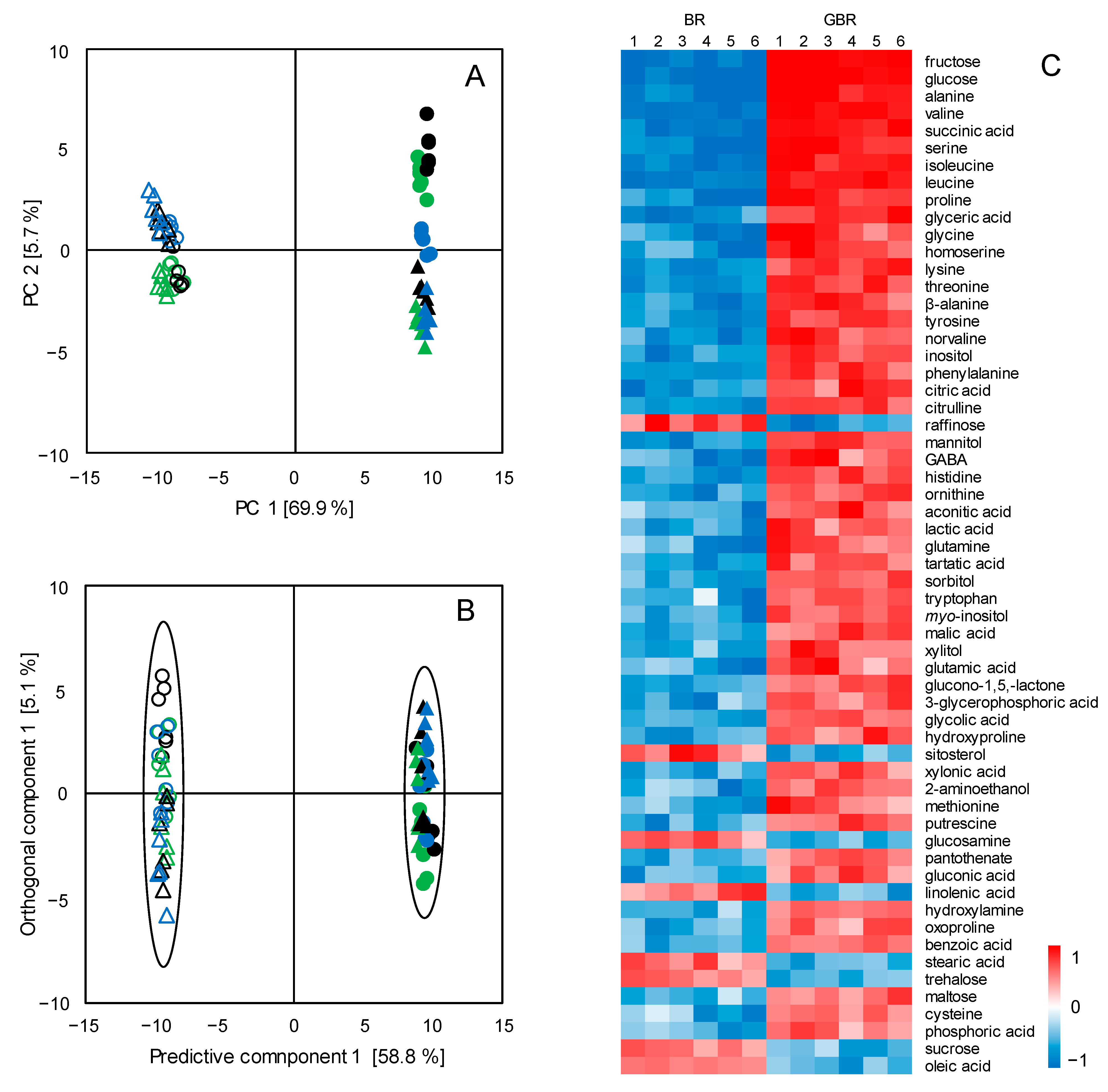
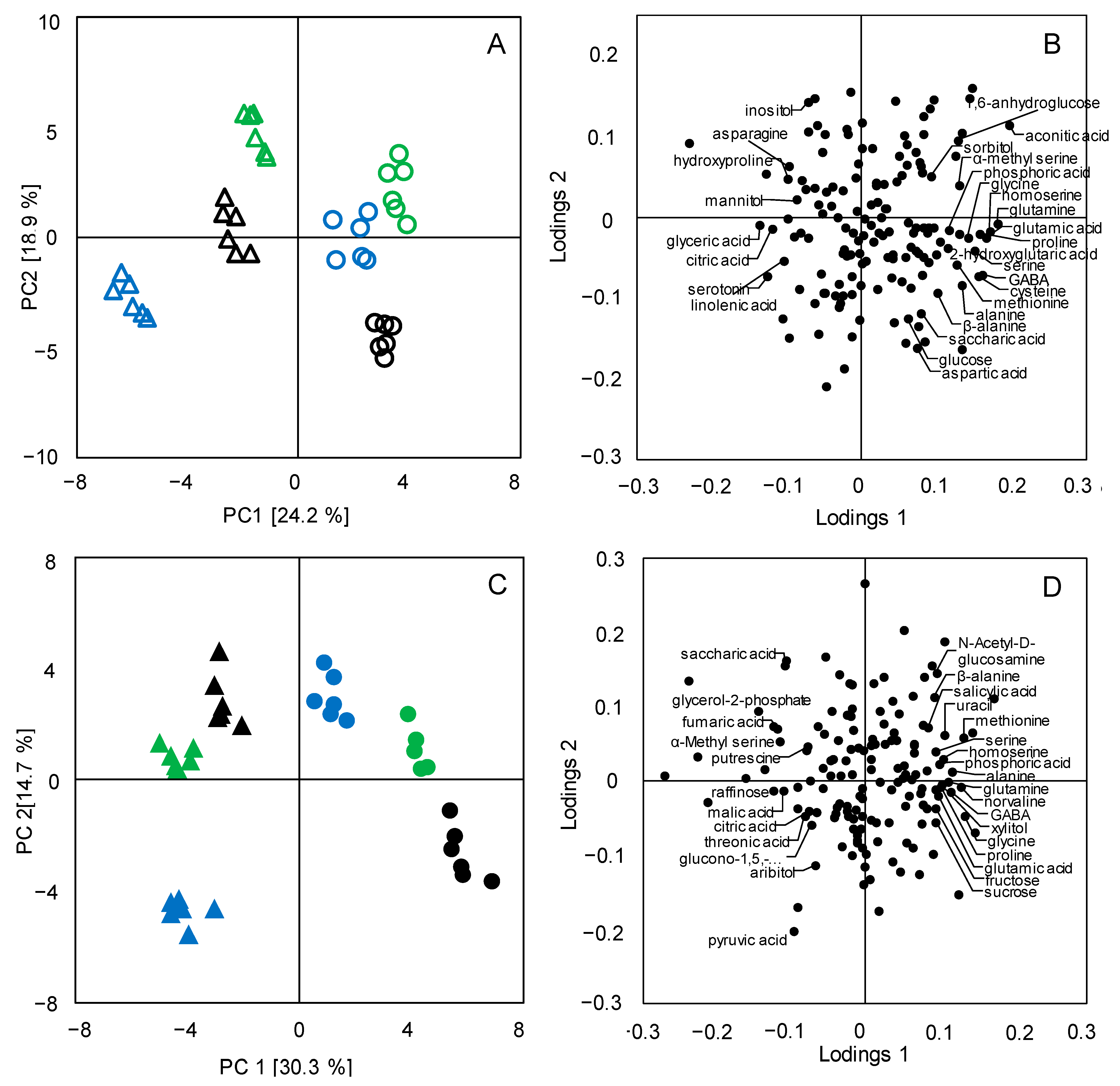
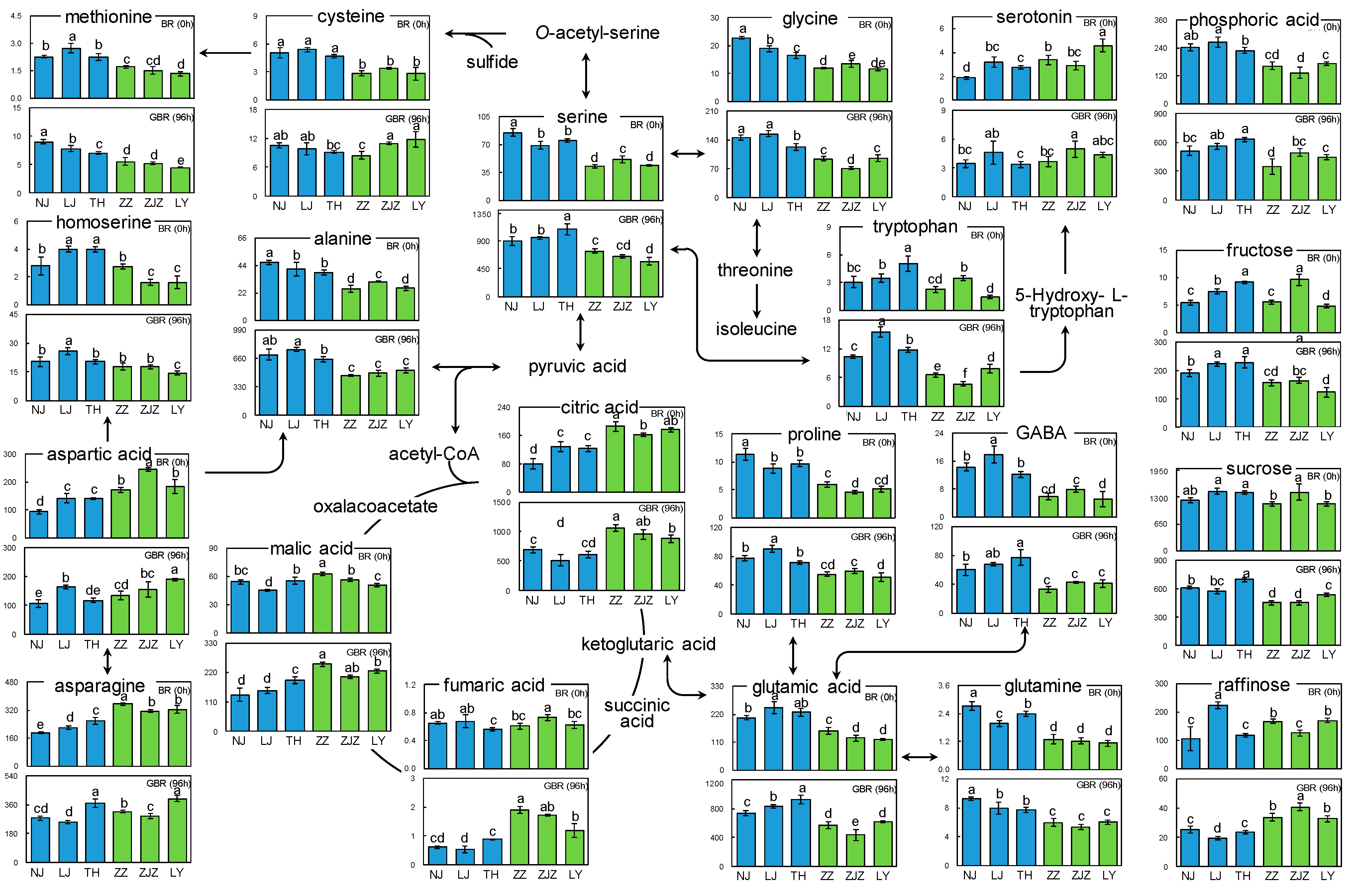
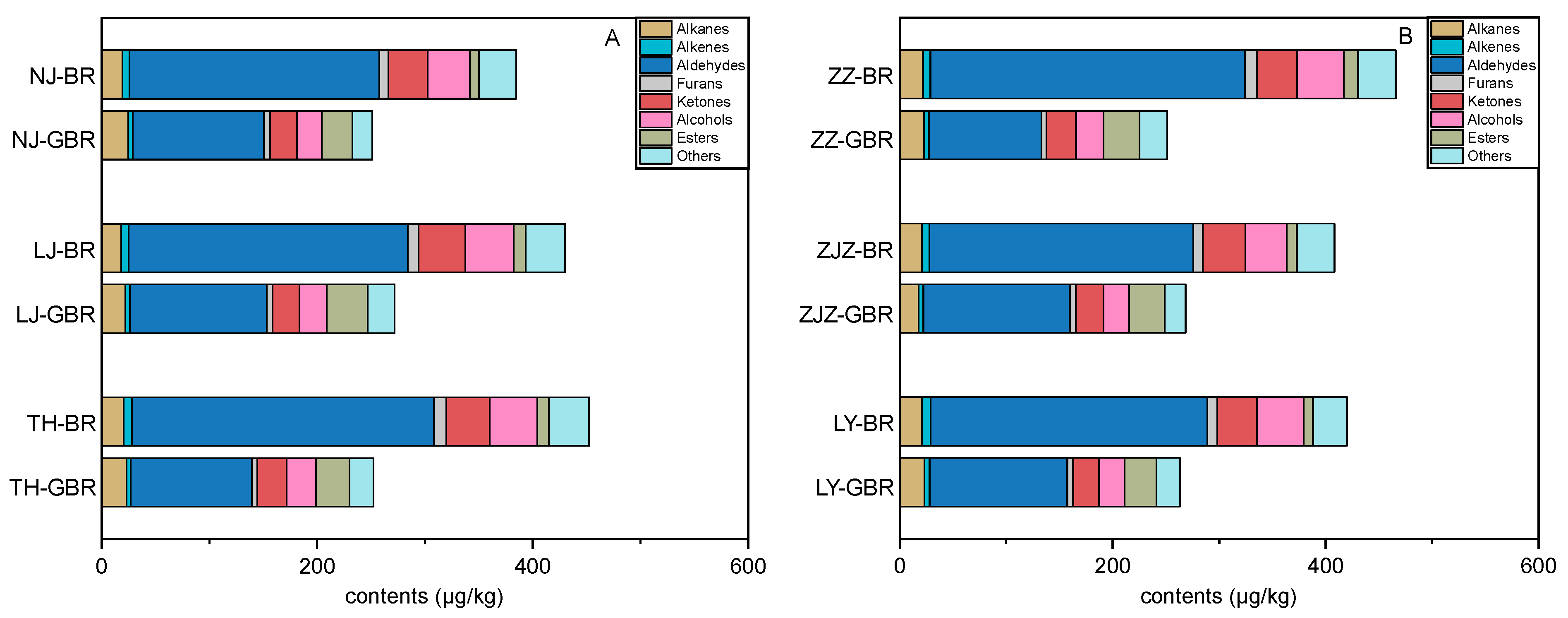
| Japonica Cultivars | Brown Rice (BR) | Germinated Brown Rice (GBR) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volatile Compounds | NJ | LJ | TH | NJ | LJ | TH | ||||||||||||||||||
| Myrcene | 1.96 | ± | 0.48 | b | 2.25 | ± | 0.49 | b | 2.85 | ± | 0.12 | a | 0.39 | ± | 0.05 | c | 0.24 | ± | 0.03 | c | 0.25 | ± | 0.06 | c |
| Butanal | 1.34 | ± | 0.06 | b | 2.28 | ± | 0.40 | a | 2.03 | ± | 0.29 | a | 0.32 | ± | 0.05 | c | 0.36 | ± | 0.04 | c | 0.35 | ± | 0.04 | c |
| 1-Octen-3-ol | 3.34 | ± | 0.41 | b | 4.04 | ± | 0.39 | a | 4.03 | ± | 0.23 | a | 1.40 | ± | 0.12 | c | 1.64 | ± | 0.19 | c | 1.40 | ± | 0.13 | c |
| Methyl acetate | 0.47 | ± | 0.02 | c | 0.60 | ± | 0.10 | c | 0.66 | ± | 0.10 | c | 1.75 | ± | 0.27 | b | 2.18 | ± | 0.19 | a | 1.87 | ± | 0.14 | b |
| (E,E)-2,4-Decadienal | 2.70 | ± | 0.31 | b | 3.51 | ± | 0.29 | a | 3.32 | ± | 0.29 | a | 0.91 | ± | 0.19 | c | 0.93 | ± | 0.28 | c | 0.82 | ± | 0.14 | c |
| Heptanal | 14.38 | ± | 1.93 | b | 15.82 | ± | 2.25 | b | 18.32 | ± | 1.50 | a | 6.33 | ± | 1.39 | c | 5.75 | ± | 1.25 | c | 5.98 | ± | 0.55 | c |
| α-Pinene | 8.80 | ± | 2.54 | a | 7.79 | ± | 1.51 | a | 7.67 | ± | 1.12 | a | 2.08 | ± | 0.27 | b | 2.59 | ± | 0.37 | b | 2.69 | ± | 0.59 | b |
| 1-Hexanol | 7.07 | ± | 0.58 | c | 8.21 | ± | 0.39 | b | 9.46 | ± | 0.48 | a | 3.13 | ± | 0.83 | d | 2.97 | ± | 0.70 | d | 3.17 | ± | 0.51 | d |
| Pentanal | 18.37 | ± | 2.78 | b | 19.03 | ± | 3.29 | b | 21.95 | ± | 0.74 | a | 7.62 | ± | 0.86 | d | 10.55 | ± | 1.02 | c | 9.32 | ± | 0.72 | cd |
| Hexanal | 131.9 | ± | 16.8 | c | 152.6 | ± | 18.7 | b | 176.4 | ± | 16.6 | a | 68.2 | ± | 12.9 | d | 72.4 | ± | 14.7 | d | 60.6 | ± | 10.4 | d |
| Ethanol | 2.78 | ± | 0.45 | c | 2.35 | ± | 0.35 | c | 2.27 | ± | 0.16 | c | 5.72 | ± | 1.06 | a | 5.67 | ± | 0.88 | a | 4.65 | ± | 0.44 | b |
| 2-Pentylfuran | 2.90 | ± | 0.62 | c | 3.33 | ± | 0.44 | b | 4.13 | ± | 0.38 | a | 0.59 | ± | 0.17 | d | 0.37 | ± | 0.16 | d | 0.36 | ± | 0.10 | d |
| Ethyl hexadecanoate | 3.35 | ± | 0.41 | b | 3.91 | ± | 0.34 | b | 3.52 | ± | 0.58 | b | 9.53 | ± | 2.13 | a | 10.67 | ± | 2.53 | a | 10.59 | ± | 0.87 | a |
| 2-Acetyl-1-pyrroline | 1.59 | ± | 0.16 | b | 1.94 | ± | 0.12 | a | 1.98 | ± | 0.19 | a | 0.73 | ± | 0.07 | d | 0.79 | ± | 0.11 | d | 0.95 | ± | 0.08 | c |
| 2-Heptanone | 17.05 | ± | 2.20 | b | 20.96 | ± | 1.19 | a | 18.91 | ± | 2.36 | ab | 10.14 | ± | 1.03 | c | 7.34 | ± | 1.30 | cd | 7.02 | ± | 1.87 | d |
| Indica cultivars | Brown rice (BR) | Germinated brown rice (GBR) | ||||||||||||||||||||||
| Volatile compounds | ZZ | ZJZ | LY | ZZ | ZJZ | LY | ||||||||||||||||||
| Myrcene | 2.60 | ± | 0.37 | b | 2.38 | ± | 0.43 | bb | 3.34 | ± | 0.66 | a | 0.22 | ± | 0.02 | c | 0.42 | ± | 0.04 | c | 0.27 | ± | 0.06 | c |
| Butanal | 2.14 | ± | 0.25 | a | 1.61 | ± | 0.16 | 1.93 | ± | 0.25 | a | 0.32 | ± | 0.01 | c | 0.32 | ± | 0.04 | c | 0.36 | ± | 0.04 | c | |
| 1-Octen-3-ol | 4.15 | ± | 0.27 | a | 3.41 | ± | 0.39 | b | 4.04 | ± | 0.38 | a | 1.45 | ± | 0.09 | c | 1.20 | ± | 0.10 | c | 1.34 | ± | 0.11 | c |
| Methyl acetate | 0.64 | ± | 0.10 | d | 0.73 | ± | 0.05 | d | 0.68 | ± | 0.10 | d | 2.37 | ± | 0.20 | a | 1.61 | ± | 0.14 | c | 2.07 | ± | 0.24 | b |
| (E,E)-2,4-Decadienal | 2.86 | ± | 0.29 | b | 3.53 | ± | 0.13 | a | 2.53 | ± | 0.33 | b | 0.92 | ± | 0.10 | c | 0.89 | ± | 0.23 | c | 0.76 | ± | 0.18 | c |
| Heptanal | 19.31 | ± | 1.77 | a | 18.44 | ± | 1.33 | a | 15.54 | ± | 2.56 | b | 5.36 | ± | 0.88 | c | 6.54 | ± | 1.14 | c | 5.78 | ± | 1.01 | c |
| α-Pinene | 7.22 | ± | 0.81 | a | 8.08 | ± | 1.15 | a | 7.33 | ± | 1.28 | a | 3.10 | ± | 0.36 | b | 2.28 | ± | 0.36 | b | 2.59 | ± | 0.44 | b |
| 1-Hexanol | 8.03 | ± | 0.37 | b | 7.41 | ± | 0.43 | b | 8.69 | ± | 0.91 | a | 3.65 | ± | 0.35 | c | 2.87 | ± | 0.49 | cd | 2.16 | ± | 0.33 | d |
| Pentanal | 20.51 | ± | 2.63 | b | 19.45 | ± | 2.45 | b | 24.44 | ± | 3.29 | a | 8.01 | ± | 0.96 | c | 11.23 | ± | 0.57 | c | 8.53 | ± | 0.80 | c |
| Hexanal | 186.3 | ± | 23.5 | a | 151.4 | ± | 14.7 | b | 157.8 | ± | 9.90 | b | 57.35 | ± | 6.03 | c | 76.90 | ± | 18.47 | c | 68.58 | ± | 6.22 | c |
| Ethanol | 2.35 | ± | 0.22 | d | 2.46 | ± | 0.23 | d | 2.03 | ± | 0.34 | d | 5.44 | ± | 0.60 | c | 6.36 | ± | 0.73 | b | 7.40 | ± | 0.36 | a |
| 2-Pentylfuran | 4.16 | ± | 0.20 | a | 3.06 | ± | 0.55 | b | 1.69 | ± | 0.21 | c | 0.37 | ± | 0.12 | d | 0.35 | ± | 0.15 | d | 0.44 | ± | 0.14 | d |
| Ethyl hexadecanoate | 3.92 | ± | 0.43 | c | 2.89 | ± | 0.30 | c | 3.34 | ± | 0.41 | c | 6.86 | ± | 1.00 | b | 9.75 | ± | 0.98 | a | 9.96 | ± | 1.36 | a |
| 2-Acetyl-1-pyrroline | 2.25 | ± | 0.31 | a | 1.66 | ± | 0.13 | b | 1.86 | ± | 0.15 | b | 0.83 | ± | 0.04 | c | 0.65 | ± | 0.04 | cd | 0.51 | ± | 0.08 | d |
| 2-Heptanone | 16.98 | ± | 1.84 | b | 21.24 | ± | 1.28 | a | 17.41 | ± | 1.49 | b | 6.85 | ± | 1.15 | d | 9.47 | ± | 1.41 | c | 6.57 | ± | 0.83 | d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Zhou, Y.; Hu, Y.; Li, B.; Zhang, R.; Zheng, K.; Liu, J.; Wang, J.; Zuo, M.; Liu, S. Integrated Analysis of Metabolome and Volatile Profiles of Germinated Brown Rice from the Japonica and Indica Subspecies. Foods 2021, 10, 2448. https://doi.org/10.3390/foods10102448
Zhou C, Zhou Y, Hu Y, Li B, Zhang R, Zheng K, Liu J, Wang J, Zuo M, Liu S. Integrated Analysis of Metabolome and Volatile Profiles of Germinated Brown Rice from the Japonica and Indica Subspecies. Foods. 2021; 10(10):2448. https://doi.org/10.3390/foods10102448
Chicago/Turabian StyleZhou, Chenguang, Yaojie Zhou, Yuqian Hu, Bin Li, Roujia Zhang, Kaiyi Zheng, Jie Liu, Jing Wang, Min Zuo, and Siyao Liu. 2021. "Integrated Analysis of Metabolome and Volatile Profiles of Germinated Brown Rice from the Japonica and Indica Subspecies" Foods 10, no. 10: 2448. https://doi.org/10.3390/foods10102448
APA StyleZhou, C., Zhou, Y., Hu, Y., Li, B., Zhang, R., Zheng, K., Liu, J., Wang, J., Zuo, M., & Liu, S. (2021). Integrated Analysis of Metabolome and Volatile Profiles of Germinated Brown Rice from the Japonica and Indica Subspecies. Foods, 10(10), 2448. https://doi.org/10.3390/foods10102448









